Assessment of Beaded, Powdered and Coated Desiccants for Atmospheric Water Harvesting in Arid Environments
Abstract
1. Introduction
2. Materials and Methods
Experimental Uncertainty
3. Results and Discussion
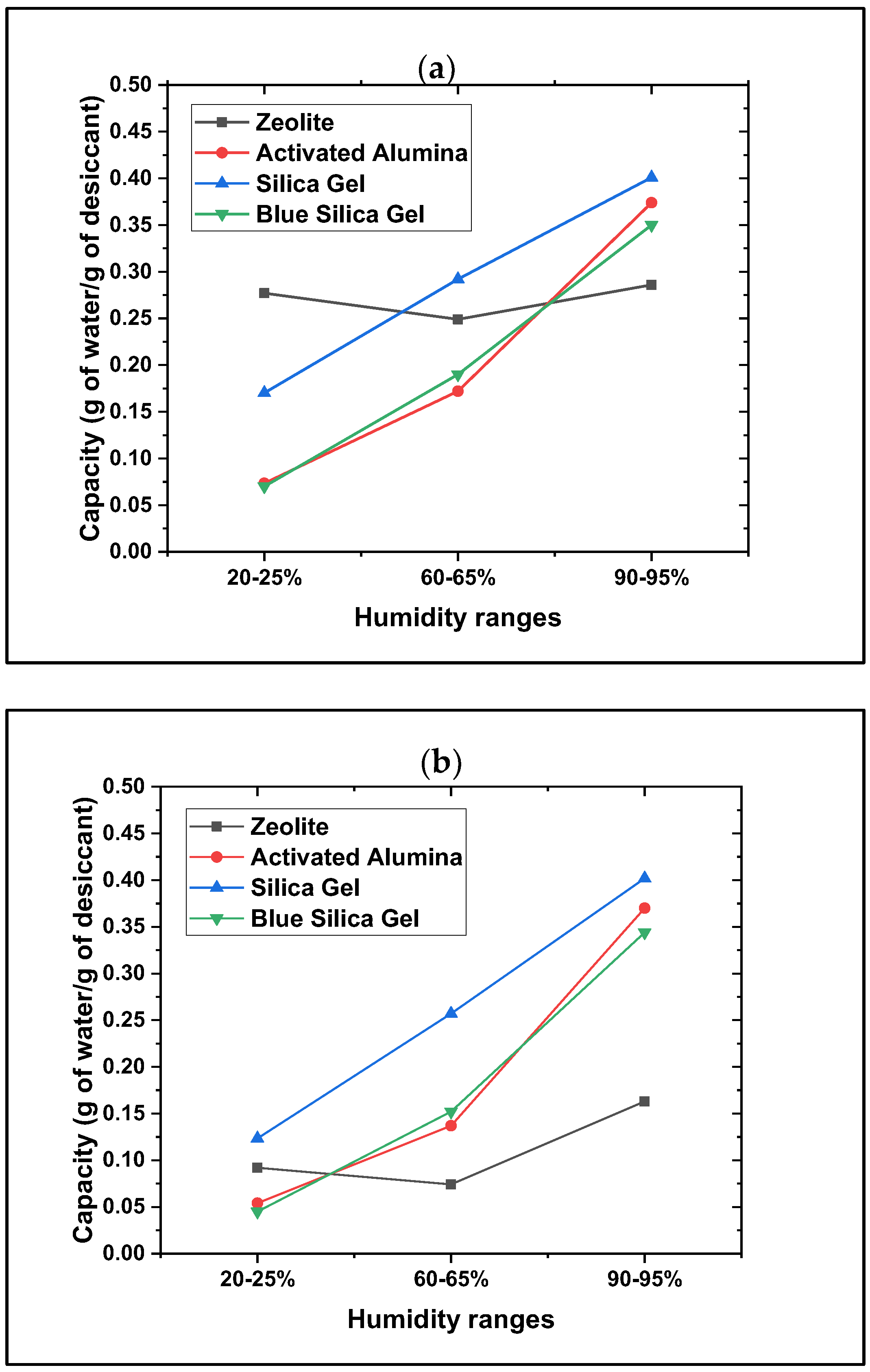
3.1. Beaded Desiccants
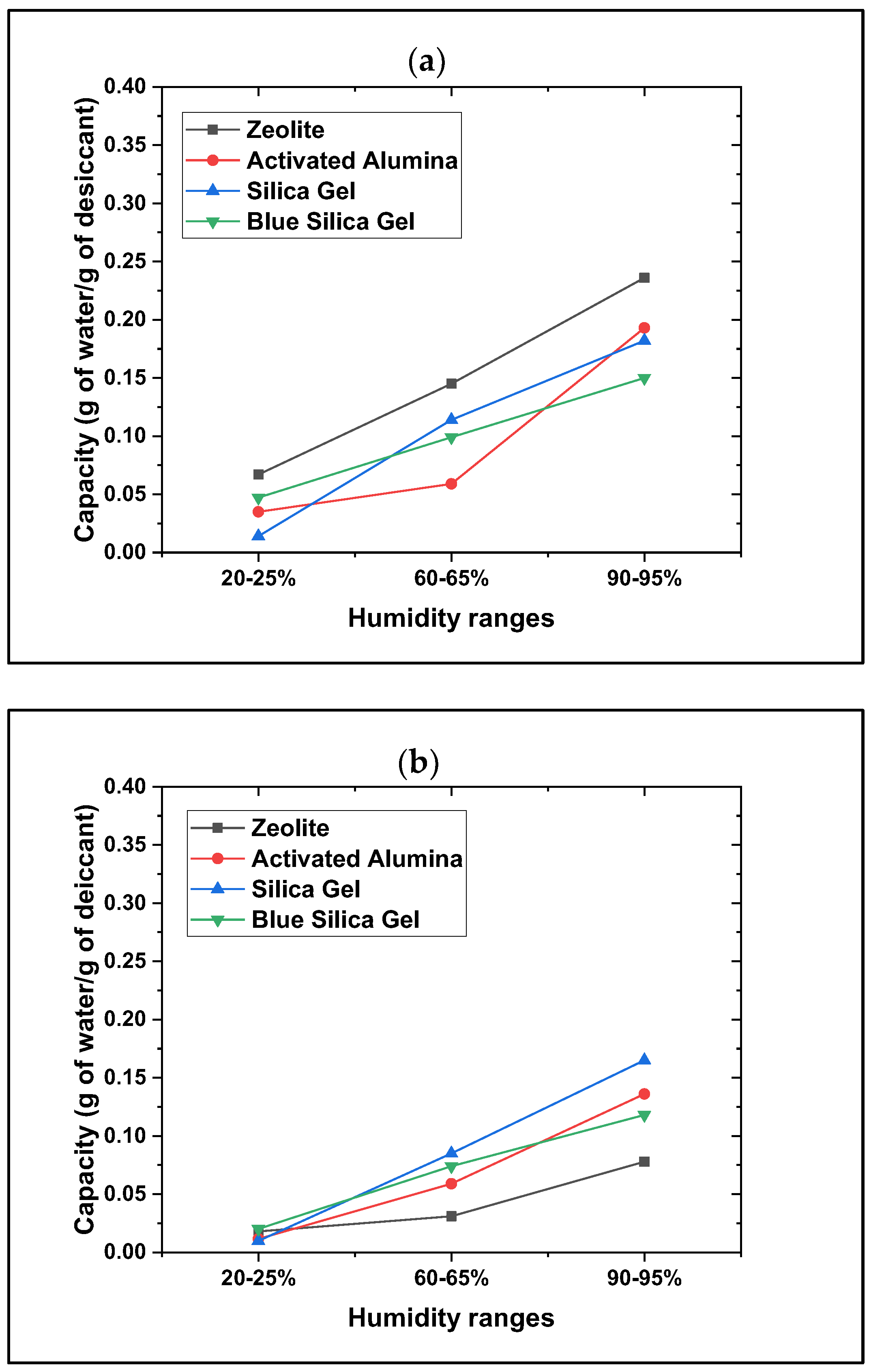
3.2. Powdered Desiccants
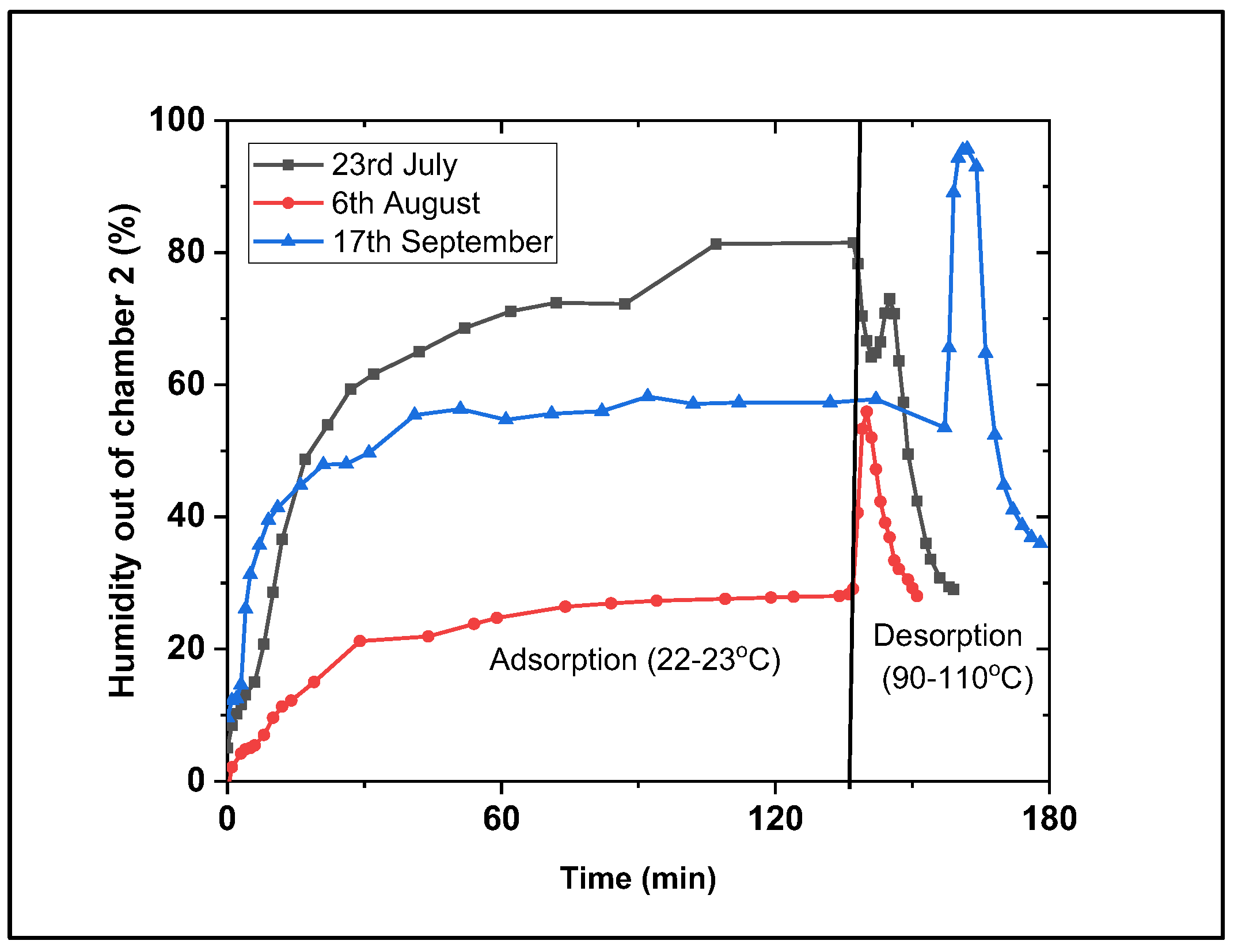
3.3. Coated Desiccants
4. Comparison Among Desiccant Forms
Characterization of the Desiccant Powders
5. Conclusions
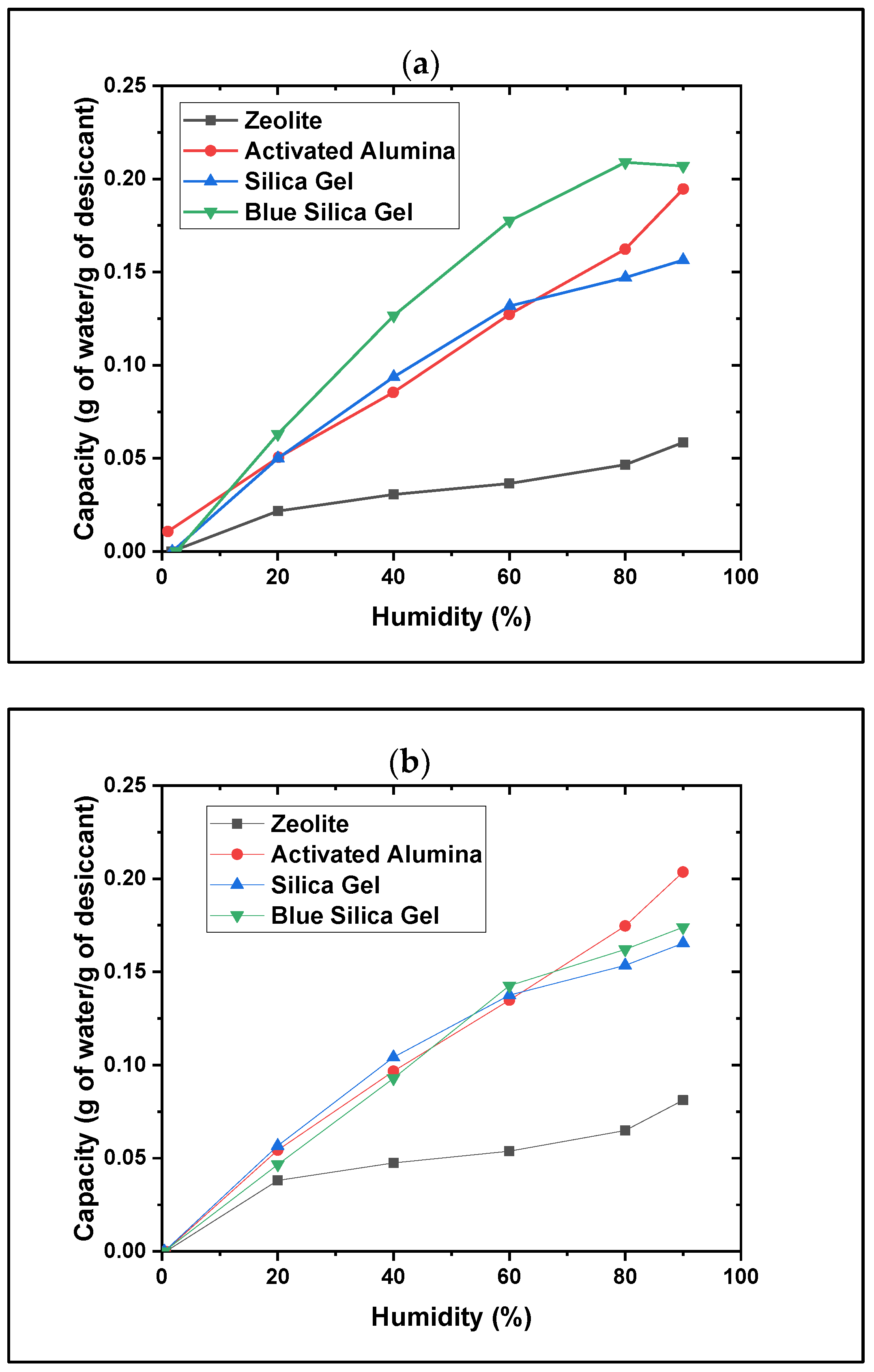
- All beaded desiccants were found to be effective. However, the effectiveness of water adsorption, desorption, and kinetics varied among the different types of desiccants. The analyses of the adsorption and desorption profiles, along with the kinetics and water adsorption and desorption capacities (g/g), reveal that beaded desiccants may require a considerably longer time (hours) to reach their saturation level, exhibiting slower water uptake followed by a slower release of water during regeneration. Additionally, they demonstrate long-term stability with no surface degradation observed over repeated tests and cycles. The results indicate that the adsorption and desorption capacities (g/g) increase as humidity rises.
- The powdered desiccants showed much faster moisture uptakes than beads, but the operation and handling of the powdered form as desiccants are more challenging than the beaded form. As powdered material, these are highly ground with fines (60–100 mesh sizes), can easily aerosolize, especially when dried, and can be harmful if inhaled; precautions are necessary during handling.
- The coated desiccant avoided the above issues of the powdered form and was observed to have the highest adsorption and desorption kinetics. Varying the air flow rate (0.6, 1.1, and 2.9 m/s) also impacts the kinetic speed but not the adsorption capacity. This indicated that it would be the best form (as opposed to bead and powder) for AWH and longer-term repeated use with effective cyclability options.
- A few experiments were repeated to ensure consistency; however, a thorough statistical analysis was not conducted. Future research could integrate rigorous statistical tests to improve the reliability and generalizability of the findings.
- The lifecycle considerations of the desiccant can be implemented for future recommendations.
- The test rig did not have a condenser in the module. The water quality can be assessed using different desiccants and types. Water quality also depends on the air. It varies from residences to labs, offices, and industries [9]. The combination of the parameters can be inculcated as a practical implementation to understand more about the water yield and quality.
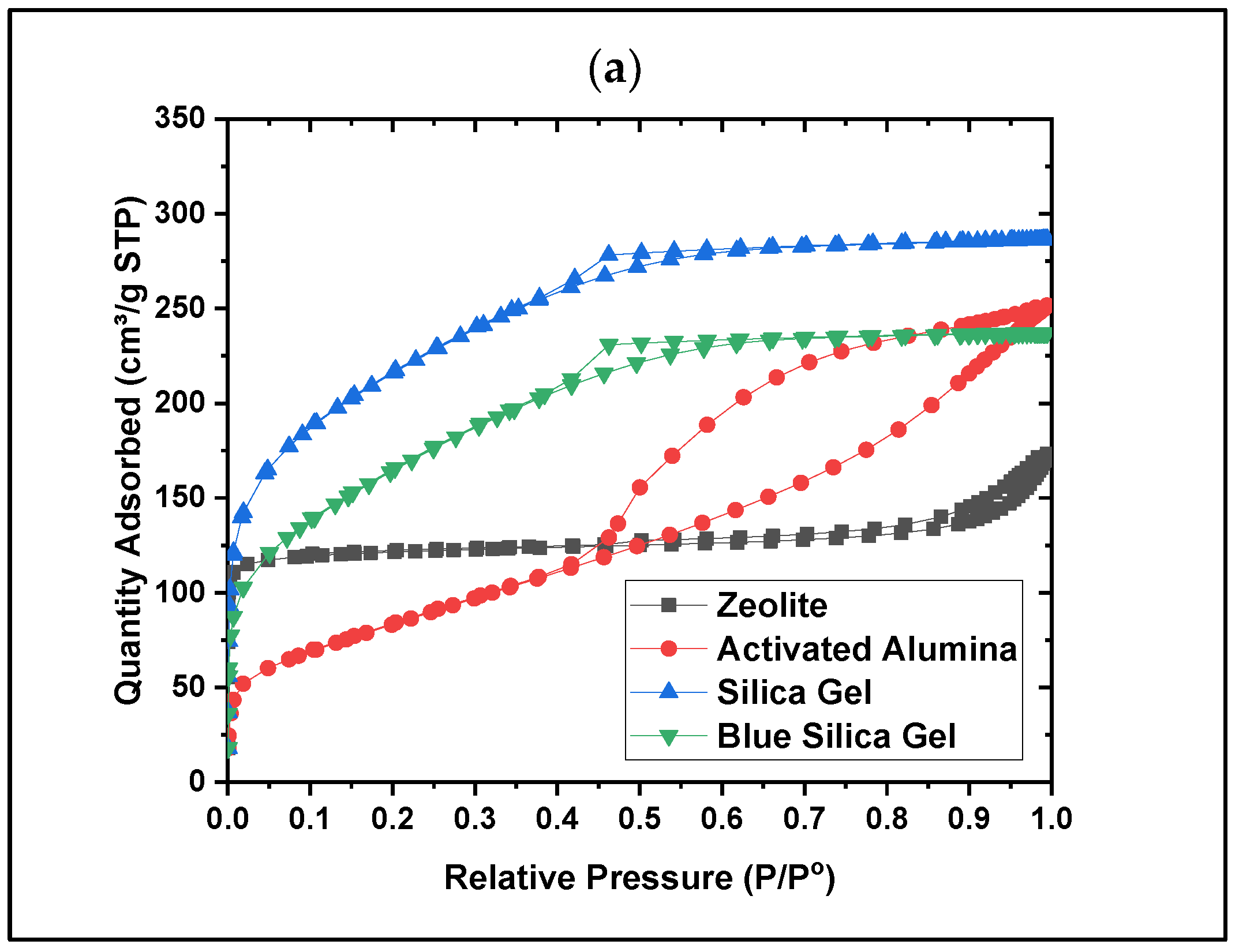
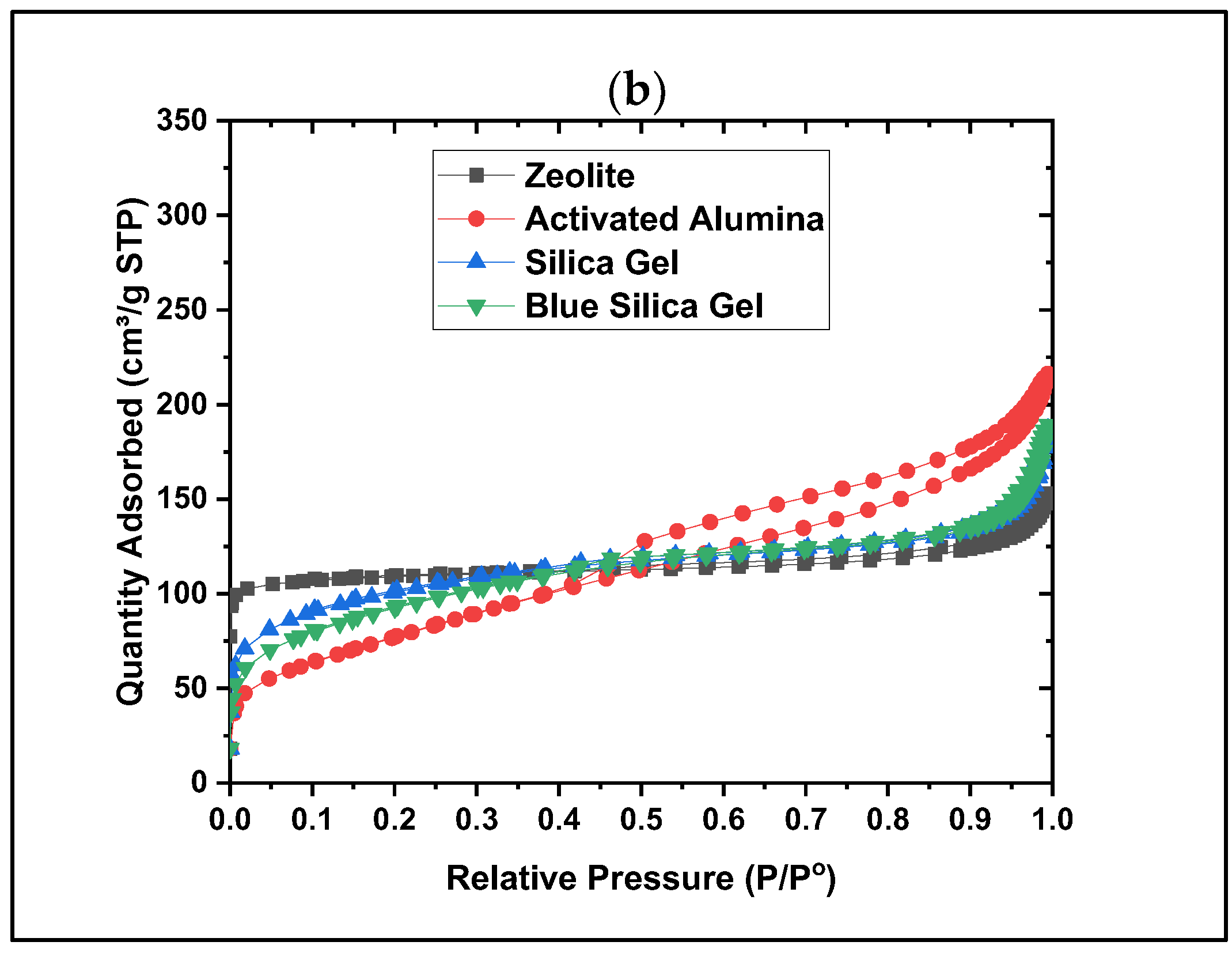
- Material characterization, such as Brunauer–Emmett–Teller (BET), has been performed to understand the pore geometry and structure. It has been shown that the mesopores for most desiccants (zeolite, activated alumina, and silica gels) are lost during the conversion of beaded to powder form (by ball-milling). In contrast, the silica gels in the beaded form showed the highest surface area (759 m2/g).
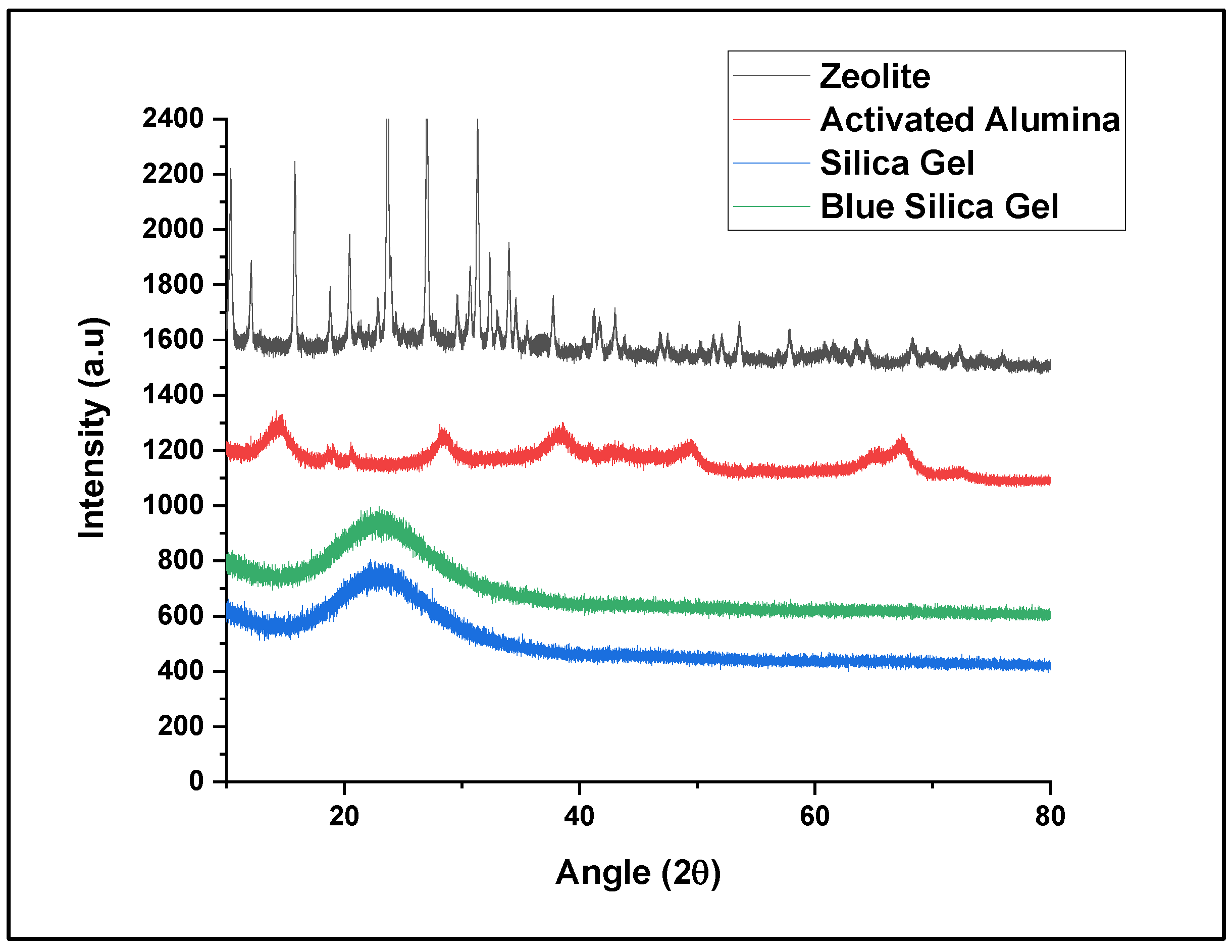
- Dynamic vapor sorption (DVS) data show that silica gel offers the highest water adsorption capacity, suggesting this material as the best water adsorber compared to the other tested materials. Based on the crystallinity analysis results obtained from XRD, no crystalline peaks were detected for the silica gel material, showing its amorphous nature. Collectively, these data show that the amorphous nature of silica gel leads to its higher water adsorption capacity due to its disordered structure and higher surface area, which provide more adsorption sites. The same behavior has been observed in the literature for amorphous adsorbents and adsorptive removal of contaminants from water [25].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruintjes, R.T. A Review of Cloud Seeding Experiments to Enhance Precipitation and Some New Prospects. Bull. Am. Meteor. Soc. 1999, 80, 805–820. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Xiang, T.; Xie, S.; Chen, G.; Zhang, C.; Guo, Z. Recent advances in atmospheric water harvesting technology and its development. Mater. Horiz. 2025, 12, 1084–1105. [Google Scholar] [CrossRef]
- Mulchandani, A.; Edberg, J.; Herckes, P.; Westerhoff, P. Seasonal atmospheric water harvesting yield and water quality using electric-powered desiccant and compressor dehumidifiers. Sci. Total Environ. 2022, 825, 153966. [Google Scholar] [CrossRef] [PubMed]
- Ahrestani, Z.; Sadeghzadeh, S.; Motejadded Emrooz, H.B. An overview of atmospheric water harvesting methods, the inevitable path of the future in water supply. RSC Adv. 2023, 13, 10273–10307. [Google Scholar] [CrossRef]
- Jarimi, H.; Powell, R.; Riffat, S. Review of sustainable methods for atmospheric water harvesting. Int. J. Low-Carbon Technol. 2020, 15, 253–276. [Google Scholar] [CrossRef]
- Valjarević, A.; Filipović, D.; Valjarević, D.; Milanović, M.; Milošević, S.; Živić, N.; Lukić, T. GIS and remote sensing techniques for the estimation of dew volume in the Republic of Serbia. Meteorol. Appl. 2020, 27, e1930. [Google Scholar] [CrossRef]
- Kaseke, K.F.; Wang, L. Fog and Dew as Potable Water Resources: Maximizing Harvesting Potential and Water Quality Concerns. GeoHealth 2018, 2, 327–332. [Google Scholar] [CrossRef]
- Zeng, C.; Mojiri, A.; Ananpattarachai, J.; Farsad, A.; Westerhoff, P. Sorption-based atmospheric water harvesting for continuous water production in the built environment: Assessment of water yield and quality. Water Res. 2024, 265, 122227. [Google Scholar] [CrossRef]
- Tian, G.; Fu, C.; Guo, Z. Metal-organic framework-based composite adsorbents for atmospheric water harvesting: Materials and devices. Mater. Today 2025, 83, 307–330. [Google Scholar] [CrossRef]
- Fanger, P.O. The New Comfort Equation for Indoor Air Quality. 1989. Available online: https://www.aivc.org/sites/default/files/airbase_4230.pdf (accessed on 1 February 2025).
- Desiccant—Molecular Sieve—Silica Gel—Activated Alumina. Available online: https://deltaadsorbents.com/ (accessed on 1 February 2025).
- Welcome to Dry & Dry—Premium Quality Silica Gel Desiccant. Available online: https://dryndry.com/ (accessed on 1 February 2025).
- Daghooghi-Mobarakeh, H.; Miner, M.; Wang, L.; Wang, R.; Phelan, P.E. Application of ultrasound in regeneration of silica gel for industrial gas drying processes. Dry. Technol. 2022, 40, 2251–2259. [Google Scholar]
- Mobarakeh, H.D.; Campbell, N.; Bertrand, W.K.; Kumar, P.G.; Tiwari, S.; Wang, L.; Wang, R.; Miner, M.; Phelan, P.E. Ultrasound-assisted regeneration of zeolite/water adsorption pair. Ultrason. Sonochem. 2020, 64, 105042. [Google Scholar]
- Daghooghi-Mobarakeh, H.; Miner, M.; Wang, L.; Wang, R.; Phelan, P.E. Ultrasound-assisted regeneration of activated alumina/water adsorption pair for drying and dehumidification processes. Ultrasonics 2022, 124, 106769. [Google Scholar]
- Mou, X.; Chen, Z. Experimental and predictive study on the performance and energy consumption characteristics for the regeneration of activated alumina assisted by ultrasound. Ultrason. Sonochem. 2021, 70, 105314. [Google Scholar] [CrossRef]
- 1 Gallon(7.5 LBS) “Dry & Dry” Premium Blue Indicating Silica Gel Desic. Available online: https://dryndry.com/products/1-gallon7-5-lbs-dry-dry-premium-blue-indicating-silica-gel-desiccant-beads (accessed on 1 February 2025).
- Wang, P.Y.; Guan, H.Y.; Liu, Z.H.; Wang, G.S.; Zhao, F.; Xiao, H.S. High temperature collecting performance of a new all-glass evacuated tubular solar air heater with U-shaped tube heat exchanger. Energy Convers. Manag. 2014, 77, 315–323. [Google Scholar]
- Hamzat, A.K.; Omisanya, M.I.; Sahin, A.Z.; Ropo Oyetunji, O.; Abolade Olaitan, N. Application of nanofluid in solar energy harvesting devices: A comprehensive review. Energy Convers. Manag. 2022, 266, 115790. [Google Scholar]
- Lei, C.; Guan, W.; Zhao, Y.; Yu, G. Chemistries and materials for atmospheric water harvesting. Chem. Soc. Rev. 2024, 53, 7328–7362. [Google Scholar]
- De Antonellis, S.; Bramanti, E.; Calabrese, L.; Campanella, B.; Freni, A. A novel desiccant compound for air humidification and dehumidification. Appl. Therm. Eng. 2022, 214, 118857. [Google Scholar]
- Eslamian, M.; Soltani-Kordshuli, F. Development of multiple-droplet drop-casting method for the fabrication of coatings and thin solid films. J. Coat. Technol. Res. 2018, 15, 271–280. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar]
- Farsad, A.; Niimi, K.; Ersan, M.S.; Gonzalez-Rodriguez, J.R.; Hristovski, K.D.; Westerhoff, P. Mechanistic Study of Arsenate Adsorption onto Different Amorphous Grades of Titanium (Hydr)Oxides Impregnated into a Point-of-Use Activated Carbon Block. ACS EST Eng. 2023, 3, 989–1000. [Google Scholar]
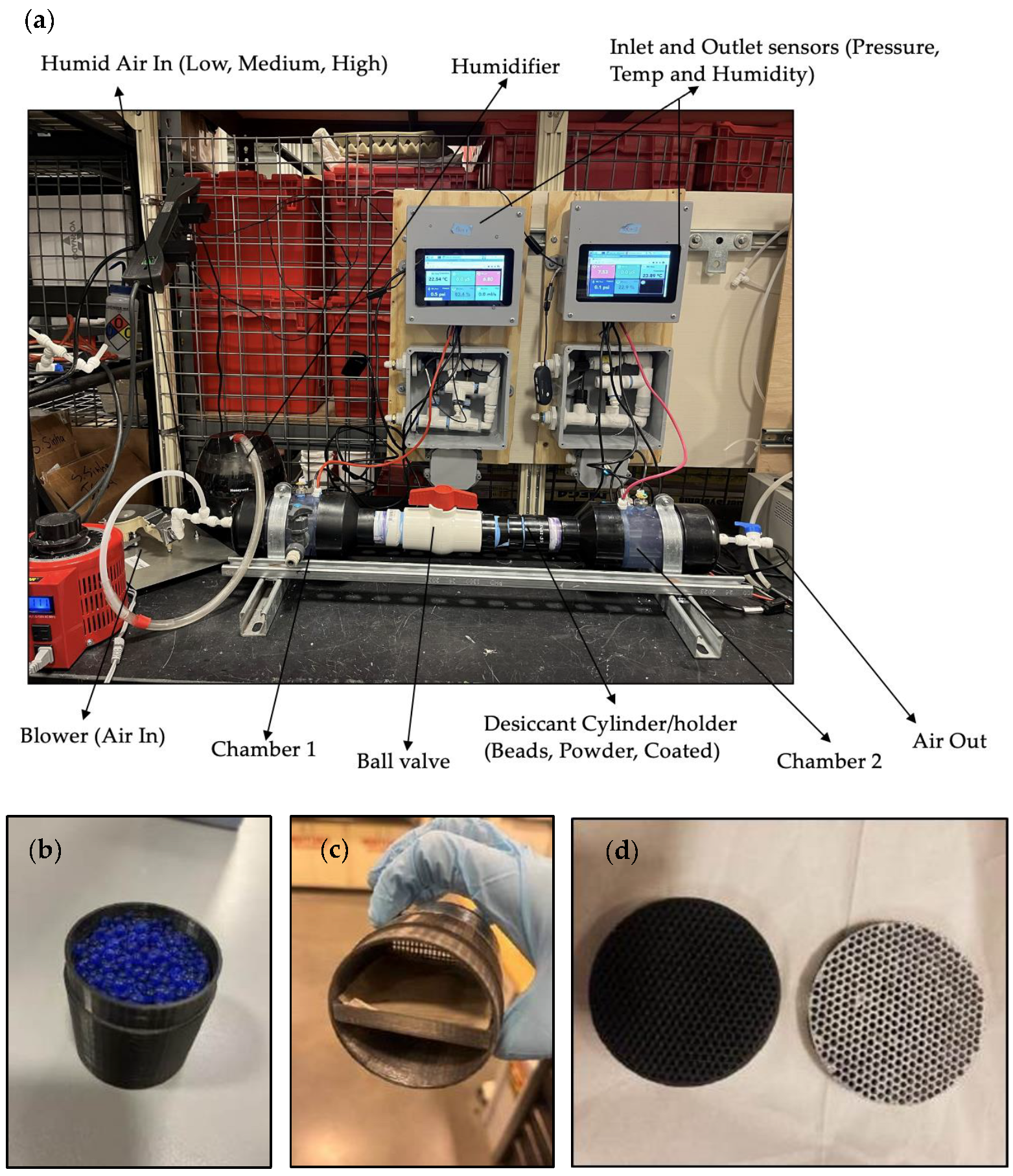



| Characteristics | Zeolite | Activated Alumina | Silica Gel | Blue Silica Gel |
|---|---|---|---|---|
| Bead diameter (mm) | 3 to 5 | 3.175 | 3.5 | 2 to 4 |
| Pore diameter (nm) | 1.3 | 4.8 | 2 to 3 | Not given |
| Density (kg/m3) | 689 | 769 | 700 | 829 |
| BET surface area (m2/g) (measured) | 781 | 298 | 759 | 578 |
| Regeneration temperature (°C) | 250 to 300 | 110 to 198 | ~100 | ~121 |
| Humidity | Range (%) | Flow Rates During Adsorption Process (m/s) |
|---|---|---|
| Low humidity | ~20 to 40 | ~1 |
| Moderate humidity | ~60 to 65 | ~1 |
| High humidity | ~90 to 95 | ~1 |
| Parameters | Uncertainty |
|---|---|
| Weighing pan (Weight) (g) | ±1% |
| Sensors: temperature, humidity, and pressure (°C,% and psi) | ±0.4%, ±2%, ±0.1% |
| Thermocouple integrated into the heater (temperature) (°C) | ±0.1% |
| Air flow rate (m/s) | ±3% |
| Characteristics | Beaded Desiccants | Powdered Desiccants | Coated Desiccants |
|---|---|---|---|
| Shape and Size | Spherical beads (2 to 5 mm) | Finely crushed particles (<500 microns) | Powder coated on the perforated disks |
| Porosity | Mesopores (2 to 50 nm) | Micropores (<2 nm) | Coated onto a highly porous substrate |
| Mechanical Stability | High durability, easy to handle | Hard to handle due to being a powder, clumping | Easy to handle |
| Airflow | Better airflow | Airflow might be affected due to clomping | Excellent airflow |
| Desiccants | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Zeolite | 781 | 0.052 | 5.04 |
| Activated Alumina | 298 | 0.380 | 4.03 |
| Silica Gel | 759 | 0.354 | 2.57 |
| Blue Silica Gel | 578 | 0.350 | 2.61 |
| Desiccants | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Zeolite | 912 | 0.045 | 4.39 |
| Activated Alumina | 276 | 0.293 | 3.64 |
| Silica Gel | 359 | 0.137 | 2.89 |
| Blue Silica Gel | 578 | 0.176 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafat, M.; Chandrasekaran, G.; Shrivastava, S.; Farsad, A.; Ananpattarachai, J.; Qiu, A.; Sinha, S.; Westerhoff, P.; Phelan, P. Assessment of Beaded, Powdered and Coated Desiccants for Atmospheric Water Harvesting in Arid Environments. Environments 2025, 12, 110. https://doi.org/10.3390/environments12040110
Rafat M, Chandrasekaran G, Shrivastava S, Farsad A, Ananpattarachai J, Qiu A, Sinha S, Westerhoff P, Phelan P. Assessment of Beaded, Powdered and Coated Desiccants for Atmospheric Water Harvesting in Arid Environments. Environments. 2025; 12(4):110. https://doi.org/10.3390/environments12040110
Chicago/Turabian StyleRafat, Mona, Gokul Chandrasekaran, Shubham Shrivastava, Alireza Farsad, Jirapat Ananpattarachai, Abigail Qiu, Shahnawaz Sinha, Paul Westerhoff, and Patrick Phelan. 2025. "Assessment of Beaded, Powdered and Coated Desiccants for Atmospheric Water Harvesting in Arid Environments" Environments 12, no. 4: 110. https://doi.org/10.3390/environments12040110
APA StyleRafat, M., Chandrasekaran, G., Shrivastava, S., Farsad, A., Ananpattarachai, J., Qiu, A., Sinha, S., Westerhoff, P., & Phelan, P. (2025). Assessment of Beaded, Powdered and Coated Desiccants for Atmospheric Water Harvesting in Arid Environments. Environments, 12(4), 110. https://doi.org/10.3390/environments12040110






