Evaluating the Efficacy of Thiolating Agents for Biochar Surface Modification
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Instrumentation
2.3. Preparation of Pristine and Thiolated Biochar
2.3.1. Chemical Impregnation with the 3-MPTS Reagent (BX-3M Thiolated Biochar Sample Series)
2.3.2. Ball Milling with the 3-MPTS Reagent (BX-BA Thiolated Biochar Sample Series)
2.3.3. Chemical Impregnation with β-Mercaptoethanol Reagent (BX-BM Thiolated Biochar Sample Series)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Isaac, D.; Labbancz, J.; Knowles, N.R.; Tenic, E.; Horgan, A.; Ghogare, R.; Dhingra, A. Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality. Horticulturae 2024, 10, 368. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Saini, I.; Parmar, S.; Pundir, V.; Kumar, V.; Kumar, V.; Naik, B.; Rustagi, S. Biochar Production Methods and Their Transformative Potential for Environmental Remediation. Discov. Appl. Sci. 2024, 6, 408. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and Beneficial Impact of Biochar for Environmental Application: A Comprehensive Review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.-K.; Jung, J.; Hyun, S. Comparison of Biochar Properties from Biomass Residues Produced by Slow Pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Kumar, R.; Sahoo, P.K.; Nadda, A.K. Recent Advances in Biochar Amendments for Immobilization of Heavy Metals in an Agricultural Ecosystem: A Systematic Review. Environ. Pollut. 2023, 319, 120937. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, S. Advances in the Study of Heavy Metal Adsorption from Water and Soil by Modified Biochar. Water 2022, 14, 3894. [Google Scholar] [CrossRef]
- Gogoi, L.; Narzari, R.; Chutia, R.S.; Borkotoki, B.; Gogoi, N.; Kataki, R. Chapter Two—Remediation of Heavy Metal Contaminated Soil: Role of Biochar. In Advances in Chemical Pollution, Environmental Management and Protection; Sarmah, A.K., Ed.; Biochar: Fundamentals and Applications in Environmental Science and Remediation Technologies; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 39–63. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, A. Effect of Biochars on Adsorption of Cu(II), Pb(II) and Cd(II) by Three Variable Charge Soils from Southern China. Environ. Sci. Pollut. Res. 2013, 20, 8491–8501. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks and Applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Baquy, M.A.-A.; Mia, S.; Odii, E.C.; Xu, R. Reduction of Heavy Metal Uptake from Polluted Soils and Associated Health Risks Through Biochar Amendment: A Critical Synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Chen, M.; Bao, C.; Hu, D.; Jin, X.; Huang, Q. Facile and Low-Cost Fabrication of ZnO/Biochar Nanocomposites from Jute Fibers for Efficient and Stable Photodegradation of Methylene Blue Dye. J. Anal. Appl. Pyrolysis 2019, 139, 319–332. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, Environmental Application and Prospect of Biochar-Supported Metal Nanoparticles: A Review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, B.; Theng, B.K.G.; Wu, P.; Liu, F.; Wang, S.; Lee, X.; Chen, M.; Li, L.; Zhang, X. Formation and Mechanisms of Nano-Metal Oxide-Biochar Composites for Pollutants Removal: A Review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar] [CrossRef] [PubMed]
- Karpuraranjith, M.; Chen, Y.; Ramadoss, M.; Wang, B.; Yang, H.; Rajaboopathi, S.; Yang, D. Magnetically Recyclable Magnetic Biochar Graphitic Carbon Nitride Nanoarchitectures for Highly Efficient Charge Separation and Stable Photocatalytic Activity Under Visible-Light Irradiation. J. Mol. Liq. 2021, 326, 115315. [Google Scholar] [CrossRef]
- Ahuja, R.; Kalia, A.; Sikka, R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review. ACS Omega 2022, 7, 45825–45836. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar Pyrolytically Produced from Municipal Solid Wastes for Aqueous As(V) Removal: Adsorption Property and Its Improvement with KOH Activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Yousaf, B.; Fu, B.; Ullah, H.; Ali, M.U.; Abbas, Q.; Mujtaba Munir, M.A.; Ruijia, L. Simultaneous Functionalization and Magnetization of Biochar Via NH3 Ambiance Pyrolysis for Efficient Removal of Cr (VI). Chemosphere 2018, 208, 712–721. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of Heavy Metals on Chitosan-Modified Biochars and Its Biological Effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of Cadmium and Lead Polluted Soil Using Thiol-Modified Biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Fang, L.; Zhao, B.; Zhang, Y.; Zhu, Y.; Wang, Z.; Wang, Q.; Li, F. Interfacial Chemistry of Mercury on Thiol-Modified Biochar and Its Implication for Adsorbent Engineering. Chem. Eng. J. 2023, 454, 140310. [Google Scholar] [CrossRef]
- Ul Hasan, I.M.; Niazi, N.K.; Bibi, I.; Younas, F.; Al-Misned, F.; Shakoor, M.B.; Ali, F.; Ilyas, S.; Hussain, M.M.; Qiao, J.; et al. Enhanced Capacity of Thiol-Functionalized Sugarcane Bagasse and Rice Husk Biochars for Arsenite Sorption in Aqueous Solutions. Environ. Sci. Pollut. Res. 2024, 31, 52293–52305. [Google Scholar] [CrossRef]
- Jiao, Z.-Q.; Ge, S.-J.; Zheng, W.-X.; Liu, J.-H.; Chen, M.; Kong, Y.-K.; Wang, Y.-Y. Stabilization of Cd-contaminated Soil with Thiol-modified Biochar and Response of Soil Microorganisms. Huan Jing Ke Xue 2024, 45, 5570–5577. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, J.; Liu, W.; Huang, S.; Chen, X.; Zhang, N.; Huang, Y. Mercury Speciation Transformation Mediated by Thiolated Biochar in High Salinity Groundwater: Interfacial Processes, Influencing Factors, and Mechanisms. Chem. Eng. J. 2024, 484, 149443. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced Heavy Metals Sorption by Modified Biochars Derived from Pig Manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Lyu, H.; Xia, S.; Tang, J.; Zhang, Y.; Gao, B.; Shen, B. Thiol-Modified Biochar Synthesized by a Facile Ball-Milling Method for Enhanced Sorption of Inorganic Hg2+ and Organic CH3Hg+. J. Hazard. Mater. 2020, 384, 121357. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Wang, L.; Lyu, H.; Xia, S.; Tang, J. Effective Removal of Hg(II) and MeHg from Aqueous Environment by Ball Milling Aided Thiol-Modification of Biochars: Effect of Different Pyrolysis Temperatures. Chemosphere 2022, 294, 133820. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Huang, Y.; Tang, J.; Wang, L. Preparation of Various Thiol-Functionalized Carbon-Based Materials for Enhanced Removal of Mercury from Aqueous Solution. Environ. Sci. Pollut. Res. 2019, 26, 8709–8720. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Marroquín, M.C.; Carbonel, D.; Esquivel, S.; Colorado, H. Thiol-Modified Olive-Stone Biochar Preparation for Hg(II) Removal from Aqueous Solutions. J. Environ. Eng. Sci. 2024, 19, 177–188. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, S.; Lyu, J.; Tang, J. Highly Efficient Removal of Aqueous Hg2+ and CH3Hg+ by Selective Modification of Biochar with 3-Mercaptopropyltrimethoxysilane. Chem. Eng. J. 2019, 360, 1646–1655. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Gai, L.; Gong, Y.; Guan, H.; He, R.; Lyu, H. Different Approaches for Preparing a Novel Thiol-Functionalized Graphene Oxide/Fe-Mn and Its Application for Aqueous Methylmercury Removal. Chem. Eng. J. 2017, 319, 229–239. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Jiang, S.-J.; Tseng, W.-L. Facile Synthesis and Characterization of Thiol-Functionalized Graphene Oxide as Effective Adsorbent for Hg(II). J. Environ. Chem. Eng. 2016, 4, 2052–2065. [Google Scholar] [CrossRef]
- Kokkinos, E.; Lampou, A.; Kellartzis, I.; Karfaridis, D.; Zouboulis, A. Thiol-Functionalization Carbonaceous Adsorbents for the Removal of Methyl-Mercury from Water in the ppb Levels. Water 2022, 14, 49. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Chai, L.; Li, Q.; Zhu, Y.; Zhang, Z.; Wang, Q.; Wang, Y.; Yang, Z. Synthesis of Thiol-Functionalized Spent Grain as a Novel Adsorbent for Divalent Metal Ions. Bioresour. Technol. 2010, 101, 6269–6272. [Google Scholar] [CrossRef]
- Matuana, L.M.; Balatinecz, J.J.; Sodhi, R.N.S.; Park, C.B. Surface Characterization of Esterified Cellulosic Fibers by XPS and FTIR Spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Boyaci, I.H.; Eksi-Kocak, H. Determination of Chemical Changes in Heat-Treated Wood Using ATR-FTIR and FT Raman Spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 395–400. [Google Scholar] [CrossRef]
- Aldana-Pérez, A.; Lartundo-Rojas, L.; Gómez, R.; Niño-Gómez, M.E. Sulfonic Groups Anchored on Mesoporous Carbon Starbons-300 and Its Use for the Esterification of Oleic Acid. Fuel 2012, 100, 128–138. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Auvergne, R.; Boutevin, B.; David, G. Biobased Cross-Linked Polyurethanes Obtained from Ester/Amide Pseudo-Diols of Fatty Acid Derivatives Synthesized by Thiol–Ene Coupling. Polym. Chem. 2012, 3, 450–457. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Y.; Tang, J.; Xia, S. Effective Removal of Inorganic Mercury and Methylmercury from Aqueous Solution Using Novel Thiol-Functionalized Graphene Oxide/Fe-Mn Composite. J. Hazard. Mater. 2019, 366, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lei, H.; Wang, L.; Yadavalli, G.; Zhang, X.; Wei, Y.; Liu, Y.; Yan, D.; Chen, S.; Ahring, B. Biochar of Corn Stover: Microwave-Assisted Pyrolysis Condition Induced Changes in Surface Functional Groups and Characteristics. J. Anal. Appl. Pyrolysis 2015, 115, 149–156. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y. Construction of a 3D Multiple Network Skeleton by the Thiol-Michael Addition Click Reaction to Fabricate Novel Polymer/Graphene Aerogels with Exceptional Thermal Conductivity and Mechanical Properties. J. Mater. Chem. A 2017, 5, 22352–22360. [Google Scholar] [CrossRef]
- Samal, S.; Geckeler, K.E. Unexpected Solute Aggregation in Water on Dilution. Chem. Commun. 2001, 21, 2224–2225. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of Ball Milling on the Physicochemical and Sorptive Properties of Biochar: Experimental Observations and Governing Mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Julien, F.; Baudu, M.; Mazet, M. Relationship Between Chemical and Physical Surface Properties of Activated Carbon. Water Res. 1998, 32, 3414–3424. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of Surface and Porosity of Biochar on Water Holding Capacity Aiming Indirectly at Preservation of the Amazon Biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhao, H.; Lyu, H.; Wang, L.; Huang, H.; Nan, Q.; Tang, J. UV Modification of Biochar for Enhanced Hexavalent Chromium Removal from Aqueous Solution. Environ. Sci. Pollut. Res. 2018, 25, 10808–10819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ling, X.Y.; Guo, B.; Hong, L.; Lee, J.Y. Pt and PtRu Nanoparticles Deposited on Single-Wall Carbon Nanotubes for Methanol Electro-Oxidation. J. Power Sources 2007, 167, 272–280. [Google Scholar] [CrossRef]
- Carriere, B.; Deville, J.P.; Brion, D.; Escard, J. X-Ray Photoelectron Study of Some Silicon-Oxygen Compounds. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 85–91. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-Doped Biochar Synthesized by a Facile Ball-Milling Method for Enhanced Sorption of CO2 and Reactive Red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]



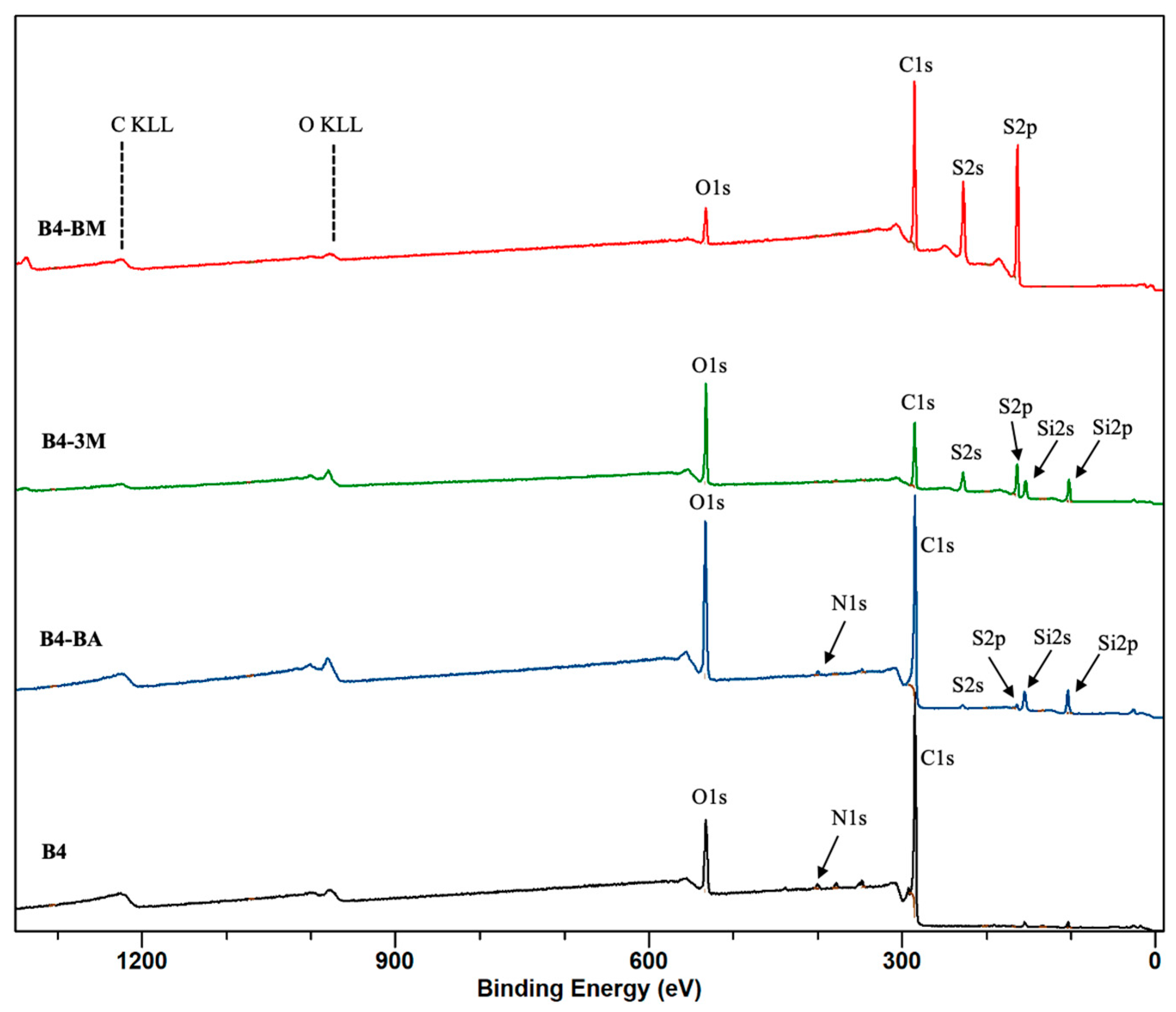
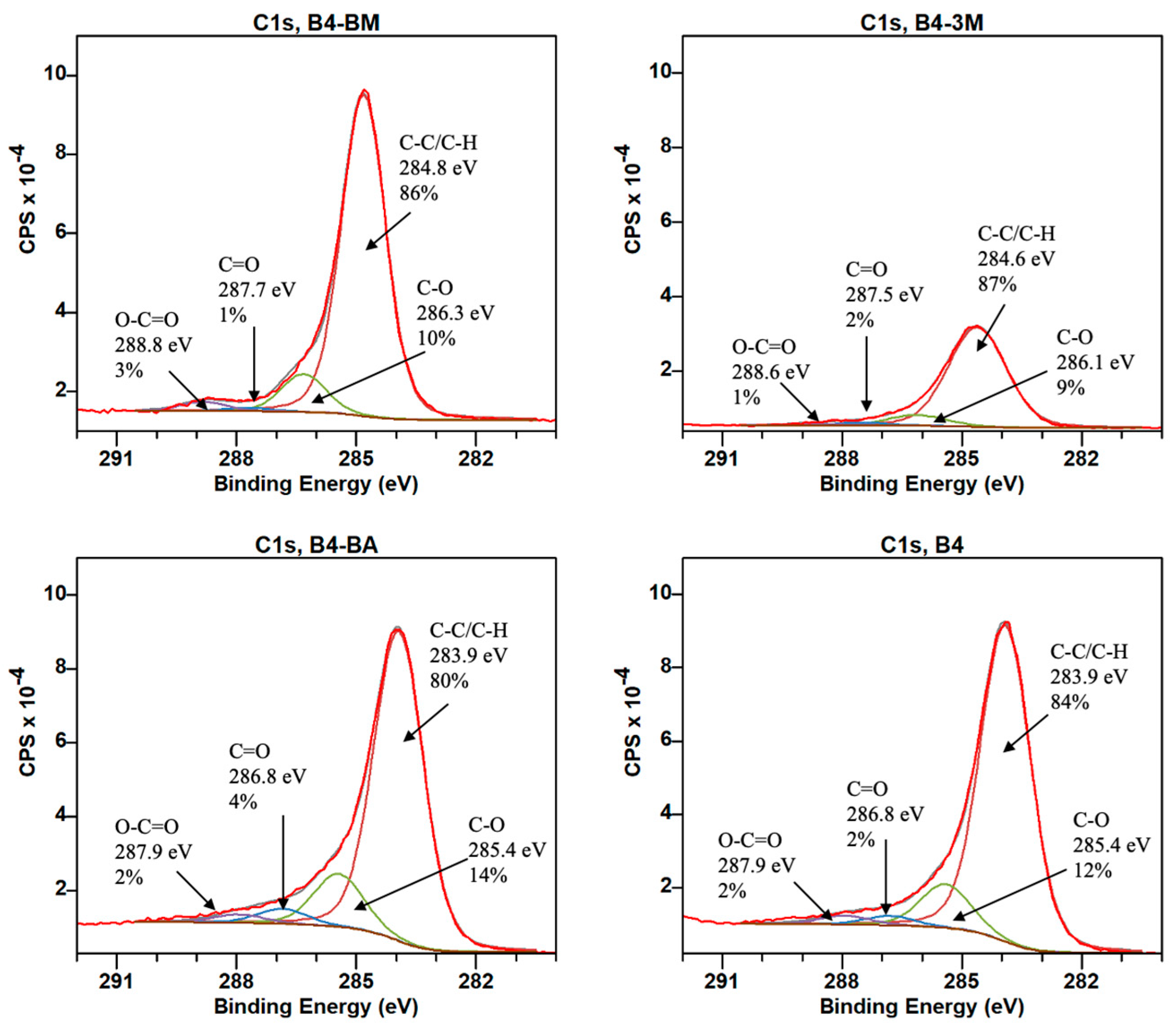

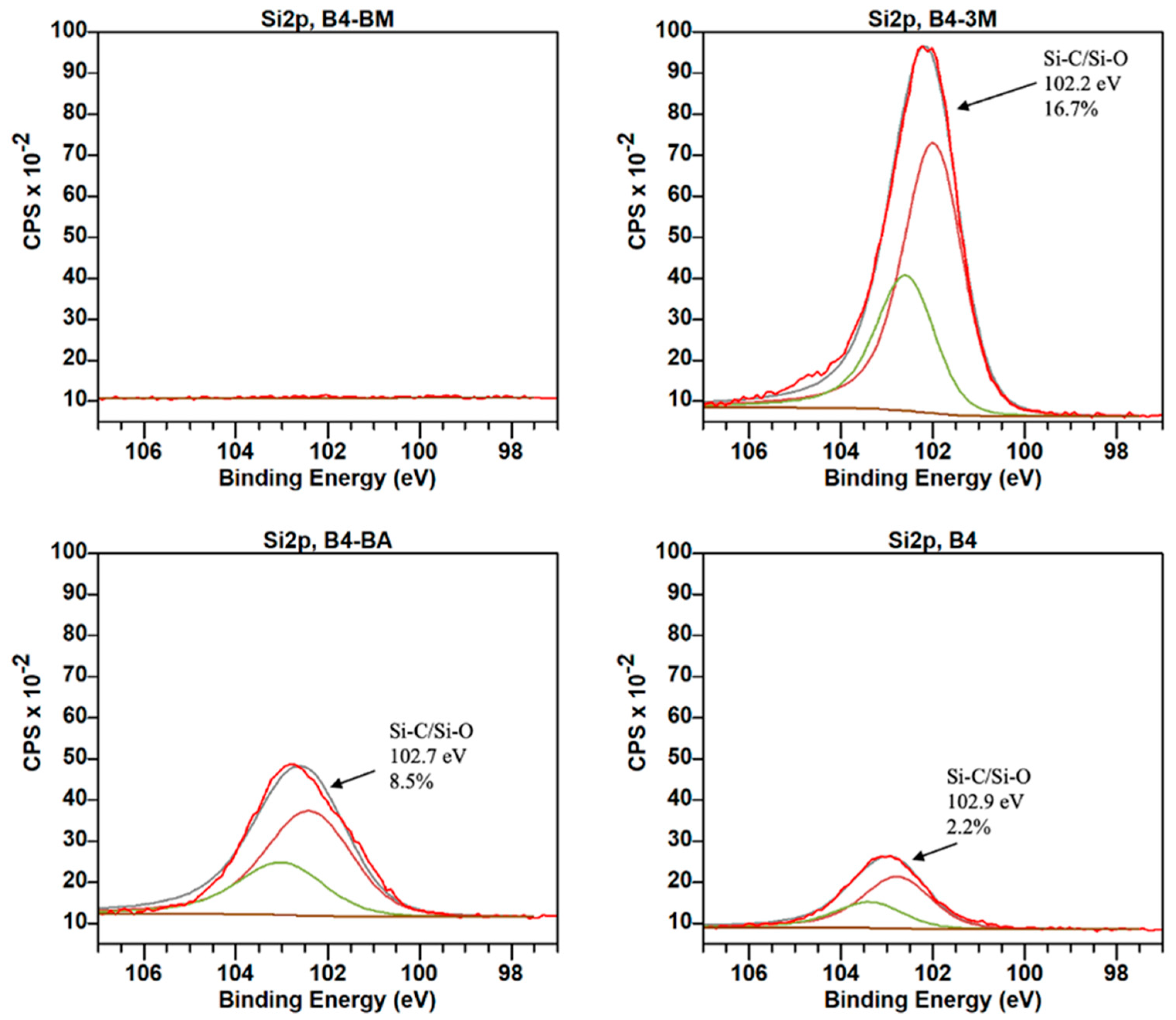
| B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | |
|---|---|---|---|---|---|---|---|---|---|
| Pristine | −26.01 ± 1.19 | −23.37 ± 0.56 | −26.00 ± 4.55 | −48.45 ± 1.92 | −24.48 ± 1.99 | −18.78 ± 1.86 | −31.78 ± 1.59 | −43.03 ± 1.67 | −40.97 ± 1.81 |
| BX-3M | −47.7 ± 1.45 | −43.08 ± 2.64 | −33.33 ± 4.06 | −50.45 ± 2.13 | −40.62 ± 2.30 | −51.38 ± 2.80 | −52.37 ± 1.95 | −35.68 ± 2.33 | −49.27 ± 1.67 |
| BX-BM | −1.91 ± 1.11 | −4.56 ± 2.25 | −2.21 ± 1.76 | −2.43 ± 2.19 | −3.23 ± 3.24 | −4.78 ± 0.44 | −0.52 ± 1.96 | −0.82 ± 1.17 | 1.72 ± 1.15 |
| BX-BA | −43.68 ± 4.59 | −44.78 ± 1.68 | −47.23 ± 2.16 | −45.00 ± 1.61 | −46.13 ± 2.11 | −48.87 ± 1.87 | −38.93 ± 1.18 | −48.55 ± 1.60 | −44.95 ± 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aduloju, O.; Pandey, A.; Eivazi, F.; Bardhan, S.; Afrasiabi, Z. Evaluating the Efficacy of Thiolating Agents for Biochar Surface Modification. Environments 2025, 12, 84. https://doi.org/10.3390/environments12030084
Aduloju O, Pandey A, Eivazi F, Bardhan S, Afrasiabi Z. Evaluating the Efficacy of Thiolating Agents for Biochar Surface Modification. Environments. 2025; 12(3):84. https://doi.org/10.3390/environments12030084
Chicago/Turabian StyleAduloju, Oluyinka, Arnav Pandey, Frieda Eivazi, Sougata Bardhan, and Zahra Afrasiabi. 2025. "Evaluating the Efficacy of Thiolating Agents for Biochar Surface Modification" Environments 12, no. 3: 84. https://doi.org/10.3390/environments12030084
APA StyleAduloju, O., Pandey, A., Eivazi, F., Bardhan, S., & Afrasiabi, Z. (2025). Evaluating the Efficacy of Thiolating Agents for Biochar Surface Modification. Environments, 12(3), 84. https://doi.org/10.3390/environments12030084







