Antibiotic Resistance Genes in Food Animal Production: Environmental Implications and One Health Challenges
Abstract
1. Introduction
2. Materials and Methods
3. AMR in Animal Sector and Aquaculture
3.1. Cattle
3.2. Small Ruminants
3.3. Poultry
3.4. Swine
3.5. Aquaculture
3.6. Mitigation Strategies
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARB | Antibiotic-resistant bacteria |
| ARGs | Antibiotic resistance genes |
| MGEs | Mobile genetic elements |
| MRSA | Staphylococcus aureus |
| HGT | Horizontal gene transfer |
| BRD | Bovine respiratory disease |
| ESBL | extended-spectrum β-lactamase |
| LMICs | low- and middle-income countries |
| PCU | Population Corrected Unit |
| DDDvet | Defined Daily Doses |
| DCDvet | Defined Course Doses |
| MDR | Multidrug-resistant |
| ESVAC | European Surveillance of Veterinary Antimicrobial Consumption |
| IMTA | integrated multi-trophic aquaculture |
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Canica, M. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Moulin, G.; Cavalié, P.; Pellanne, I.; Chevance, A.; Laval, A.; Millemann, Y.; Colin, P.; Chauvin, C.; Antimicrobial Resistance ad hoc Group of the French Food Safety Agency. A comparison of antimicrobial usage in human and veterinary medicine in France from 1999 to 2005. J. Antimicrob. Chemother. 2008, 62, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Ikhimiukor, O.O.; Okeke, I.N. A snapshot survey of antimicrobial resistance in food-animals in low and middle-income countries. One Health 2023, 16, 100489. [Google Scholar] [CrossRef]
- De Alcântara Rodrigues, I.; Ferrari, R.G.; Panzenhagen, P.H.N.; Mano, S.B.; Conte-Junior, C.A. Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 2020, 112, 143–183. [Google Scholar] [CrossRef]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. London: Wellcome Trust and HM Government. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 11 August 2025).
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 September 2025).
- Centers for Disease Control and Prevention. Antibiotic Resistance and Food Are Connected. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/causes/environmental-food.html?CDC_AAref_Val=https://www.cdc.gov/drugresistance/food.html (accessed on 12 August 2025).
- Food and Agriculture Organization of the United Nations. Food Safety. Antimicrobial Resistance. 2019. Available online: http://www.fao.org/antimicrobial-resistance/key-sectors/food-safety/en (accessed on 11 August 2025).
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 3rd ed.; WHO: Geneva, Switzerland, 2011. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 September 2025).
- Klous, G.; Huss, A.J.; Heederik, D.J.J.; Coutinho, R.A. Human–livestock contacts and their relationship to transmission of zoonotic pathogens: A systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef]
- Abdugheni, R.; Li, L.; Yang, Z.N.; Huang, Y.; Fang, B.Z.; Shurigin, V.; Mohamad, O.A.A.; Liu, Y.-H.; Li, W.-J. Microbial risks caused by livestock excrement: Current research status and prospects. Microorganisms 2023, 11, 1897. [Google Scholar] [CrossRef]
- Conceição, S.; Queiroga, M.C.; Laranjo, M. Antimicrobial resistance in bacteria from meat and meat products: A One Health perspective. Microorganisms 2023, 11, 2581. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Lawlor, P.G. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) prevalence in humans in close contact with animals and measures to reduce on-farm colonization. Ir. Vet. J. 2021, 74, 21. [Google Scholar] [CrossRef]
- Pouquet, M.; Bareille, N.; Guatteo, R.; Moret, L.; Beaudeau, F. Coxiella burnetii infection in humans: To what extent do cattle in infected areas free from small ruminants play a role? Epidemiol. Infect. 2020, 148, e232. [Google Scholar] [CrossRef]
- Mayo-Yáñez, M.; González-Torres, L. Recurrent penicillin-resistant tonsillitis due to Lactococcus garvieae, a new zoonosis from aquaculture. Zoonotic Dis. 2023, 3, 1–5. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Rosner, M. Meat and Dairy Production. Data Adapted from Food and Agriculture Organization of the United Nations—With Major Processing by Our World in Data. 2023. Available online: https://ourworldindata.org/meat-production (accessed on 12 September 2025).
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [CrossRef]
- Luo, T.; Dai, X.; We, W.; Xu, Q.; Ni, B.J. Microplastics enhance the prevalence of antibiotic resistance genes in anaerobic sludge digestion by enriching antibiotic-resistant bacteria in surface biofilm and facilitating the vertical and horizontal gene transfer. Environ. Sci. Technol. 2023, 57, 14611–14621. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Hyde, R.M.; Remnant, J.G.; Bradley, A.J.; Breen, J.E.; Hudson, C.D.; Davies, P.L.; Clarke, T.; Critchell, Y.; Hylands, M.; Linton, E.; et al. Quantitative analysis of antimicrobial use on British dairy farms. Vet. Rec. 2017, 181, 683. [Google Scholar] [CrossRef]
- Crosby, S.; Credille, B.; Giguère, S.; Berghaus, R. Comparative efficacy of enrofloxacin to that of tulathromycin for the control of bovine respiratory disease and prevalence of antimicrobial resistance in Mannheimia haemolytica in calves at high risk of developing bovine respiratory disease. J. Anim. Sci. 2018, 96, 1259–1267. [Google Scholar] [CrossRef]
- Woolums, A.R.; Karisch, B.B.; Frye, J.G.; Epperson, W.; Smith, D.R.; Blanton, J., Jr.; Austin, F.; Kaplan, R.; Hiott, L.; Woodley, T.; et al. Multidrug resistant Mannheimia haemolytica isolated from high-risk beef stocker cattle after antimicrobial metaphylaxis and treatment for bovine respiratory disease. Vet. Microbiol. 2018, 221, 143–152. [Google Scholar] [CrossRef]
- Het Lam, J.; Derkman, T.H.J.; van Garderen, E.; Dijkman, R.; van Engelen, E. Distinct Mannheimia haemolytica serotypes isolated from fatal infections in veal calves and dairy cows. Vet. J. 2023, 292, 105940. [Google Scholar] [CrossRef] [PubMed]
- Lupindu, A.M.; Dalsgaard, A.; Msoffe, P.L.; Ngowi, H.A.; Mtambo, M.M.; Olsen, J.E. Transmission of antibiotic-resistant Escherichia coli between cattle, humans and the environment in peri-urban livestock keeping communities in Morogoro, Tanzania. Prev. Vet. Med. 2015, 118, 477–482. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, M.; Won, H.G.; Belaynehe, K.M.; Yoon, I.J.; Yoo, H.S. Characteristics of transmissible CTX-M- and CMY-type β-lactamase-producing Escherichia coli isolates collected from pig and chicken farms in South Korea. J. Microbiol. Biotechnol. 2017, 27, 307–316. [Google Scholar] [CrossRef]
- Aslam, M.; Diarra, M.S.; Service, C.; Rempel, H. Antimicrobial resistance genes in Escherichia coli isolates recovered from a commercial beef processing plant. J. Food Prot. 2009, 72, 1089–1093. [Google Scholar] [CrossRef]
- Shoaib, M.; He, Z.; Geng, X.; Tang, M.; Hao, R.; Wang, S.; Shang, R.; Wang, X.; Zhang, H.; Pu, W. The emergence of multi-drug resistant and virulence gene carrying Escherichia coli strains in the dairy environment: A rising threat to the environment, animal, and public health. Front. Microbiol. 2023, 14, 1197579. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef]
- Osman, K.; Alvarez-Ordóñez, A.; Ruiz, L.; Badr, J.; ElHofy, F.; Al-Maary, K.S.; Moussa, I.M.M.; Hessain, A.M.; Orabi, A.; Saad, A.; et al. Antimicrobial resistance and virulence characterization of Staphylococcus aureus and coagulase-negative staphylococci from imported beef meat. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 35. [Google Scholar] [CrossRef]
- Thomas, V.; de Jong, A.; Moyaert, H.; Simjee, S.; El Garch, F.; Morrissey, I.; Marion, H.; Vallé, M. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int. J. Antimicrob. Agents. 2015, 46, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.J.; Jeckel, S.; Ridler, A. Characteristics of sheep flocks affected by Streptococcus dysgalactiae arthritis. Vet. Rec. 2015, 176, 435–437. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C.; et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef]
- Cheney, T.E.A.; Smith, R.P.; Hutchinson, J.P.; Brunton, L.A.; Pritchard, G.; Teale, C.J. Cross-sectional survey of antibiotic resistance in Escherichia coli isolated from diseased farm livestock in England and Wales. Epidemiol. Infect. 2015, 143, 2653–2659. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Krikó, E.; Tőzsér, D.; Maróti, G.; Patai, Á.V.; Makrai, L.; Szita, G.; Solymosi, N. Antimicrobial resistance genes in raw milk for human consumption. Sci. Rep. 2020, 10, 7464. [Google Scholar] [CrossRef]
- Borin Nobrega, D. Antimicrobial Resistance: Prevalence, Genetics and Associations with Antimicrobial Use in Food-Producing Animals. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2020. Available online: http://hdl.handle.net/1880/112352 (accessed on 14 September 2025).
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Murray, L.M.; Hayes, A.; Snape, J.; Kasprzyk-Hordern, B.; Gaze, W.H.; Murray, A.K. Co-selection for antibiotic resistance by environmental contaminants. Npj Antimicrob. Resist. 2024, 2, 9. [Google Scholar] [CrossRef]
- Baghdadi, M.; Brassard, P.; Godbout, S.; Létourneau, V.; Turgeon, N.; Rossi, F.; Lachance, É.; Veillette, M.; Gaucher, M.-L.; Duchaine, C. Contribution of manure-spreading operations to bioaerosols and antibiotic resistance genes’ emission. Microorganisms 2023, 11, 1797. [Google Scholar] [CrossRef]
- Usui, M.; Shirakawa, T.; Fukuda, A.; Tamura, Y. The role of flies in disseminating plasmids with antimicrobial-resistance genes between farms. Microb. Drug Resist. 2015, 21, 562–569. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. Npj Clean. Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. Biol. Sci. 2018, 285, 20180332. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Herawati, O.; Bejo, S.K.; Zakaria, Z.; Ramanoon, S.Z. The global profile of antibiotic resistance in bacteria isolated from goats and sheep: A systematic review. Vet. World 2023, 16, 977–986. [Google Scholar] [CrossRef]
- Davies, P.; Remnant, J.G.; Green, M.J.; Gascoigne, E.; Gibbon, N.; Hyde, R.; Porteous, J.R.; Schubert, K.; Lovatt, F.; Corbishley, A. Quantitative analysis of antibiotic usage in British sheep flocks. Vet. Rec. 2017, 181, 511. [Google Scholar] [CrossRef]
- Wu, G.; Ehricht, R.; Mafura, M.; Stokes, M.; Smith, N.; Pritchard, G.C.; Woodward, M.J. Escherichia coli isolates from extraintestinal organs of livestock animals harbour diverse virulence genes and belong to multiple genetic lineages. Vet. Microbiol. 2012, 160, 197–206. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Bi, W.; Shan, H.; Wang, J.; Yang, Z. Coexistence and genomics characterization of mcr-1 and extended-spectrum-β-lactamase-producing Escherichia coli, an emerging extensively drug-resistant bacteria from sheep in China. Sci. Total Environ. 2024, 955, 177016. [Google Scholar] [CrossRef]
- Askari, N.; Momtaz, H.; Tajbakhsh, E. Acinetobacter baumannii in sheep, goat, and camel raw meat: Virulence and antibiotic resistance pattern. AIMS Microbiol. 2019, 5, 272–281. [Google Scholar] [CrossRef]
- Da Silva-Guedes, J.; Martinez-Laorden, A.; Gonzalez-Fandos, E. Effect of the presence of antibiotic residues on the microbiological quality and antimicrobial resistance in fresh goat meat. Foods 2022, 11, 3030. [Google Scholar] [CrossRef]
- Bonilla Cedrez, C.; Andeweg, K.; Casu, F.A.M. Circular Food Systems Around the World: Exploring Concepts, Ideas and Opportunities; Wageningen Livestock Research: Wageningen, The Netherlands, 2023; Report No.: 1–102. [Google Scholar]
- Beni, N.N.; Snow, D.D.; Berry, E.D.; Mittelstet, A.R.; Messer, T.L.; Bartelt-Hunt, S. Measuring the occurrence of antibiotics in surface water adjacent to cattle grazing areas using passive samplers. Sci. Total Environ. 2020, 726, 138296. [Google Scholar] [CrossRef]
- de Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Berchieri Júnior, B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.E.; Fayyaz, A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 2016, 20, 1433–1446. [Google Scholar] [CrossRef]
- Edqvist, L.E.; Pedersen, K.B. Antimicrobials as growth promoters: Resistance to common sense. In The Precautionary Principle in the 20th Century; Routledge: Abingdon, UK, 2013; pp. 100–110. [Google Scholar]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ashworth, A.J.; Willett, C.; Cook, K.; Upadhyay, A.; Owens, P.R.; Ricke, S.C.; DeBruyn, J.M.; Moore, P.A., Jr. Review of antibiotic resistance, ecology, dissemination, and mitigation in U.S. broiler poultry systems. Front. Microbiol. 2019, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, A.E.; Willems, R.; London, N.; Top, J.; Stobberingh, E.E. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 2002, 49, 497–505. [Google Scholar] [CrossRef]
- El-Tras, W.F.; Holt, H.R.; Tayel, A.A.; El-Kady, N.N. Campylobacter infections in children exposed to infected backyard poultry in Egypt. Epidemiol. Infect. 2015, 143, 308–315. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del, R.C.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; Schwarz, S., Cavaco, L.M., Shen, J., Eds.; Wiley Online Library: London, UK, 2018; pp. 185–227. [Google Scholar]
- Maurer, J.J.; Hoke, A.; Das, K.C.; Wu, J.; Williams, M.A.; Kinstler, S.; Ritz, C.; Pittman, G.P.; Berghaus, R.; Lee, M.D. The Impact Aerobic and Anaerobic Incubations of Poultry Litter Have on Class 1 Integron Resistome and Microbiome. Agriculture 2025, 15, 398. [Google Scholar] [CrossRef]
- Sant’Anna, G.S.L.; de Carvalho, L.A.L.; da Silva, M.S.R.d.A.; Gonçalves, J.V.d.S.; Pinheiro, D.G.; Zonta, E.; Coelho, I.d.S. Short-Term Effects of Poultry Litter and Cattle Manure on Soil’s Chemical Properties and Bacterial Community. Agronomy 2024, 14, 1382. [Google Scholar] [CrossRef]
- Henson, F. Evaluating the Effects of Poultry Litter Amendments on Escherichia coli Populations, Virulence Genes, and Antimicrobial-Resistance Genes in Soil and Vegetables. Ph.D. Thesis, Mississippi State University, Starkville, MS, USA, 2024. Available online: https://scholarsjunction.msstate.edu/cgi/viewcontent.cgi?article=7129&context=td (accessed on 1 September 2025).
- Chen, Y.; Liu, Y.; Zhao, C.; Ma, J.; Guo, J. Antibiotic Resistance Gene Pollution in Poultry Farming Environments and Approaches for Mitigation: A System Review. Poult. Sci. 2025, 104, 104858. [Google Scholar] [CrossRef]
- Laghari, A.A. Bioaerosol Is an Important Source for Dissemination of Antimicrobial Resistance Genes from Poultry Farms. Aerosol Sci. Technol. 2025, 59, 1234–1246. [Google Scholar] [CrossRef]
- Song, L.; Ma, J.; Jiang, G.; Wang, C.; Zhang, Y.; Chen, H.; Huang, R.J. Comparison of Airborne Antibiotic Resistance Genes in the Chicken Farm during Winter and Summer. Indoor Air 2024, 34, e1707863. [Google Scholar] [CrossRef]
- Habibi, N. Aerosol-Mediated Spread of Antibiotic Resistance Genes from Intensive Animal Farming. Int. J. Environ. Res. Public. Health 2024, 21, 983. [Google Scholar] [CrossRef]
- Subirats, J.; Murray, R.; Yin, X.; Zhang, T.; Topp, E. Impact of Chicken Litter Pre-Application Treatment on the Abundance, Field Persistence, and Transfer of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes to Vegetables. Sci. Total Environ. 2021, 801, 149718. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Z.; Li, Z.; Zhang, X.; Jia, C.; Li, T.; Wang, X. Antimicrobial Resistance and Population Genomics of Multidrug-Resistant Escherichia coli in Pig Farms in Mainland China. Nat. Commun. 2022, 13, 1116. [Google Scholar] [CrossRef]
- Pollock, J.; Muwonge, A.; Hutchings, M.R.; Mainda, G.; Bronsvoort, B.M.; Gally, D.L.; Corbishley, A. Resistance to Change: AMR Gene Dynamics on a Commercial Pig Farm with High Antimicrobial Usage. Sci. Rep. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Apley, M.D.; Bush, E.J.; Morrison, R.B.; Singer, R.S.; Snelson, H. Use Estimates of In-Feed Antimicrobials in Swine Production in the United States. Foodborne Pathog. Dis. 2012, 9, 272–279. [Google Scholar] [CrossRef]
- Maron, D.F.; Smith, T.J.; Nachman, K.E. Restrictions on Antimicrobial Use in Food Animal Production: An International Regulatory and Economic Survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef]
- de O Ventura, N.K.; Freitas, L.R.; Sousa, F.A.; Cossi, M.V.C.; Nero, L.A.; Yamatogi, R.S. Colistin and β-Lactam Resistance in Escherichia coli Isolates from Bovines, Swine, and Humans. J. Infect. Dev. Ctries. 2025, 19, 49–57. [Google Scholar] [CrossRef]
- Zihan, W.; Hongna, L.; Wang, X.; Li, S. Distribution and Influencing Factors of Antibiotics and Antibiotic Resistance Genes in Soil-Plant Systems Induced by Manure Return. J. Agric. Resour. Environ. 2024, 41, 1144. [Google Scholar]
- Bai, H.; He, L.Y.; Wu, D.L.; Gao, F.Z.; Zhang, M.; Zou, H.Y.; Ying, G.G. Spread of Airborne Antibiotic Resistance from Animal Farms to the Environment: Dispersal Pattern and Exposure Risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef]
- Holda, E.B. Antimicrobial Resistance Dynamics in Poultry Environment and the Role of Insects as Vectors of Resistance. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2024. [Google Scholar]
- Mencía-Ares, O.; Cabrera-Rubio, R.; Cobo-Díaz, J.F.; Álvarez-Ordóñez, A.; Gómez-García, M.; Puente, H.; Argüello, H. Antimicrobial Use and Production System Shape the Fecal, Environmental, and Slurry Resistomes of Pig Farms. Microbiome 2020, 8, 164. [Google Scholar] [CrossRef]
- Bednorz, C.; Oelgeschläger, K.; Kinnemann, B.; Hartmann, S.; Neumann, K.; Pieper, R.; Guenther, S. The Broader Context of Antibiotic Resistance: Zinc Feed Supplementation of Piglets Increases the Proportion of Multi-Resistant Escherichia coli In Vivo. Int. J. Med. Microbiol. 2013, 303, 396–403. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Chen, X.; Jiang, X.; Li, J.; Yang, L.; Zhang, X. Occurrence and Distribution of Microplastics in Organic Fertilizers in China. Sci. Total Environ. 2022, 844, 157061. [Google Scholar] [CrossRef]
- Dewulf, J.; Joosten, P.; Chantziaras, I.; Bernaerdt, E.; Vanderhaeghen, W.; Postma, M.; Maes, D. Antibiotic Use in European Pig Production: Less Is More. Antibiotics 2022, 11, 1493. [Google Scholar] [CrossRef]
- Ma, R.; Wang, J.; Liu, Y.; Wang, G.; Yang, Y.; Liu, Y.; Yuan, J. Dynamics of Antibiotic Resistance Genes and Bacterial Community during Pig Manure, Kitchen Waste, and Sewage Sludge Composting. J. Environ. Manag. 2023, 345, 118651. [Google Scholar] [CrossRef]
- Maes, D.G.; Dewulf, J.; Piñeiro, C.; Edwards, S.; Kyriazakis, I. A Critical Reflection on Intensive Pork Production with an Emphasis on Animal Health and Welfare. J. Anim. Sci. 2020, 98 (Suppl. 1), S15–S26. [Google Scholar] [CrossRef]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendaño-Herrera, R.; Rodkhum, C. Tenacibaculosis Caused by Tenacibaculum Maritimum: Updated Knowledge of This Marine Bacterial Fish Pathogen. Front. Cell. Infect. Microbiol. 2023, 12, 1068000. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Ivanova, L.; Shah, S.Q.A.; Sørum, H.; Tomova, A. Freshwater Salmon Aquaculture in Chile and Transferable Antimicrobial Resistance. Environ. Microbiol. 2020, 22, 559–563. [Google Scholar] [CrossRef]
- Morrison, D.B.; Saksida, S. Trends in antimicrobial use in Marine Harvest Canada farmed salmon production in British Columbia (2003–2011). Can. Veterinary J. 2013, 54, 1160. [Google Scholar]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial Resistance in Aquaculture: A Crisis for Concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Pärnänen, K.; Johnson, T.A.; Lyra, C.; Karkman, A.; Stedtfeld, R.D.; Virta, M. Aquaculture Changes the Profile of Antibiotic Resistance and Mobile Genetic Element Associated Genes in Baltic Sea Sediments. FEMS Microbiol. Ecol. 2016, 92, fiw052. [Google Scholar] [CrossRef]
- Lin, Z.; Yuan, T.; Zhou, L.; Cheng, S.; Qu, X.; Lu, P.; Feng, Q. Impact Factors of the Accumulation, Migration and Spread of Antibiotic Resistance in the Environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Feng, W.Q.; Han, Y.; Zheng, J.; Chen, T.; Wei, Y.Y.; Gillings, M.; Zhu, Y.G.; Chen, H. Prevalence and transmission of antibiotic resistance and microbiota between humans and water environments. Environ. Int. 2018, 121 (Suppl. 2), 1155–1161. [Google Scholar] [CrossRef]
- Kampouris, I.D.; Klümper, U.; Kramer, L.; Sorum, H.; Wedekind, H.; Berendonk, T.U. Dissemination of antibiotic resistance in antibiotic-free recirculating aquaculture systems. J. Hazard. Mater. Adv. 2022, 8, 100201. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Salgueiro, V.; Manageiro, V.; Rosado, T.; Bandarra, N.M.; Botelho, M.J.; Dias, E.; Caniça, M. Snapshot of resistome, virulome and mobilome in aquaculture. Sci. Total Environ. 2023, 905, 166351. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, X.H.; Li, J.B. Emergence of CTX-M-3, TEM-1 and a new plasmid-mediated MOX-4 AmpC in a multiresistant Aeromonas caviae isolate from a patient with pneumonia. J. Med. Microbiol. 2010, 59, 843–847. [Google Scholar] [CrossRef][Green Version]
- Shen, X.; Jin, G.; Zhao, Y.; Shao, X. Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci. Total Environ. 2020, 711, 134626. [Google Scholar] [CrossRef]
- Cabello, F.C.; Tomova, A.; Ivanova, L.; Godfrey, H.P. Aquaculture and mcr Colistin Resistance Determinants. mBio 2017, 3, e01229-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreas, P.; Anders, D. Species composition and antimicrobial resistance genes of Enterococcus spp., isolated from integrated and traditional fish farms in Thailand. Environ. Microbiol. 2003, 5, 395–402. [Google Scholar] [CrossRef]
- Barros, J.; Margarida, A.; Hajer, R.; Maria, L.; Gilberto, I.; Patricia, P.; Carmen, T. Detection of vanA-Containing Enterococcus Species in Faecal Microbiota of Gilthead Seabream (Sparus aurata). Microbes Environ. 2012, 27, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Desbois, A.P. The Global Approach: Aquaculture and National Action Plans for Antimicrobial Resistance. In Antimicrobial Resistance in Aquaculture and Aquatic Environments; Springer Nature: Singapore, 2025; pp. 385–412. [Google Scholar]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Robinson, T.P.; Wertheim, H.F.; Kakkar, M.; Kariuki, S.; Bu, D.; Price, L.B. Animal Production and Antimicrobial Resistance in the Clinic. Lancet 2016, 387, e1–e3. [Google Scholar] [CrossRef]
- Palić, D.; Aksentijević, K. Autogenous Vaccines in Aquaculture: Tool to Combat Resistance of Bacteria to Antibiotics? Vet. Glasnik 2022, 76, 91–102. [Google Scholar] [CrossRef]
- Scarfe, A.D.; Palić, D. Aquaculture Biosecurity: Practical Approach to Prevent, Control, and Eradicate Diseases. In Aquaculture Health Management; Academic Press: Cambridge, MA, USA, 2020; pp. 75–116. [Google Scholar]
- Sun, G.; Wu, S.; Shou, B.; Tan, X.; Fang, H.; Zhang, Y.; Wan, Y. Antibiotic Residues: Status, Hotspots and Trends. Hotspots Trends 2024. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries 2022: Trends from 2010–2022. Thirteenth ESVAC Report. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf (accessed on 15 August 2025).
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Heuer, H.; Smalla, K. Plasmids Foster Diversification and Adaptation of Bacterial Populations in Soil. FEMS Microbiol. Rev. 2012, 36, 1083–1104. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.I.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Su, J.Q. Continental-Scale Pollution of Estuaries with Antibiotic Resistance Genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Gillings, M.R. Integrons: Past, Present, and Future. Microbiol. Mol. Biol. Rev. 2014, 78, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family—A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Smalla, K. Manure and Sulfadiazine Synergistically Increased Bacterial Antibiotic Resistance in Soil over at Least Two Months. Environ. Microbiol. 2007, 9, 657–666. [Google Scholar] [CrossRef]
- Graham, J.P.; Price, L.B.; Evans, S.L.; Graczyk, T.K.; Silbergeld, E.K. Antibiotic Resistant Enterococci and Staphylococci Isolated from Flies Collected near Confined Poultry Feeding Operations. Sci. Total Environ. 2009, 407, 2701–2710. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.H.; Muurinen, J.; Virta, M.P.; Zhu, Y.G. Antibiotic Resistome in the Livestock and Aquaculture Industries: Status and Solutions. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2159–2196. [Google Scholar] [CrossRef]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef]
- Joy, S.R.; Bartelt-Hunt, S.L.; Snow, D.D.; Gilley, J.E.; Woodbury, B.L.; Parker, D.B.; Li, X. Fate and Transport of Antimicrobials and Antimicrobial Resistance Genes in Soil and Runoff Following Land Application of Swine Manure Slurry. Environ. Sci. Technol. 2013, 47, 12081–12088. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Nobrega, D.B.; De Buck, J.; Barkema, H.W. Antimicrobial Resistance in Non-Aureus Staphylococci Isolated from Milk Is Associated with Systemic but Not Intramammary Administration of Antimicrobials in Dairy Cattle. J. Dairy. Sci. 2018, 101, 7425–7436. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Thériault, W.; Beaudry, F.; Bergeron, N.; Laurent-Lewandowski, S.; Letellier, A. The Fecal Presence of Enterotoxin and F4 Genes as an Indicator of Efficacy of Treatment with Colistin Sulfate in Pigs. BMC Microbiol. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Chow, L.; Waldron, L.; Gillings, M.R. Potential Impacts of Aquatic Pollutants: Sub-Clinical Antibiotic Concentrations Induce Genome Changes and Promote Antibiotic Resistance. Front. Microbiol. 2015, 6, 803. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse Abroad in the Land’: The Role of Wildlife in the Dissemination of Antimicrobial Resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.F.; Gaze, W.H.; Kuroda, M.; et al. Critical Knowledge Gaps and Research Needs Related to the Environmental Dimensions of Antibiotic Resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef]
- Li, D.; Li, X.Y.; Schwarz, S.; Yang, M.; Zhang, S.M.; Hao, W.; Du, X.D. Tn6674 Is a Novel Enterococcal optrA-Carrying Multiresistance Transposon of the Tn554 Family. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Jensen, J.S.; Unemo, M. Antimicrobial Treatment and Resistance in Sexually Transmitted Bacterial Infections. Nat. Rev. Microbiol. 2024, 22, 435–450. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current Status of the Use of Antibiotics and the Antimicrobial Resistance in the Chilean Salmon Farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Grace, D.; Dessie, T.; Dione, M.; Kiara, H.; Liljander, A.; Mariner, J.; Wieland, B. Transboundary Animal Diseases. In The Impact of the International Livestock Research Institute; CABI: Wallingford, UK, 2020; pp. 274–301. [Google Scholar]
- European Commission. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_05_1687 (accessed on 1 September 2025).
- Qian, X.; Gu, J.; Sun, W.; Wang, X.J.; Su, J.Q.; Stedfeld, R. Diversity, Abundance, and Persistence of Antibiotic Resistance Genes in Various Types of Animal Manure Following Industrial Composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Piddock, L.J. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
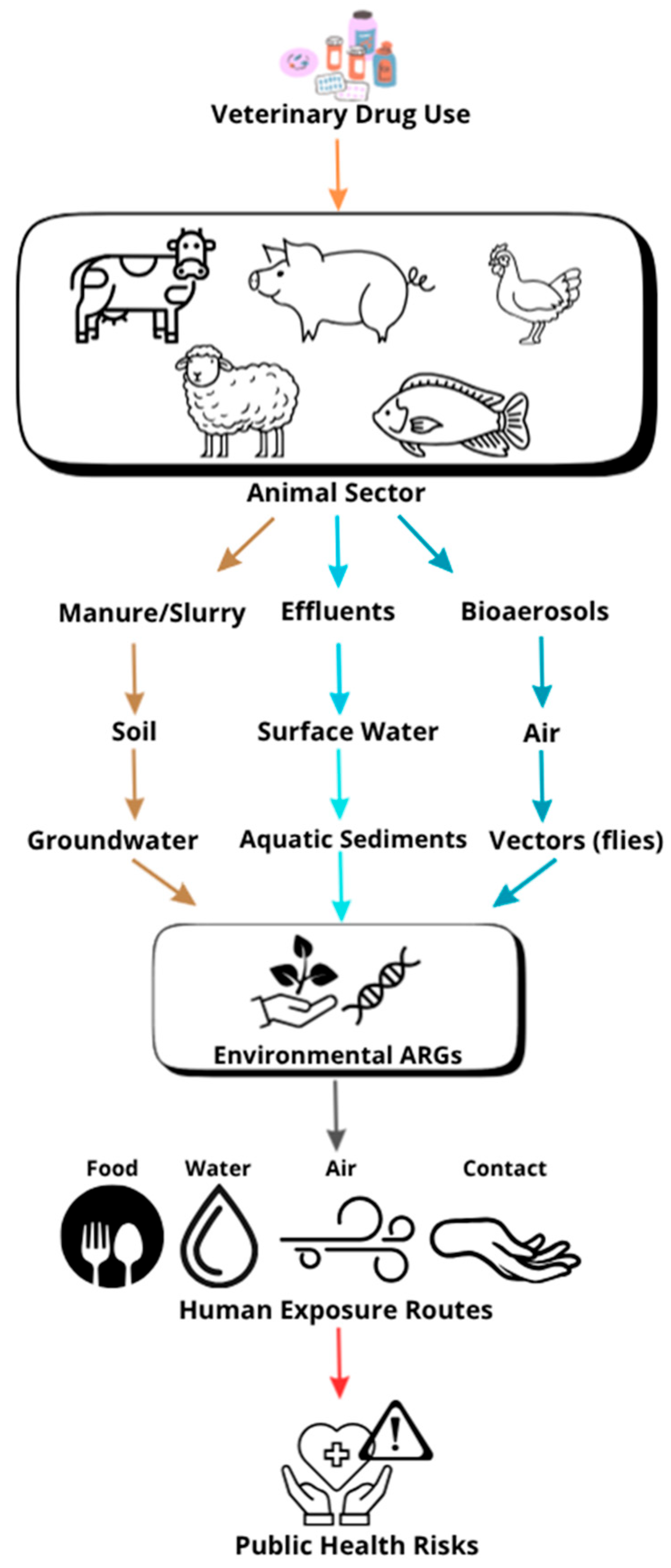
| Food Animal Sector | Representative ARB | Key ARGs | Representative MGEs | Type of Resistance Mediated | References |
|---|---|---|---|---|---|
| Cattle | M. haemolytica, E. coli, S. uberis, MRSA, Enterococcus spp. | blaCTX-M, blaARL, erm, tet(A/B/C), mcr-1, norA, mepA, aadA, sul1, VanA | Conjugative plasmids (IncF, IncI1), integrons (intl 1), transposons (Tn916- Tn1545) | Macrolides, lincosamides, β-lactam, tetracycline, colistin, fluoroquinolones, sulfonamide resistance | [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] |
| Small ruminants (sheep/goats) | S. aureus, C. jejuni, Enterococcus spp., E. coli | blaCTX-M, erm(B), tet(A/B/C), aac(6′)-aph(2″), sul, mcr-1, VanA | Plasmids (IncI2, IncFIB-IncFIC), transposons (Tn1545), integrons | Macrolide, tetracycline, β-lactam, aminoglycoside, colistin, sulfonamide resistance | [54,55,56,57,58,59,60] |
| Poultry | Salmonella spp., C. jejuni, E. coli (ESBL), Enterococcus spp., S. aureus | blaTEM, blaSHV, qnrS, tet(A/B/C), erm(B), qnr, sul2, VanA | IncI1/IncK plasmids, class 1 integrons, IS26 transposase | β-lactam, Cephalosporin, quinolone, tetracycline, macrolide, sulfonamide resistance | [61,62,63,64,65,66,67,68,69,70,71,72,73,74] |
| Swine | E. coli, Salmonella spp., E. faecalis, MRSA | blaCTX-M, mecA, erm(B), tet(M), aadA2, floR, sul1, VanA, mcr-1 | SCCmec cassette, Tn916, IncI1/IncI2 plasmids, class 1 integrons | β-lactam, macrolide, tetracycline, amphenicol, Fluoroquinolones, sulfonamide resistance | [5,7,21,76,77,78,79,80,81,82,83,84,85,86,87,88,89] |
| Aquaculture | A. hydrophila, Vibrio spp., Pseudomonas spp., Edwardsiella tarda, Lactococcus garvieae | tet(A/B), qnrS, sul1, floR, catA, blaCTX-M, blaNDM, mcr, VanA | Plasmids (IncQ, IncP), integrons, gene cassettes, ISCR elements, Tn1721 transposase | Tetracycline, quinolone, sulfonamide, chloramphenicol, β-lactam resistance | [4,8,27,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] |
| Aspect | Cattle | Small Ruminants (Sheep/Goats) | Poultry | Swine | Aquaculture |
|---|---|---|---|---|---|
| Main antimicrobial use | Therapeutic and metaphylactic treatments for mastitis, respiratory and enteric infections | Preventive or therapeutic use against pneumonia and footrot | Routine prophylaxis in intensive systems; feed additives in some regions | High usage for growth promotion and prophylaxis in intensive farming | Mixed in feed or water to prevent bacterial diseases |
| Mechanisms of resistance generation | HGT in gut and manure microbiota | HGT among commensal and pathogenic bacteria in mixed herds | Plasmid and integronmediated resistance | Co-selection through mobile genetic elements in gut microbiota | HGT in biofilm-associated bacteria and sediments |
| Environmental reservoirs | Manure, slurry, and pasture soils | Manure and grazing areas | Litter, dust, and runoff from poultry houses | Manure lagoons and contaminated soils, microplastics in manure and effluents | Sediments, water columns, and biofilms |
| Routes of AMR spread | Manure application to fields, milk waste, farm runoff, airborne routes, arthropod vectors | Open grazing, manure deposition, bedding and slurry accumulation | Airborne dust, litter disposal, food chain | Manure application to fields, Airborne dust, arthropod vectors, contact with workers, meat contamination | Effluent discharge (fish farms-human wastewater), fish trade, and bioaerosols |
| Public health relevance | E. coli, Salmonella, Staphylococcus aureus, Streptococcus uberis | Campylobacter, Staphylococcus spp., Macrococcus caseolyticus, Enterococcus faecium, MRSA | Salmonella, Campylobacter, Enterococcus | E. coli, Salmonella, Yersinia, LA-MRSA | Aeromonas, Vibrio, Pseudomonas, Flavobacterium, Acinetobacter, Lactococcus, Edwardsiella |
| References | [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] | [54,55,56,57,58,59,60,61] | [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] | [5,7,78,79,80,81,82,83,84,85,86,87,88,89,90,91] | [4,8,27,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnarowski, K.; Cholewińska, P.; Zhao, D.; Pacoń, J.; Bodkowski, R. Antibiotic Resistance Genes in Food Animal Production: Environmental Implications and One Health Challenges. Environments 2025, 12, 427. https://doi.org/10.3390/environments12110427
Wojnarowski K, Cholewińska P, Zhao D, Pacoń J, Bodkowski R. Antibiotic Resistance Genes in Food Animal Production: Environmental Implications and One Health Challenges. Environments. 2025; 12(11):427. https://doi.org/10.3390/environments12110427
Chicago/Turabian StyleWojnarowski, Konrad, Paulina Cholewińska, Dongqinq Zhao, Jakub Pacoń, and Robert Bodkowski. 2025. "Antibiotic Resistance Genes in Food Animal Production: Environmental Implications and One Health Challenges" Environments 12, no. 11: 427. https://doi.org/10.3390/environments12110427
APA StyleWojnarowski, K., Cholewińska, P., Zhao, D., Pacoń, J., & Bodkowski, R. (2025). Antibiotic Resistance Genes in Food Animal Production: Environmental Implications and One Health Challenges. Environments, 12(11), 427. https://doi.org/10.3390/environments12110427







