Impact of Pollution on Physico-Chemical Parameters and Diatom Communities Diversity in the Main Tributaries of the Arieș River, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Its Characteristics
2.2. Water Sampling
2.3. Analysis of River Water Samples
2.3.1. River Water Quality Parameters
2.3.2. River Water Salinity Parameters
2.3.3. ICP-MS Metal Analysis in River Water Samples
2.4. Sampling of Periphytic Biofilm from Submerged Surfaces
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Diatom Responses and Teratologies as Indicators of Trace Element Pollution in Mining-Affected Rivers
4.2. Statistical Evaluation of Results
4.2.1. Pearson Correlation Between Trace Elements and Diatom Species Distribution [65]
4.2.2. Multivariate Analysis of Water Quality: PCA Insights into Metal and Physico-Chemical Gradients [68]
4.2.3. Canonical Correspondence Analysis (CCA): Linking Environmental Variables to Species Distribution [68]
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Calmus, T.; Valencia-Moreno, M.; Del Río-Salas, R.; Ochoa-Landín, L.; Mendivil-Quijada, H. A multi-elemental study to establish the natural background and geochemical anomalies in rocks from the Sonora River upper basin, NW Mexico. Rev. Mex. Cienc. Geol. 2018, 35, 158–167. [Google Scholar] [CrossRef]
- Dumitrel, G.A.; Glevitzky, M.; Popa, M.; Vica, M.L. Studies Regarding the Heavy Metals Pollution of Streams and Rivers in Rosia Montana Area, Romania. J. Environ. Prot. Ecol. 2015, 16, 850–860. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Environmental Pollution Series; Springer: Dordrecht, The Netherlands, 2002; Volume 5. [Google Scholar]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid mine drainage control and treatment. In Reclamation of Drastically Disturbed Lands; Barnhisel, R.I., Darmody, R.G., Daniels, W.L., Eds.; American Society of Agronomy: Madison, WI, USA, 2000; Volume 41, p. 154. [Google Scholar] [CrossRef]
- Battes, K.P.; Cîmpean, M.; Momeu, L.; Avram, A.; Battes, K.W.; Stoica, I.V. Multiple impact assessment and water quality based on diatom, benthic invertebrate and fish communities in the Arieș River catchment area (Transylvania, Romania). Stud. Univ. Babeș-Bolyai Biol. 2018, 63, 27–40. [Google Scholar] [CrossRef]
- Szekely-Andorko, J.; Peterfi, L.Ș.; Momeu, L. Benthic Diatoms Used as Bioindicators for Water Quality Evaluation in the Drainage Basin of the Arieș River (Transylvania, Romania). Contrib. Bot. 2011, 46, 107–115. [Google Scholar]
- Butiuc-Keul, A.; Momeu, L.; Craciunas, C.; Dobrota, C.; Cuna, S.; Balas, G. Physico-chemical and biological studies on water from Aries River (Romania). J. Environ. Manag. 2012, 95, S3–S8. [Google Scholar] [CrossRef]
- Olenici, A.; Blanco, S.; Jiménez-Gómez, F.; Borrego-Ramos, M.; Baciu, C. Effects of Water Pollution on Diatom Communities of Roșia Montană Mining Area, Romania. Sustainability 2025, 17, 4592. [Google Scholar] [CrossRef]
- Mamanazarova, K.; Alimjanova, K.; Barinova, S. Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan. Land 2024, 13, 1809. [Google Scholar] [CrossRef]
- Gloaguen, R.; Ali, S.H.; Herrington, R.; Ajjabou, L.; Downey, E.; Stewart, I.S. Mineral revolution for the wellbeing economy. Glob. Sustain. 2022, 5, e15. [Google Scholar] [CrossRef]
- Dumitrel, G.A.; Glevitzky, M.; Popa, M.; Chirila, D.; Palea, A. Monitoring of Lead, Copper and Cadmium Contamination Level of Soil from Zlatna Region Romania. J. Environ. Prot. Ecol. 2017, 18, 55–62. [Google Scholar]
- Glevitzky, M.; Bostan, R.; Varvara, S.; Corcheş, M.-T.; Dumitrel, G.-A.; Popa, M. Impact of Mining on River Water Quality in Roșia Montană Area, Romania, and the Use of Zeolites for Acid Mine Drainage Remediation. Clean. Technol. 2025, 7, 41. [Google Scholar] [CrossRef]
- Lobo, E.A.; Heinrich, C.G.; Schuch, M.; Wetzel, C.E.; Ector, L. Diatoms as Bioindicators in Rivers. In River Algae; Necchi, O., Jr., Ed.; Springer: Cham, Switzerland, 2016; pp. 245–272. [Google Scholar] [CrossRef]
- Shibabaw, T.; Beyene, A.; Awoke, A.; Tirfie, M.; Azage, M.; Triest, L. Diatom community structure in relation to environmental factors in human influenced rivers and streams in tropical Africa. PLoS ONE 2021, 2021 16, e0246043. [Google Scholar] [CrossRef]
- Hustedt, F. Kieselalgen (Diatomeen). Einführung in die Kleinlebwelt; Kosmos Verlag: Stuttgart, Germany, 1956; 70p. [Google Scholar]
- SR EN 872:2005; Water Quality—Determination of Suspended Solids—Method by Filtration through Glass Fibre Filters. ASRO: Bucharest, Romania, 2005.
- SR EN 25813:2000; Water Quality—Determination of Dissolved Oxygen. Iodometric Method. ASRO: Bucharest, Romania, 2000.
- SR EN 1899-2:2002; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BODn)—Part 2: Method for Undiluted Samples (ISO 5815:1989, Modified). ASRO: Bucharest, Romania, 2002.
- SR ISO 15705:2022; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD). ASRO: Bucharest, Romania, 2022.
- SR ISO 7150-1:2001; Water Quality—Determination of Ammonium Content—Part 1: Manual Spectrometric Method. ASRO: Bucharest, Romania, 2001.
- SR EN 26777:2002; Water Quality—Determination of Nitrite Content—Molecular Absorption Spectrometric Method. ASRO: Bucharest, Romania, 2002.
- BSI. SR ISO 7890-3:2000; Water Quality—Determination of Nitrate Content—Part 3: Spectrometric Method Using Sulfosalicylic Acid. BSI: London, UK, 2000.
- ASRO. STAS 9187:1984; Surface, Underground and Waste Waters—Residuum Determination. ASRO: Bucharest, Romania, 1984.
- STAS 6194/1-85; Water Quality—Determination of Bicarbonates (HCO3−) in Water. ASRO: Bucharest, Romania, 1985.
- SR ISO 9297:2001; Water Quality—Determination of Chloride Content. Titration with Silver Nitrate Using Chromate as Indicator (Mohr Method). ASRO: Bucharest, Romania, 2001.
- APHA. Method 4500-SO42−; Standard Methods for the Examination of Water and Wastewater. Available online: https://law.resource.org/pub/us/cfr/ibr/002/apha.method.4500-so42.1992.pdf (accessed on 8 January 2022).
- Fernández-Turiel, J.L.; Llorens, J.F.; López-Vera, F.; Gómez-Artola, C.; Morell, I.; Gimeno, D. Strategy for Water Analysis Using ICP-MS. Fresenius J. Anal. Chem. 2000, 368, 601–606. [Google Scholar] [CrossRef]
- ASRO. SR EN 13946:2014; Water Quality—Guidance for the Routine Sampling and Pretreatment of Benthic Diatoms from Rivers and Lakes. ASRO: Bucharest, Romania, 2014.
- Pantle, E.; Buck, H. Die Biologische Überwachung der Gewässer und die Darstellung der Ergebnisse (Biological Monitoring of Waterbodies and the Presentation of Results). Gas Wasserfach 1955, 96, 604–618. [Google Scholar]
- Ministry of Environment and Water Management. Normative of 16 February 2006 on the Classification of Surface Water Quality for Establishing the Ecological Status of Water Bodies (in Romanian), Approved by Order No. 161/2006; Official Gazette of Romania: Bucharest, Romania, 2006; Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/74255 (accessed on 14 April 2025).
- SR EN 14407:2014; Water Quality—Guidance for the Identification and Counting of Benthic Diatom Samples from Rivers and Lakes. ASRO: Bucharest, Romania, 2014.
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (Water Framework Directive). Off. J. Eur. Communities 2000, L327, 1–72. [Google Scholar]
- Halmagyi, A.; Butiuc-Keul, A.; Keul, M.; Dobrotă, C.; Fodorpataki, L.; Pintea, A.; Mocan, A.; Pop, V.; Coste, A. Impact of Arieş River Contaminants on Algae and Plants. Toxics 2023, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, A.; Török, A.I.; Kovacs, E.; Cadar, O.; Mirea, I.C.; Micle, V. Metal Contents and Pollution Indices Assessment of Surface Water, Soil, and Sediment from the Arieș River Basin Mining Area, Romania. Sustainability 2022, 14, 8024. [Google Scholar] [CrossRef]
- Olenici, A.; Blanco, S.; Borrego-Ramos, M.; Jiménez-Gómez, F.; Guerrero, F.; Momeu, L.; Baciu, C. A New Diatom Teratology Driven by Metal Pollution in a Temperate River (Roșia Montană, Romania). Ann. Di Bot. 2019, 9, 113–118. [Google Scholar] [CrossRef]
- Leguay, S.; Lavoie, I.; Levy, J.L.; Fortin, C. Using biofilms for monitoring metal contamination in lotic ecosystems: The protective effects of hardness and pH on metal bioaccumulation. Environ. Toxicol. Chem. 2016, 35, 1489–1501. [Google Scholar] [CrossRef]
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in diatom response to metal-contaminated environments. In Handbook of Environmental Chemistry; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Heidelberg, Germany, 2012; Volume 19, pp. 117–146. [Google Scholar]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175. [Google Scholar] [CrossRef]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Fortin, C. Littoral diatoms as indicators of recent water and sediment contamination by metals in lakes. J. Environ. Monit. 2011, 13, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.; Morin, S.; Herlory, O.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Seasonal effects of cadmium accumulation in periphytic diatom communities of freshwater biofilms. Aquat. Toxicol. 2008, 90, 19–28. [Google Scholar] [CrossRef]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Ector, L.; Matos, J.X.; Ferreira da Silva, E.A. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167. [Google Scholar] [CrossRef]
- Tolotti, R.; Consani, S.; Carbone, C.; Vagge, G.; Capello, M.; Cutroneo, L. Benthic Diatom Community Response to Metal Contamination from an Abandoned Cu Mine: Case Study of the Gromolo Torrent (Italy). J. Environ. Sci. 2019, 75, 233–246. [Google Scholar] [CrossRef]
- Gonçalves, S.; Almeida, S.F.P.; Figueira, E.; Kahlert, M. Valve Teratologies and Chl c in the Freshwater Diatom Tabellaria flocculosa as Biomarkers for Metal Contamination. Ecol. Indic. 2019, 101, 476–485. [Google Scholar] [CrossRef]
- Gonçalves, S.; Kahlert, M.; Almeida, S.F.P.; Figueira, E. Assessing Cu Impacts on Freshwater Diatoms: Biochemical and Metabolomic Responses of Tabellaria flocculosa (Roth) Kützing. Sci. Total Environ. 2018, 625, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, I.; Hamilton, P.B.; Morin, S.; Kim Tiam, S.; Kahlert, M.; Gonçalves, S.; Falasco, E.; Fortin, C.; Gontero, B.; Heudre, D.; et al. Diatom Teratologies as Biomarkers of Contamination: Are All Deformities Ecologically Meaningful? Ecol. Indic. 2017, 82, 539–550. [Google Scholar] [CrossRef]
- Cantonati, M.; Angeli, N.; Virtanen, L.; Wojtal, A.Z.; Gabrieli, J.; Falasco, E.; Lavoie, I.; Morin, S.; Marchetto, A.; Fortin, C.; et al. Achnanthidium minutissimum (Bacillariophyta) Valve Deformities as Indicators of Metal Enrichment in Diverse Widely-Distributed Freshwater Habitats. Sci. Total Environ. 2014, 475, 201–215. [Google Scholar] [CrossRef]
- Neidoni, D.G.; Nicorescu, V.; Negrea, S.C.; Stoica, C.; Pahomi, A.; Niță-Lăzar, M. Diatoms: A Viable Tool for Quantifying Surface Water Pollution. Rom. J. Ecol. Environm. Chem. 2023, 5, 31–41. [Google Scholar] [CrossRef]
- Pandey, L.K.; Sharma, Y.C.; Park, J.; Choi, S.; Lee, H.; Lyu, J.; Han, T. Evaluating Features of Periphytic Diatom Communities as Biomonitoring Tools in Fresh, Brackish and Marine Waters. Aquat. Toxicol. 2018, 194, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Falasco, E.; Ector, L.; Wetzel, C.E.; Badino, G.; Bona, F. Looking Back, Looking Forward: A Review of the New Literature on Diatom Teratological Forms (2010–2020). Hydrobiologia 2021, 848, 1675–1753. [Google Scholar] [CrossRef]
- Olenici, A.; Blanco, S.; Borrego-Ramos, M.; Momeu, L.; Baciu, C. Exploring the Effects of Acid Mine Drainage on Diatom Teratology Using Geometric Morphometry. Ecotoxicology 2017, 26, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Cerisier, A.; Vedrenne, J.; Lavoie, I.; Morin, S. Assessing the Severity of Diatom Deformities Using Geometric Morphometry. Bot. Lett. 2018, 166, 32–40. [Google Scholar] [CrossRef]
- Morin, S.; Duong, T.T.; Dabrin, A.; Coynel, A.; Herlory, O.; Baudrimont, M.; Delmas, F.; Durrieu, G.; Schäfer, J.; Winterton, P.; et al. Long-Term Survey of Heavy-Metal Pollution, Biofilm Contamination and Diatom Community Structure in the Riou Mort Watershed, South-West France. Environ. Pollut. 2008, 151, 532–542. [Google Scholar] [CrossRef]
- Fernández, M.R.; Martín, G.; Corzo, J.; de la Linde, A.; García, E.; López, M.; Sousa, M. Design and Testing of a New Diatom-Based Index for Heavy Metal Pollution. Arch. Environ. Contam. Toxicol. 2018, 74, 170–192. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.G.; Herrero, F.S.; Pitt, J.A.; Jarvie, H.P.; Claussen, U.; Leujak, W.; Solheim, A.L.; Teixeira, H.; Phillips, G. Nutrient criteria for surface waters under the European Water Framework Directive: Current state-of-the-art, challenges and future outlook. Sci. Total Environ. 2019, 695, 133888. [Google Scholar] [CrossRef]
- Kelly, M.; Teixeira, H.; Lyche Solheim, A.; Free, G.; Phillips, G.; Salas Herrero, M.F.; Kolada, A.; Varbiro, G.; Poikane, S. Physico-Chemical Criteria to Support Good Ecological Status in Europe; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Kelly, M.G.; Free, G.; Kolada, A.; Phillips, G.; Warner, S.; Wolfram, G.; Poikane, S. Warding off freshwater salinization: Do current criteria measure up? Wiley Interdiscip. Rev. Water 2024, 11, e1694. [Google Scholar] [CrossRef]
- Poikane, S.; Várbíró, G.; Kelly, M.G.; Birk, S.; Phillips, G. Estimating river nutrient concentrations consistent with good ecological condition: More stringent nutrient thresholds needed. Ecol. Indic. 2021, 121, 107017. [Google Scholar] [CrossRef]
- Gordon, N.; García-Rodríguez, F.; Adams, J.B. Paleolimnology of a Coastal Lake on the Southern Cape Coast of South Africa: Sediment Geochemistry and Diatom Distribution. J. Afr. Earth Sci. 2012, 75, 14–24. [Google Scholar] [CrossRef]
- Titu, M.; Oprean, C.; Cicala, E. Technical Statistics and Statistical Process Control; Lucian Blaga University Publishing House: Sibiu, Romania, 2001. (In Romanian) [Google Scholar]
- Martín, G.; Toja, J.; Sala, S.E.; de los Reyes Fernández, M.; Reyes, I.; Casco, M.A. Application of Diatom Biotic Indices in the Guadalquivir River Basin, a Mediterranean Basin: Which One Is the Most Appropriate? Environ. Monit. Assess. 2010, 170, 519–534. [Google Scholar] [CrossRef]
- Mijbas, Y.A.; Fayidh, M.A.; Nasif, R.M. Evaluation of Heavy Metals Pollution in the Sediments of Diyala River Lower Reaches, Eastern Iraq. Iraqi Geol. J. 2024, 57, 32–45. [Google Scholar] [CrossRef]
- Tănăsescu, N. Mathematical Modeling and Numerical Simulation of Technological Processes in the Food Industry; Matrix Rom Publishing House: Bucharest, Romania, 2000. (In Romanian) [Google Scholar]
- Liu, J.; Kang, H.; Tao, W.; Li, H.; He, D.; Ma, L.; Tang, H.; Wu, S.; Yang, K.; Li, X. A spatial distribution–Principal component analysis (SD-PCA) model to assess pollution of heavy metals in soil. Sci. Total Environ. 2023, 859, 160112. [Google Scholar] [CrossRef] [PubMed]
- Gergen, I.; Harmanescu, M. Application of principal component analysis in the pollution assessment with heavy metals of vegetable food chain in the old mining areas. Chem. Cent. J. 2012, 6, 156. [Google Scholar] [CrossRef]
- Resz, M.-A.; Roman, C.; Senila, M.; Török, A.I.; Kovacs, E. A Comprehensive Approach to the Chemistry, Pollution Impact and Risk Assessment of Drinking Water Sources in a Former Industrialized Area of Romania. Water 2023, 15, 1180. [Google Scholar] [CrossRef]
- Willems, W.; Rees, H.L.; Vincx, M.; Goethals, P.; Degraer, S. Relations and Interactions Between Environmental Factors and Biotic Properties; ICES Cooperative Research Report No. 288; ICES: Copenhagen, Denmark, 2004; p. 69. [Google Scholar]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.F.; Almeida, S.F.P.; Nunes, M.L.; Luís, A.T.; Borg, F.; Hedlund, M.; Marques de Sá, C.; Patinha, C.; Teixeira, P. Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci. Total Environ. 2009, 407, 5620–5636. [Google Scholar] [CrossRef] [PubMed]
- Kargıoğlu, M.; Serteser, A.; Kıvrak, E.; İçağa, Y.; Konuk, M. Relationships between Epipelic Diatoms, Aquatic Macrophytes, and Water Quality in Akarçay Stream, Afyonkarahisar, Turkey. Oceanol. Hydrobiol. Stud. 2012, 41, 74–84. [Google Scholar] [CrossRef]
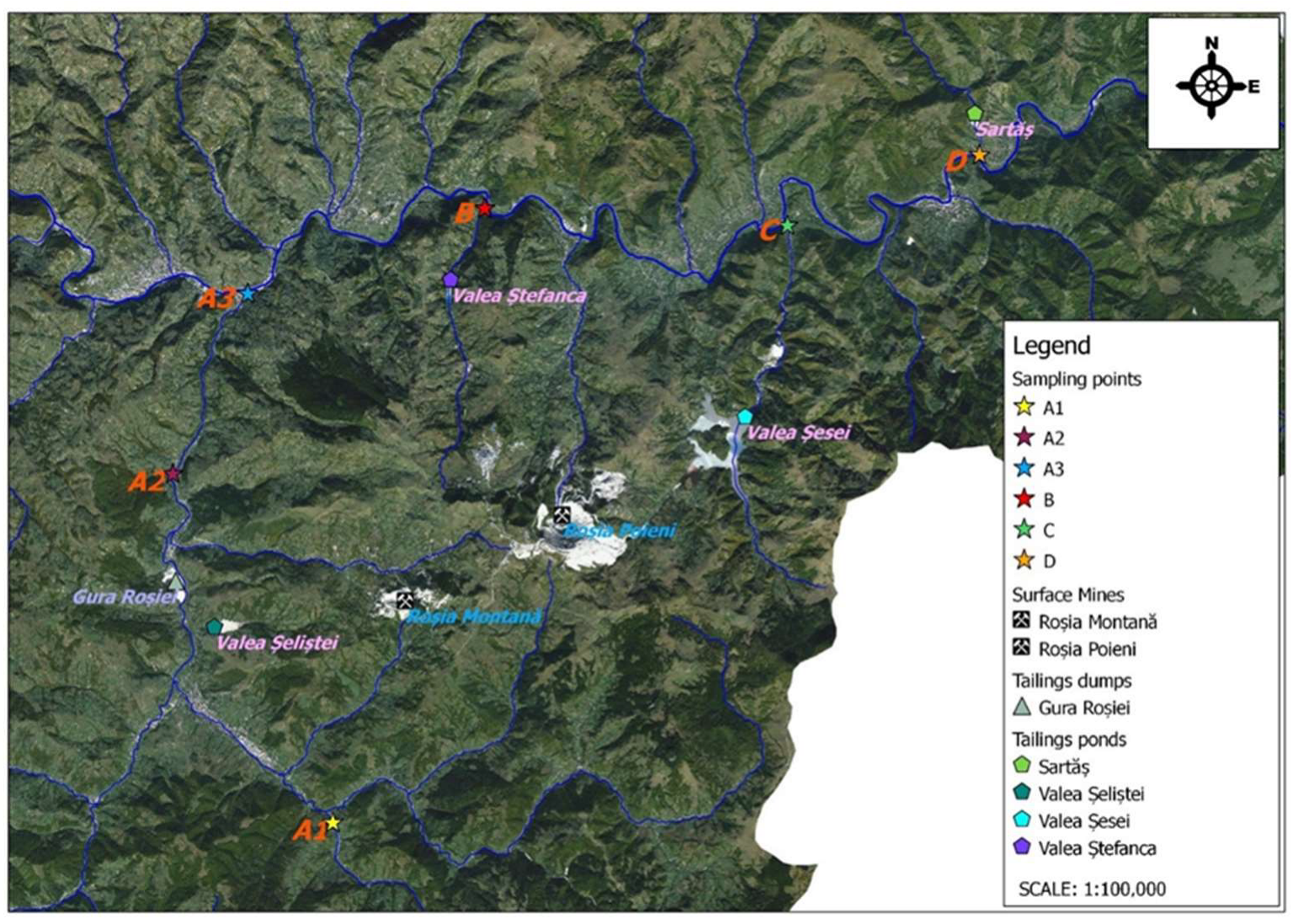
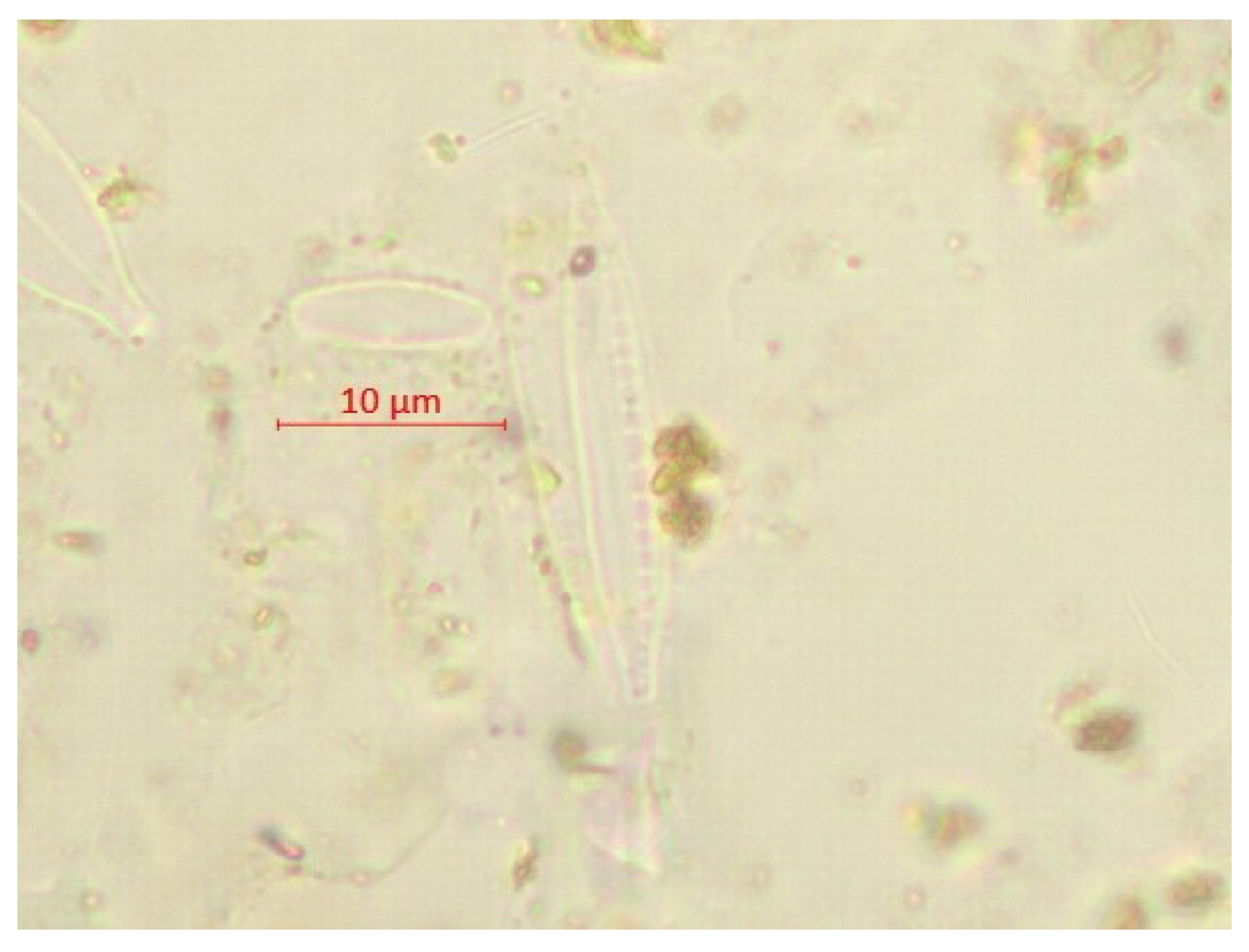
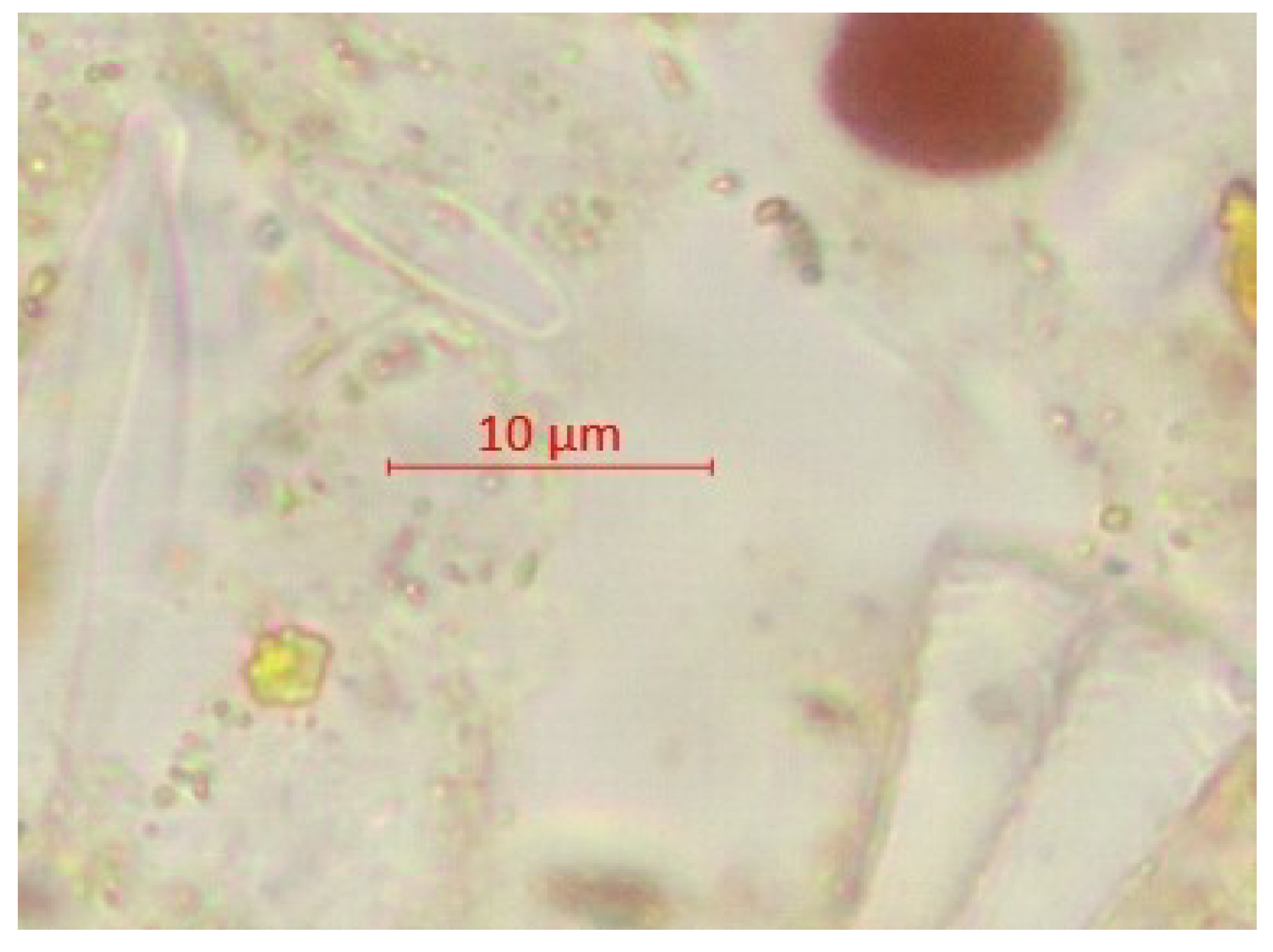
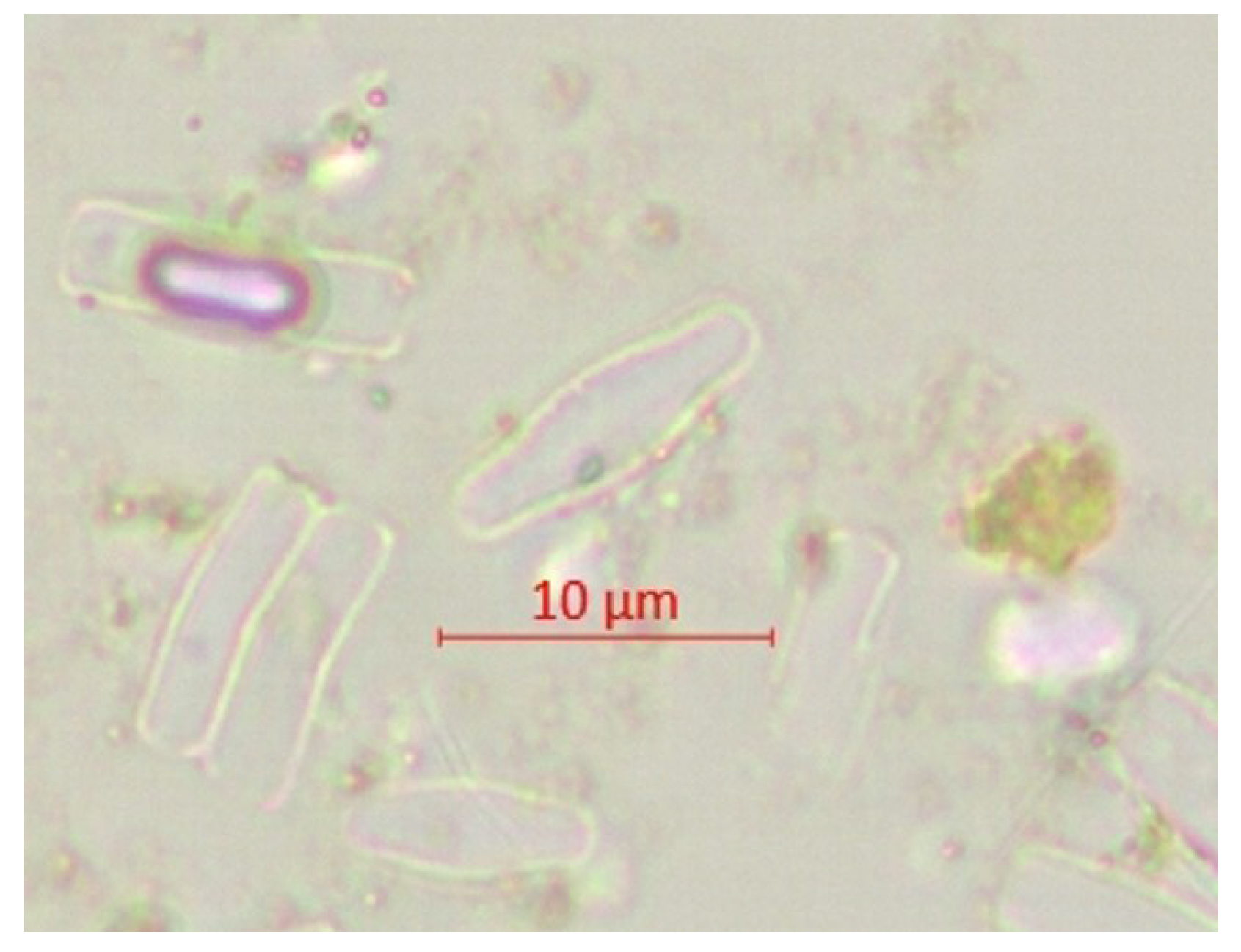
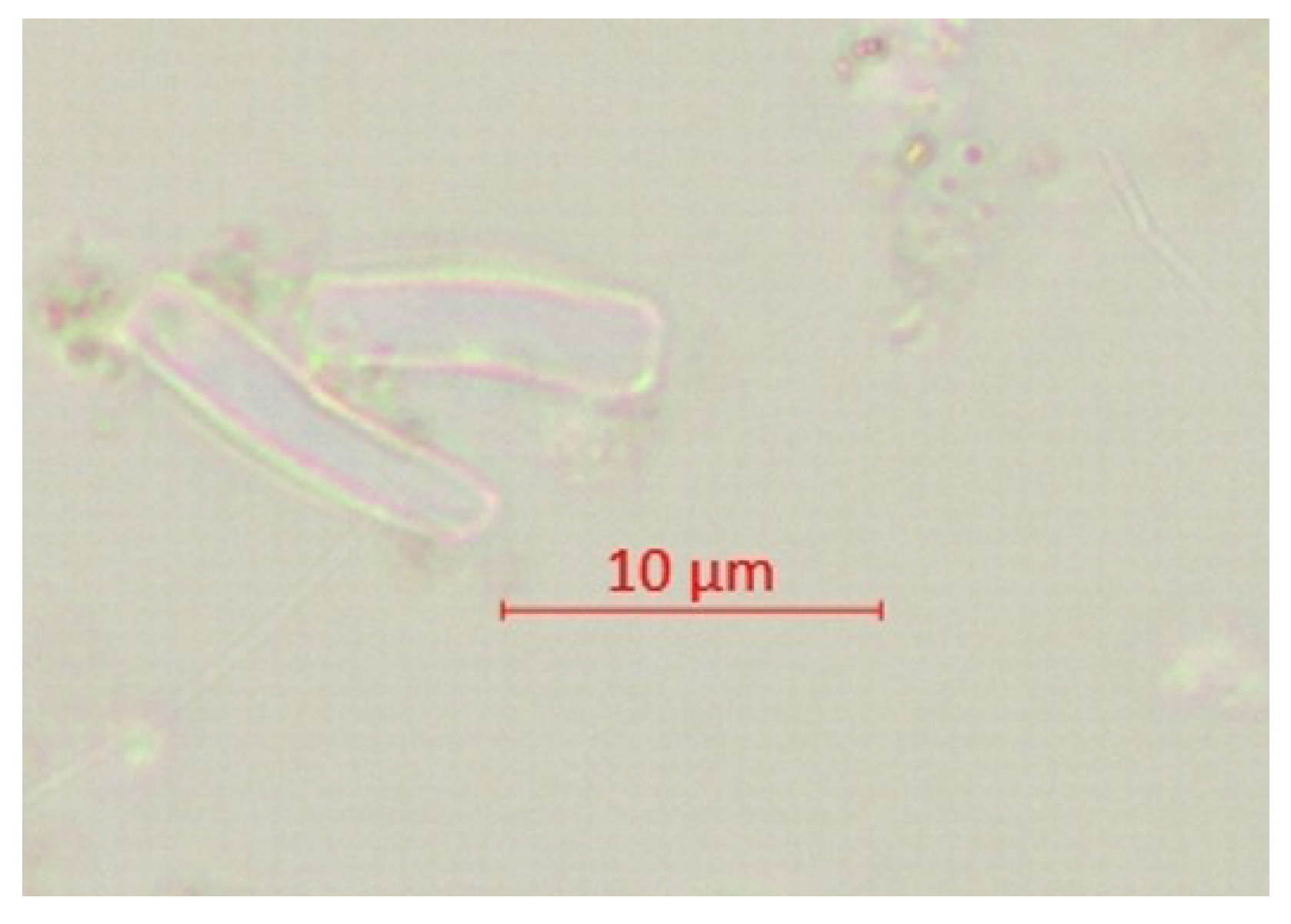

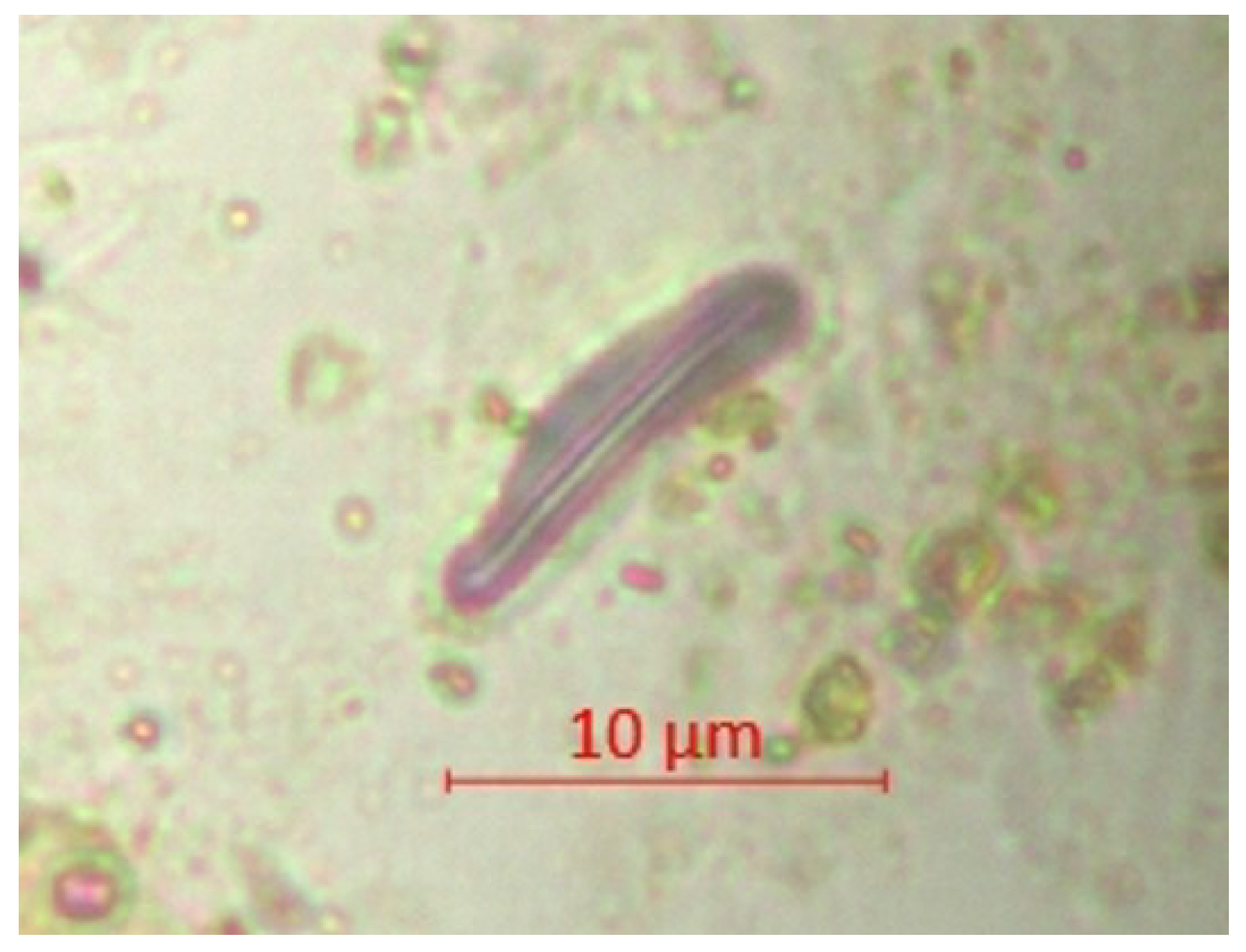
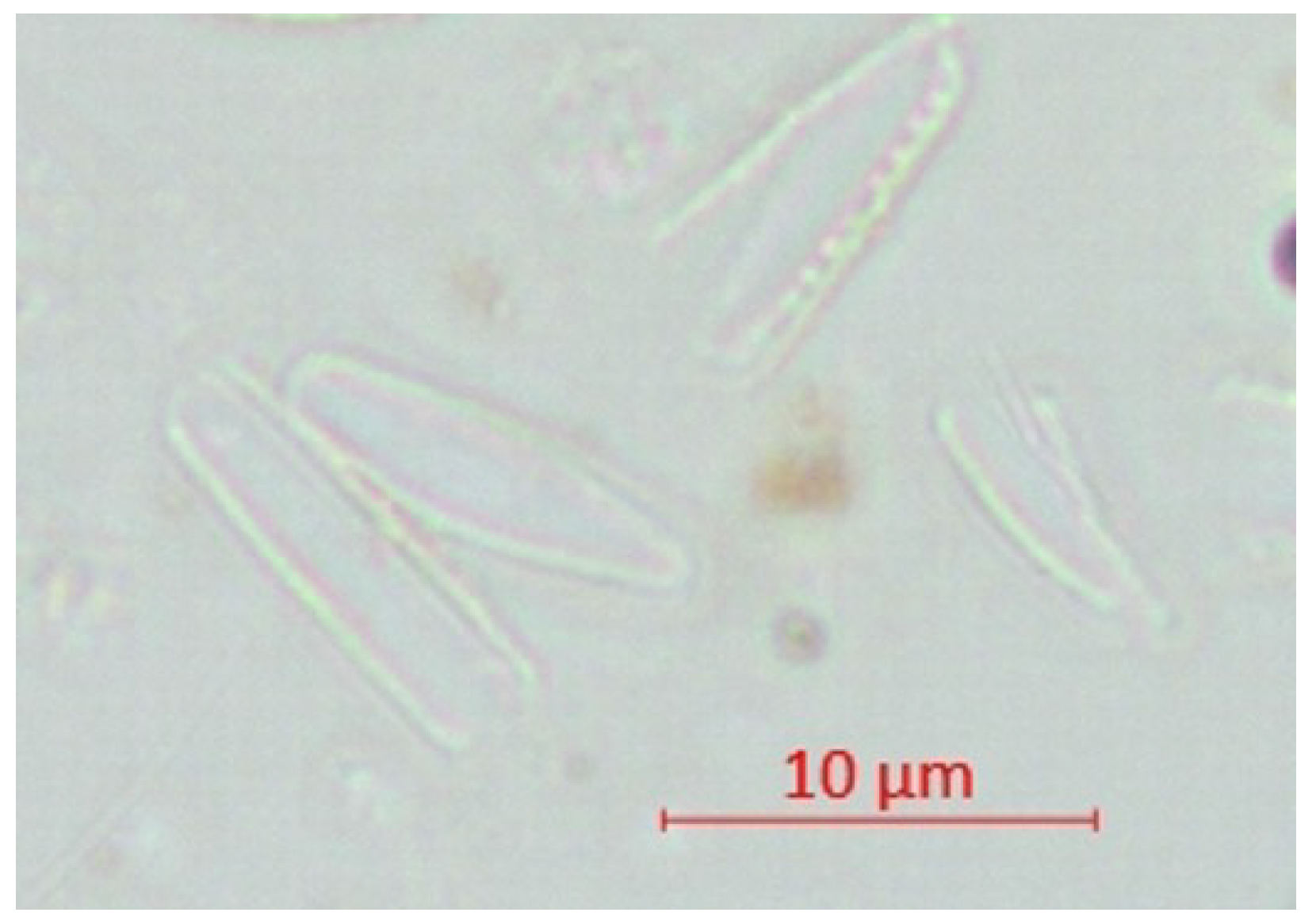
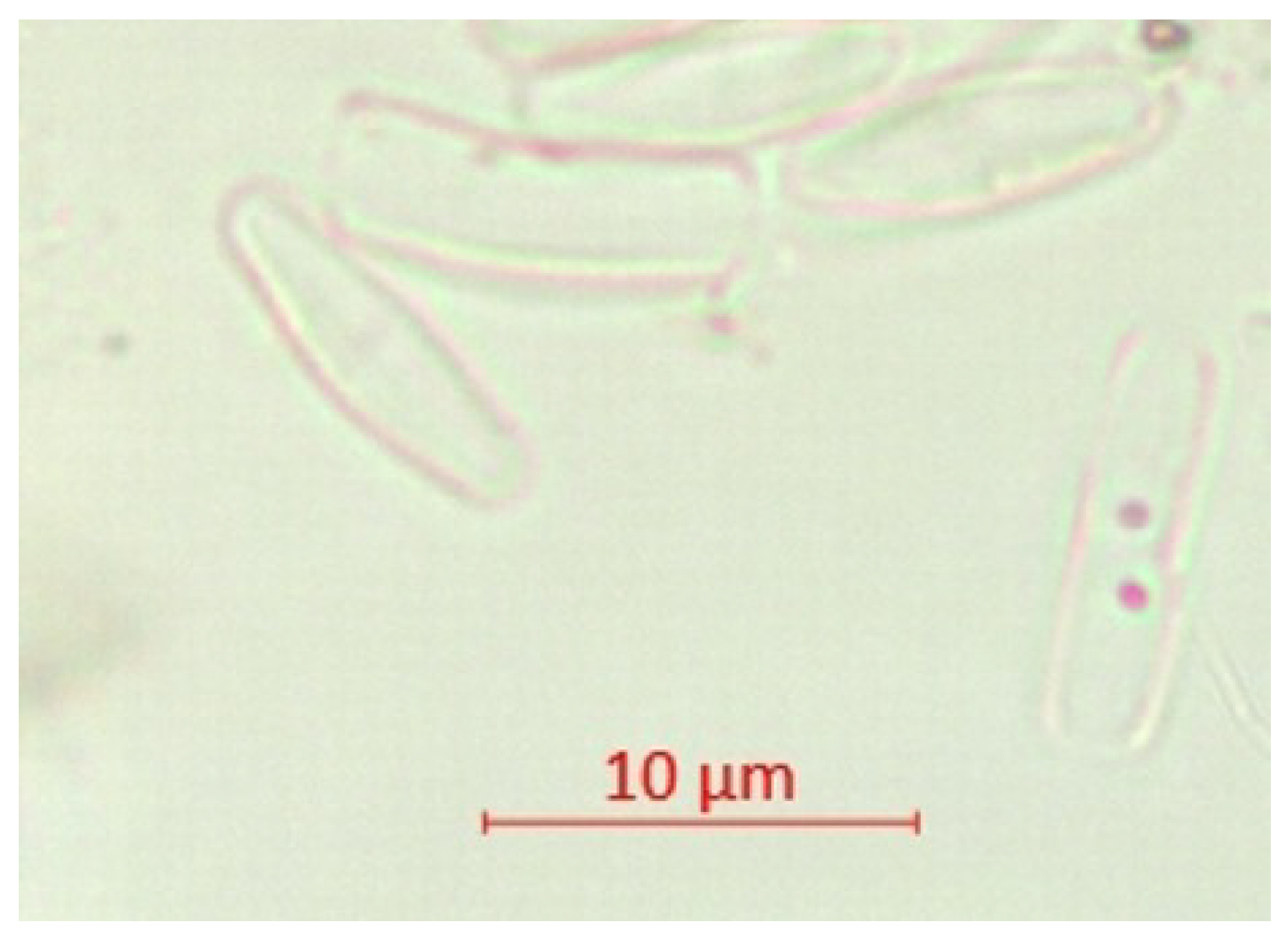

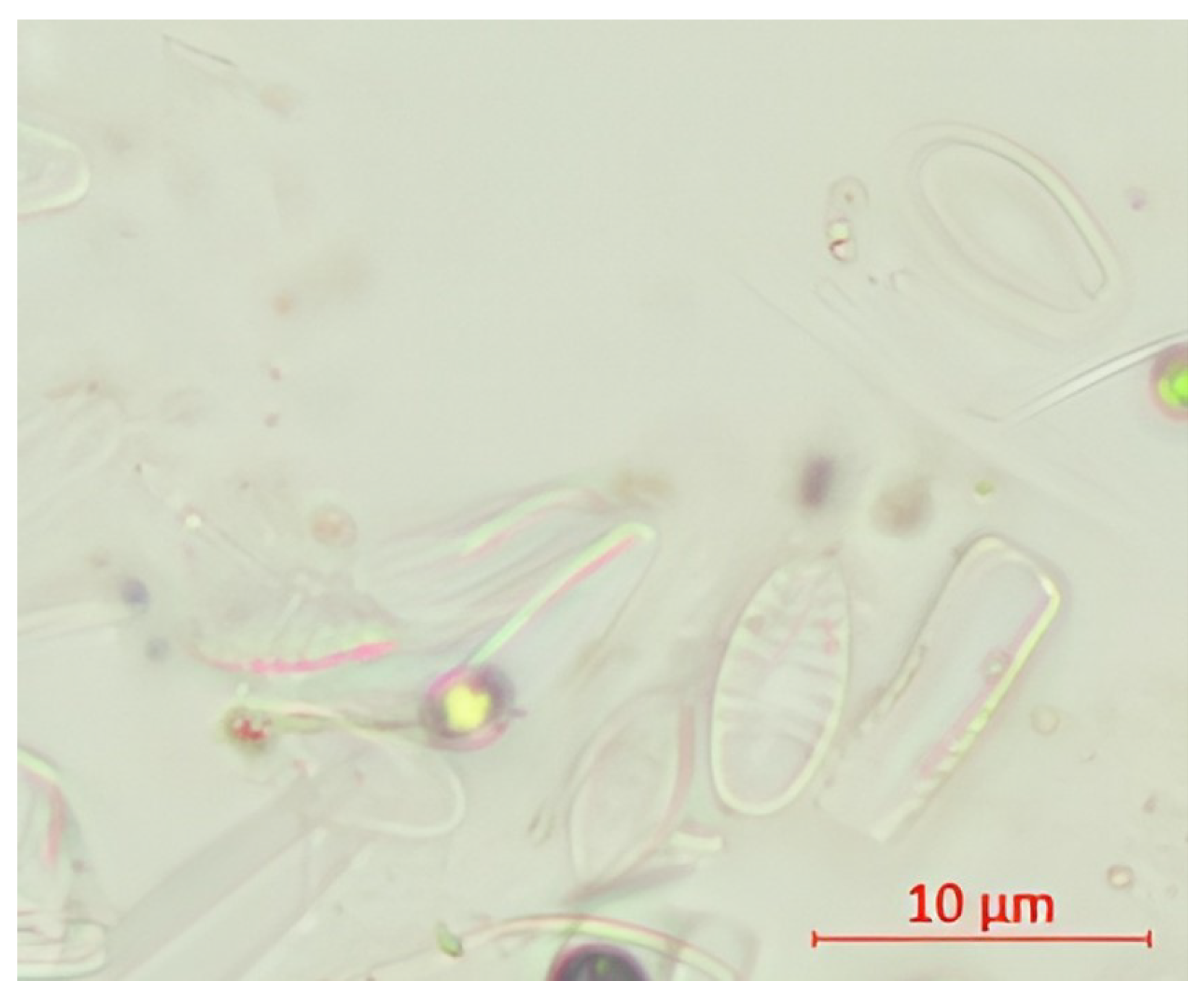

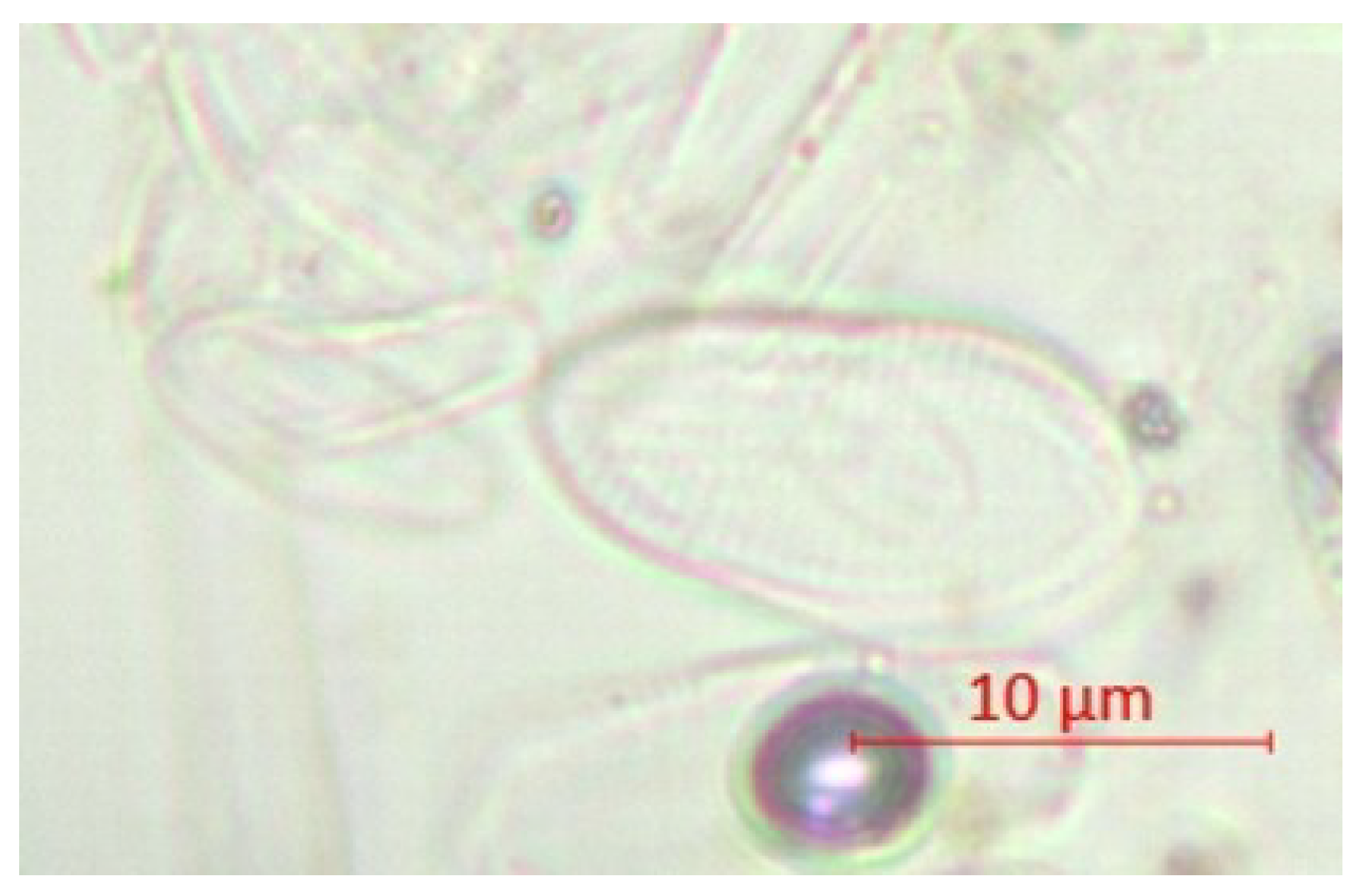

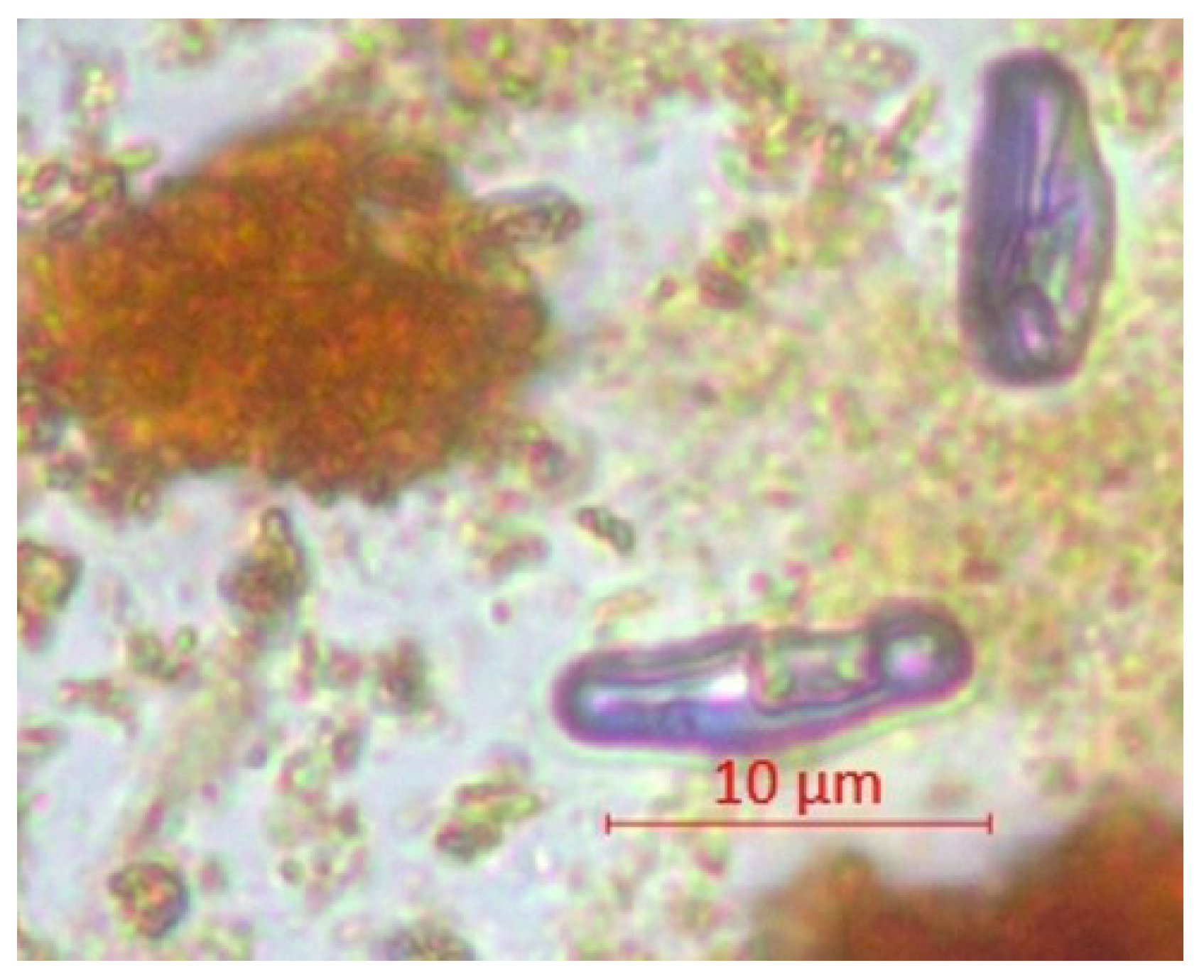

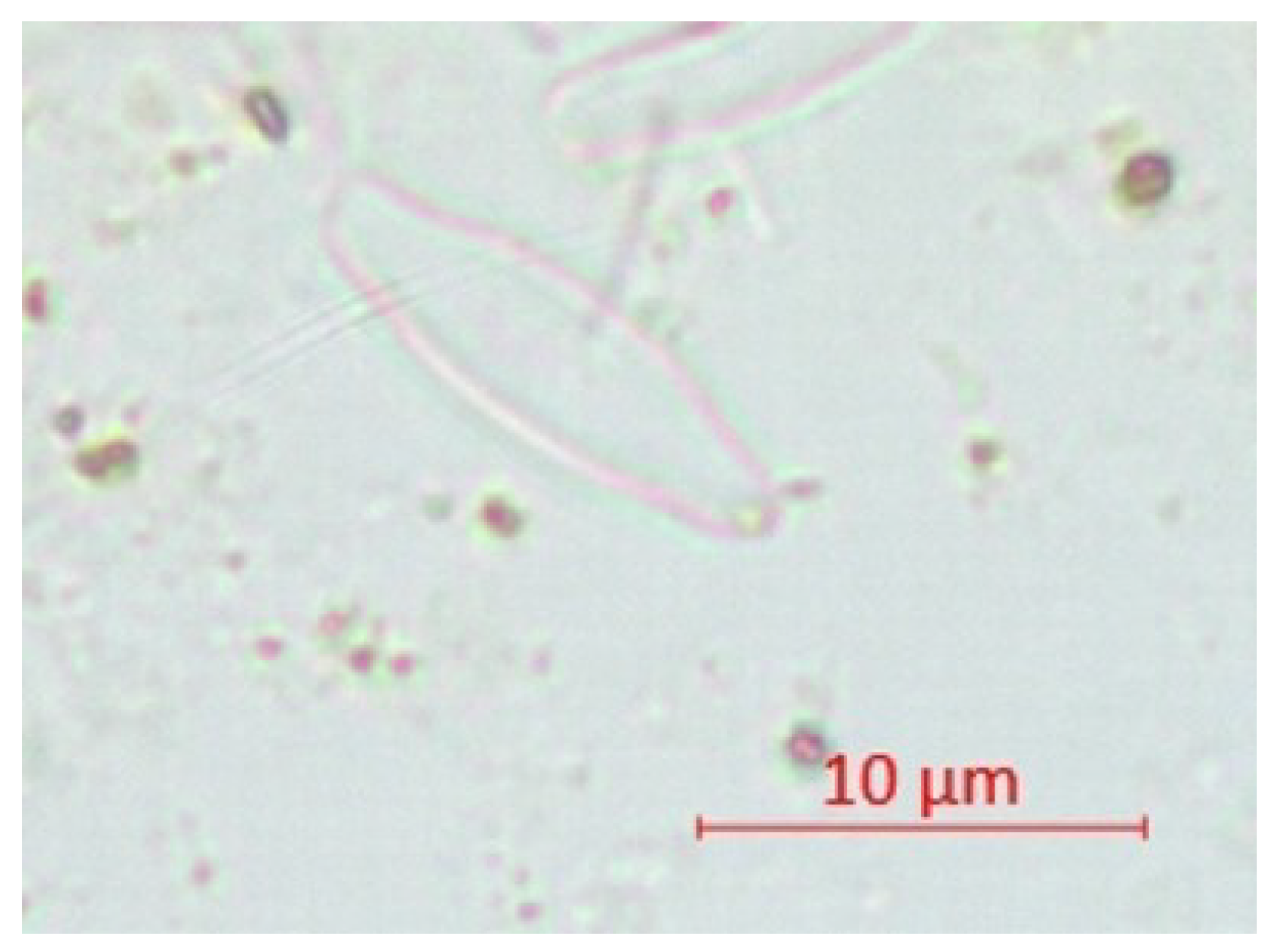
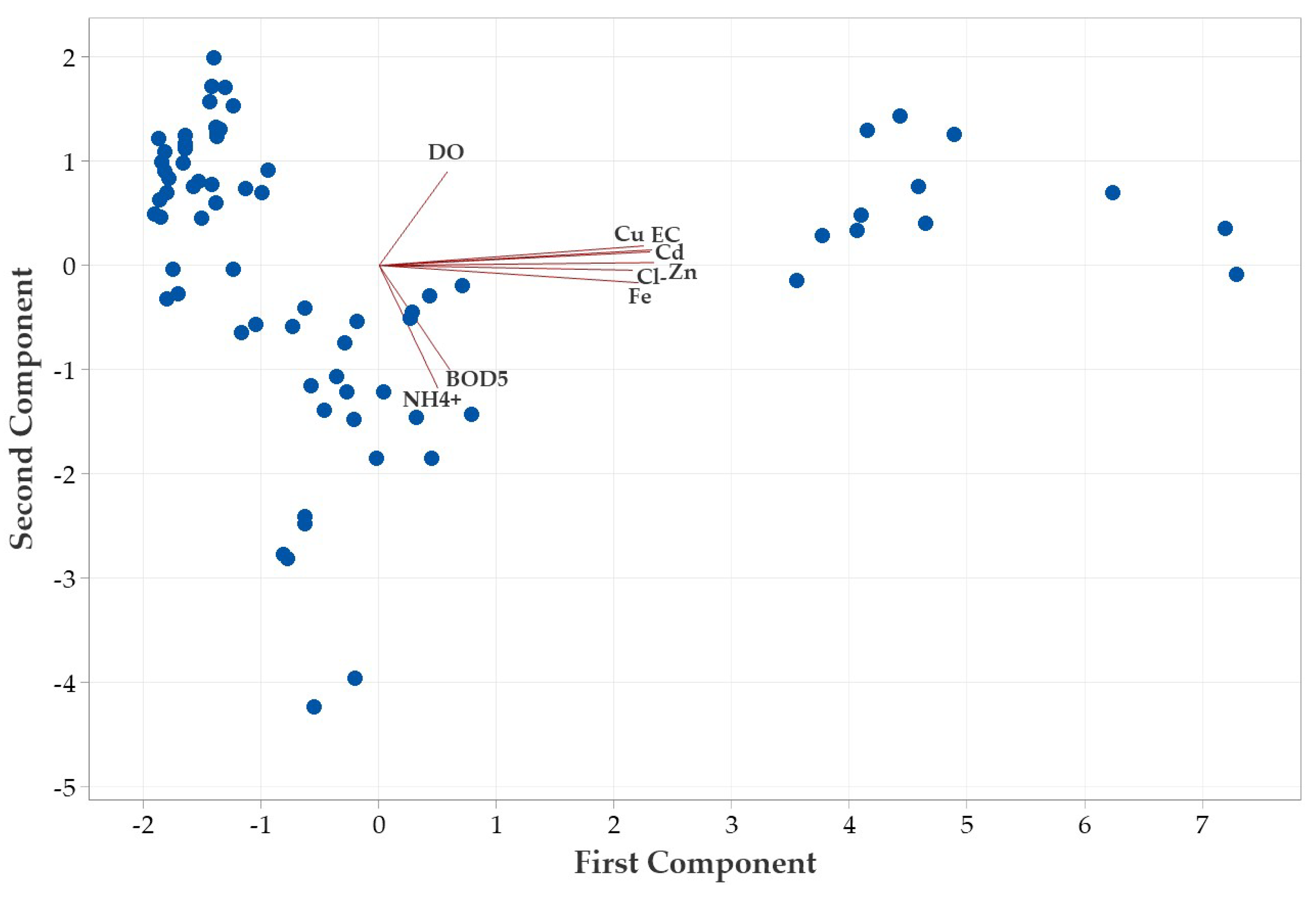
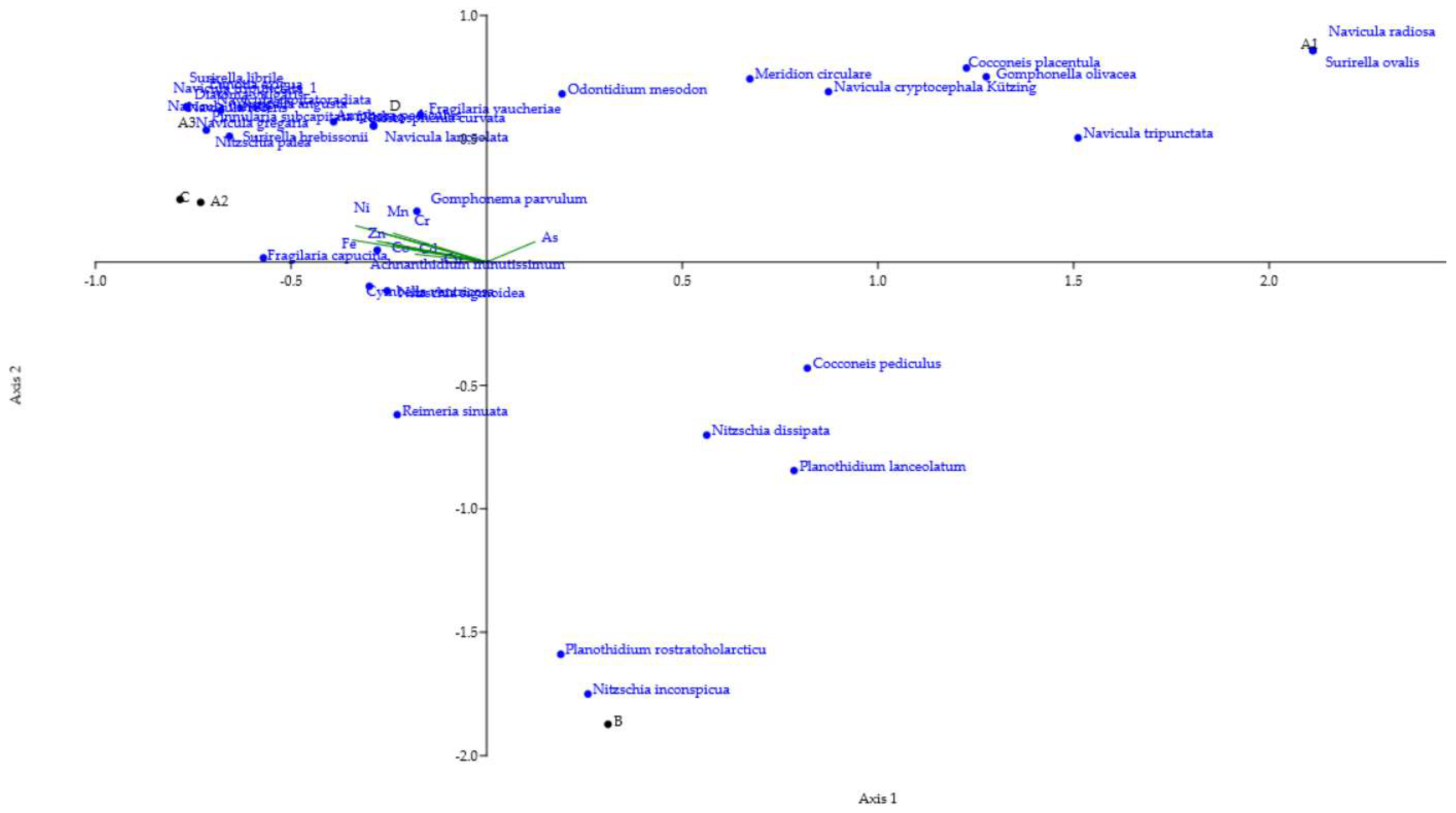
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 7.9 | 24.3 | 9.12 | 0.76 | 2.16 | 0.015 | 0.001 | 0.025 |
| Apr. | 8.2 | 31.2 | 8.46 | 0.91 | 1.73 | 0.023 | 0.002 | 0.031 | |
| Jul. | 8.1 | 14.1 | 7.73 | 1.07 | 2.25 | 0.048 | 0.005 | 0.052 | |
| Oct. | 7.7 | 19.7 | 8.37 | 0.85 | 1.96 | 0.031 | 0.004 | 0.019 | |
| 2023 | Jan. | 7.5 | 22.3 | 8.92 | 0.81 | 1.87 | 0.019 | 0.002 | 0.018 |
| Apr. | 8.0 | 31.4 | 8.81 | 1.01 | 1.34 | 0.028 | 0.003 | 0.027 | |
| Jul. | 7.9 | 24.2 | 7.94 | 1.23 | 2.36 | 0.034 | 0.006 | 0.046 | |
| Oct. | 7.6 | 32.4 | 8.35 | 0.67 | 1.24 | 0.027 | 0.001 | 0.029 | |
| 2024 | Jan. | 7.8 | 27.1 | 9.04 | 0.83 | 1.33 | 0.011 | 0.004 | 0.022 |
| Apr. | 7.7 | 32.6 | 8.84 | 0.89 | 1.57 | 0.022 | 0.003 | 0.038 | |
| Jul. | 7.5 | 28.0 | 8.01 | 1.04 | 2.67 | 0.038 | 0.009 | 0.044 | |
| Oct. | 8.7 | 32.8 | 8.76 | 0.77 | 1.56 | 0.013 | 0.002 | 0.033 | |
| Quality class [35] | I | 6.5–8.5 | - | 6.2, not less than 80% oxygen saturation | 6 | 10 | 0.4 | 0.01 | 1 |
| II | 25 | 0.8 | 0.03 | 3 | |||||
| III | 50 | 1.2 | 0.06 | 5.6 | |||||
| IV | 125 | 3.2 | 0.3 | 11.2 | |||||
| V | >125 | >3.2 | >0.3 | >11.2 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 136 | 153 | 38 | 2.13 | 4.1 | 78.7 | 0.8 | 9.8 |
| Apr. | 167 | 169 | 42 | 3.39 | 3.8 | 83.5 | 1.3 | 8.1 | |
| Jul. | 251 | 234 | 66 | 3.85 | 4.5 | 89,4 | 3.7 | 12.4 | |
| Oct. | 196 | 206 | 59 | 1.89 | 3.4 | 73.2 | 2.4 | 11.1 | |
| 2023 | Jan. | 194 | 174 | 41 | 1.66 | 3.7 | 72.7 | 1.1 | 7.6 |
| Apr. | 184 | 182 | 57 | 2.47 | 4.2 | 86.7 | 1.5 | 9.3 | |
| Jul. | 279 | 213 | 61 | 2.98 | 4.0 | 102.4 | 4.1 | 14.6 | |
| Oct. | 213 | 168 | 46 | 2.09 | 3.4 | 84.6 | 2.9 | 8.4 | |
| 2024 | Jan. | 164 | 158 | 48 | 2.17 | 3.9 | 87.4 | 1.5 | 10.2 |
| Apr. | 183 | 184 | 43 | 3.14 | 4.3 | 84.3 | 2.3 | 9.7 | |
| Jul. | 267 | 227 | 55 | 4.16 | 4.8 | 111.5 | 3.8 | 14.5 | |
| Oct. | 173 | 173 | 40 | 2.17 | 3.5 | 91.0 | 2.5 | 10.3 | |
| Quality class [35] | I | - | 500 | 50 | 12 | 25 | - | 25 | 60 |
| II | 750 | 100 | 50 | 50 | 50 | 120 | |||
| III | 1000 | 200 | 100 | 100 | 250 | 250 | |||
| IV | 1300 | 300 | 200 | 200 | 300 | 300 | |||
| V | >1300 | >300 | >200 | >200 | >300 | >300 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fetotal [mg/L] | Mntotal [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.32 | 0.31 | 0.91 | 1.47 | 2.54 | 0.02 | 0.054 | 1.64 | 7.69 |
| Apr. | 0.41 | 0.45 | 1.13 | 2.80 | 3.17 | 0.04 | 0.047 | 2.37 | 9.14 | |
| Jul. | 0.77 | 0.63 | 3.47 | 5.78 | 9.76 | 0.09 | 0.124 | 4.46 | 10.54 | |
| Oct. | 0.28 | 0.43 | 2.34 | 2.34 | 3.15 | 0.06 | 0.067 | 2.48 | 8.42 | |
| 2023 | Jan. | 0.35 | 0.28 | 0.24 | 2.01 | 3.09 | 0.01 | 0.049 | 1.87 | 6.43 |
| Apr. | 0.46 | 0.51 | 0.69 | 3.17 | 4.17 | 0.03 | 0.061 | 2.27 | 9.84 | |
| Jul. | 0.69 | 0.57 | 2.64 | 6.15 | 10.46 | 0.07 | 0.115 | 3.94 | 13.47 | |
| Oct. | 0.25 | 0.39 | 1.86 | 2.36 | 3.18 | 0.04 | 0.055 | 3.04 | 7.62 | |
| 2024 | Jan. | 0.39 | 0.41 | 0.36 | 2.17 | 4.64 | 0.01 | 0.049 | 2.07 | 6.64 |
| Apr. | 0.51 | 0.37 | 0.62 | 2.48 | 5.20 | 0.05 | 0.062 | 2.41 | 7.30 | |
| Jul. | 0.59 | 0.58 | 3.42 | 6.37 | 8.12 | 0.08 | 0.097 | 3.97 | 14.52 | |
| Oct. | 0.33 | 0.34 | 1.84 | 3.14 | 4.38 | 0.02 | 0.043 | 1.95 | 11.34 | |
| Quality class [35] | I | 10 | 0.5 | 10 | 25 | 20 | 0.3 | 0.05 | 10 | 100 |
| II | 20 | 1 | 20 | 50 | 30 | 0.5 | 0.1 | 25 | 200 | |
| III | 50 | 2 | 50 | 100 | 50 | 1.0 | 0.3 | 50 | 500 | |
| IV | 100 | 5 | 100 | 250 | 100 | 2 | 1 | 100 | 1000 | |
| V | >100 | >5 | >100 | >250 | >100 | >2 | >1 | >100 | >1000 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 62 | 0 | β | 2 |
| Achnanthidium minutissimum Kützing) Czarnecki | 129 | 8 | o-β | 1.5 |
| Cocconeis pediculus Ehrenberg | 24 | 0 | β | 2 |
| Cocconeis placentula Ehrenberg | 9 | 0 | β | 2 |
| Cymbella ventricosa Agardh | 1 | 0 | o-β | 1.5 |
| Odontidium mesodon (Ehrenberg) Kützing | 3 | 0 | o | 1 |
| Fragilaria vaucheriae (Kützing) J.B.Petersen | 5 | 0 | β | 2 |
| Gomphonella olivacea (Hornemann) Rabenhorst | 15 | 0 | β | 2 |
| Gomphonema parvulum Kützing | 3 | 0 | β | 2 |
| Meridion circulare (Greville) C.Agardh | 3 | 0 | o | 1 |
| Navicula cryptocephala Kützing | 27 | 0 | α | 3 |
| Navicula radiosa Kützing | 59 | 0 | β | 2 |
| Navicula tripunctata (O.F.Müller) Bory | 13 | 0 | β-α | 2.5 |
| Nitzschia dissipata (Kützing) Rabenhorst | 13 | 0 | o-β | 1.5 |
| Surirella ovalis Brébisson | 40 | 0 | α | 3 |
| Total | 414 | |||
| Saprobic index value | 1.91 | |||
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 7.1 | 28.5 | 8.43 | 1.27 | 1.41 | 0.357 | 0.006 | 0.246 |
| Apr. | 6.9 | 35.1 | 8.67 | 1.53 | 1.87 | 0.413 | 0.009 | 0.740 | |
| Jul. | 5.1 | 18.2 | 8.04 | 0.89 | 0.95 | 0.281 | 0.004 | 0.204 | |
| Oct. | 7.3 | 41.0 | 8.27 | 2.31 | 2.16 | 0.981 | 0.013 | 1.012 | |
| 2023 | Jan. | 7.7 | 36.7 | 8.79 | 1.46 | 2.04 | 0.652 | 0.008 | 0.314 |
| Apr. | 7.2 | 47.9 | 8.68 | 1.90 | 1.98 | 0.607 | 0.012 | 0.681 | |
| Jul. | 5.5 | 39.4 | 8.37 | 1.14 | 0.68 | 0.194 | 0.007 | 0.181 | |
| Oct. | 7.0 | 65.3 | 8.07 | 1.82 | 2.43 | 1.147 | 0.015 | 0.998 | |
| 2024 | Jan. | 7.6 | 32.0 | 8.62 | 1.51 | 1.74 | 0.842 | 0.012 | 0.372 |
| Apr. | 6.9 | 47.2 | 8.48 | 1.82 | 1.37 | 0.701 | 0.008 | 0.593 | |
| Jul. | 4.5 | 26.1 | 8.18 | 0.97 | 1.18 | 1.027 | 0.006 | 0.167 | |
| Oct. | 7.5 | 74.7 | 8.35 | 2.59 | 2.35 | 1.842 | 0.011 | 0.847 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 294 | 233 | 43 | 10.3 | 7.6 | 63.4 | 10.4 | 102.1 |
| Apr. | 374 | 261 | 86 | 12.7 | 9.1 | 74.3 | 11.6 | 132.5 | |
| Jul. | 514 | 313 | 102 | 16.3 | 11.3 | 96.0 | 16.1 | 194.3 | |
| Oct. | 263 | 179 | 43 | 4.7 | 5.5 | 61.2 | 9.2 | 77.2 | |
| 2023 | Jan. | 332 | 231 | 52 | 7.9 | 7.8 | 68.3 | 12.6 | 116.4 |
| Apr. | 347 | 246 | 74 | 17.3 | 9.2 | 89.1 | 11.3 | 126.8 | |
| Jul. | 411 | 374 | 137 | 22.2 | 10.7 | 109.8 | 15.0 | 214.1 | |
| Oct. | 363 | 262 | 63 | 8.8 | 6.3 | 55.6 | 8.2 | 51.7 | |
| 2024 | Jan. | 384 | 263 | 74 | 10.0 | 7.7 | 74.1 | 10.9 | 113.0 |
| Apr. | 473 | 324 | 94 | 17.8 | 9.8 | 74.3 | 11.7 | 141.2 | |
| Jul. | 562 | 364 | 93 | 27.4 | 10.1 | 79.7 | 13.5 | 183.4 | |
| Oct. | 318 | 189 | 46 | 11.1 | 8.4 | 41.0 | 9.8 | 52.3 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fe total [mg/L] | Mn total [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.35 | 3.68 | 6.84 | 23.41 | 127.31 | 1.28 | 10.43 | 21.68 | 412.35 |
| Apr. | 0.42 | 4.87 | 8.14 | 25.12 | 135.07 | 1.67 | 15.17 | 32.17 | 617.22 | |
| Jul. | 0.47 | 8.64 | 9.63 | 28.86 | 187.35 | 3.07 | 18.84 | 48.47 | 896.74 | |
| Oct. | 0.36 | 1.37 | 3.61 | 11.58 | 104.28 | 1.08 | 8.67 | 18.09 | 233.17 | |
| 2023 | Jan. | 0.38 | 3.02 | 7.45 | 17.61 | 94.36 | 1.69 | 12.20 | 25.34 | 387.19 |
| Apr. | 0.41 | 3.77 | 8.74 | 22.27 | 125.07 | 2.41 | 14.64 | 39.41 | 697.23 | |
| Jul. | 0.37 | 9.14 | 11.08 | 28.94 | 154.08 | 4.44 | 24.04 | 48.30 | 967.74 | |
| Oct. | 0.33 | 2.35 | 5.12 | 15.6 | 112.32 | 1.32 | 12.71 | 17.40 | 214.78 | |
| 2024 | Jan. | 0.31 | 3.62 | 7.38 | 17.08 | 89.70 | 1.87 | 11.74 | 21.77 | 386.42 |
| Apr. | 0.38 | 6.42 | 8.42 | 28.17 | 131.22 | 3.24 | 14.32 | 23.48 | 612.37 | |
| Jul. | 0.47 | 7.84 | 12.28 | 61.74 | 166.74 | 4.74 | 21.74 | 42.07 | 814.37 | |
| Oct. | 0.39 | 2.47 | 3.18 | 16.37 | 99.71 | 1.39 | 13.62 | 19.42 | 274.62 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 1 | 0 | β | 2 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 353 | 99 | o-β | 1.5 |
| Fragilaria vaucheriae (Kützing) J.B.Petersen | 2 | 0 | β | 2 |
| Navicula cryptocephala Kützing | 2 | 0 | α | 3 |
| Nitzschia dissipata (Kützing) Rabenhorst | 2 | 0 | o-β | 1.5 |
| Total | 459 | |||
| Saprobic index value | 1.22 | |||
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 7.2 | 24.4 | 8.35 | 1.37 | 1.51 | 0.368 | 0.007 | 0.254 |
| Apr. | 7.4 | 31.3 | 8.27 | 1.68 | 1.63 | 0.443 | 0.011 | 0.769 | |
| Jul. | 5.5 | 17.9 | 8.08 | 1.12 | 1.12 | 0.312 | 0.007 | 0.274 | |
| Oct. | 7.7 | 38.3 | 8.15 | 2.34 | 2.27 | 1.113 | 0.016 | 1.117 | |
| 2023 | Jan. | 7.9 | 31.3 | 8.53 | 1.64 | 2.21 | 0.674 | 0.011 | 0.427 |
| Apr. | 7.6 | 45.7 | 8.44 | 1.97 | 2.17 | 0.617 | 0.014 | 0.719 | |
| Jul. | 5.8 | 32.0 | 8.24 | 1.27 | 0.84 | 0.243 | 0.009 | 0.227 | |
| Oct. | 7.3 | 58.4 | 8.04 | 1.94 | 2.67 | 1.297 | 0.017 | 1.098 | |
| 2024 | Jan. | 7.8 | 28.8 | 8.57 | 1.63 | 1.93 | 0.863 | 0.014 | 0.432 |
| Apr. | 7.4 | 43.1 | 8.24 | 1.92 | 1.40 | 0.784 | 0.011 | 0.613 | |
| Jul. | 5.6 | 26.2 | 8.07 | 1.27 | 1.31 | 1.084 | 0.009 | 0.218 | |
| Oct. | 7.2 | 69.5 | 8.17 | 2.62 | 2.48 | 1.927 | 0.014 | 0.917 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 279 | 201 | 38 | 9.3 | 7.6 | 52.1 | 9.9 | 98.7 |
| Apr. | 356 | 249 | 74 | 10.2 | 8.7 | 67.9 | 11.2 | 124.4 | |
| Jul. | 487 | 289 | 98 | 14.1 | 10.2 | 84.4 | 15.4 | 189.1 | |
| Oct. | 237 | 156 | 34 | 3.4 | 4.7 | 48.3 | 8.7 | 34.1 | |
| 2023 | Jan. | 302 | 197 | 41 | 7.1 | 6.8 | 56.4 | 10.1 | 101.5 |
| Apr. | 318 | 212 | 62 | 14.4 | 7.3 | 74.2 | 10.4 | 117.9 | |
| Jul. | 392 | 328 | 103 | 18.4 | 9.1 | 96.4 | 13.7 | 201.5 | |
| Oct. | 321 | 187 | 49 | 5.9 | 5.9 | 41.7 | 7.9 | 42.5 | |
| 2024 | Jan. | 342 | 231 | 59 | 8.6 | 6.9 | 61.2 | 10.1 | 108.7 |
| Apr. | 408 | 281 | 81 | 11.4 | 8.1 | 67.1 | 10.7 | 134.9 | |
| Jul. | 512 | 301 | 87 | 21.5 | 8.7 | 73.8 | 11.8 | 174.8 | |
| Oct. | 298 | 141 | 28 | 6.7 | 6.4 | 33.4 | 9.3 | 41.4 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fetotal [mg/L] | Mntotal [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.26 | 3.27 | 6.47 | 17.83 | 94.70 | 1.09 | 9.87 | 19.72 | 384.17 |
| Apr. | 0.31 | 4.46 | 7.68 | 19.71 | 114.61 | 1.47 | 13.40 | 27.81 | 547.70 | |
| Jul. | 0.38 | 8.12 | 9.14 | 24.52 | 157.14 | 2.48 | 16.41 | 42.71 | 847.41 | |
| Oct. | 0.26 | 1.07 | 3.24 | 4.56 | 45.78 | 0.87 | 1.24 | 8.41 | 147.79 | |
| 2023 | Jan. | 0.29 | 2.79 | 7.04 | 16.37 | 84.87 | 1.27 | 10.37 | 20.17 | 327.18 |
| Apr. | 0.32 | 3.29 | 8.14 | 18.19 | 104.73 | 1.86 | 11.71 | 32.18 | 647.08 | |
| Jul. | 0.27 | 7.14 | 10.24 | 23.74 | 137.37 | 3.07 | 14.62 | 41.83 | 941.73 | |
| Oct. | 0.25 | 1.18 | 4.18 | 5.14 | 65.17 | 0.76 | 2.38 | 7.53 | 178.14 | |
| 2024 | Jan. | 0.26 | 3.14 | 6.87 | 15.74 | 79.09 | 1.37 | 8.82 | 17.86 | 357.14 |
| Apr. | 0.28 | 5.07 | 7.63 | 20.16 | 117.49 | 2.34 | 10.08 | 21.18 | 593.41 | |
| Jul. | 0.39 | 6.87 | 11.42 | 21.31 | 146.91 | 3.65 | 13.48 | 39.07 | 796.01 | |
| Oct. | 0.33 | 1.32 | 2.78 | 6.37 | 39.70 | 0.39 | 3.14 | 6.07 | 207.78 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Achnanthidium minutissimum Kützing) Czarnecki | 214 | 71 | o-β | 1.5 |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 3 | 0 | β | 2 |
| Planothidium rostratoholarcticum Lange-Bertalot & Bak | 1 | 0 | β | 2 |
| Amphora pediculus (Kützing) Grunow | 3 | 0 | β | 2 |
| Cocconeis pediculus Ehrenberg | 5 | 0 | β | 2 |
| Cocconeis placentula Ehrenberg | 4 | 0 | β | 2 |
| Surirella librile (Ehrenberg) Ehrenberg | 1 | 0 | β-α | 2.5 |
| Cymbella ventricosa Agardh | 17 | 0 | o-β | 1.5 |
| Odontidium mesodon (Ehrenberg) Kützing | 5 | 0 | o | 1 |
| Diatoma vulgaris Bory | 1 | 0 | β-α | 2.5 |
| Eunotia exigua (Brébisson ex Kützing) Rabenhorst | 1 | 0 | β | 2 |
| Fragilaria capucina Desmazières | 7 | 0 | β | 2 |
| Fragilaria vaucheriae (Kützing) J.B.Petersen | 13 | 0 | β | 2 |
| Gomphonema parvulum Kützing | 15 | 0 | β | 2 |
| Meridion circulare (Greville) C.Agardh | 3 | 0 | o | 1 |
| Navicula capitatoradiata H.Germain ex Gasse | 10 | 0 | o-β | 1.5 |
| Navicula gregaria Donkin | 5 | 0 | β | 2 |
| Navicula recens (H.Lange-Bertalot) H.Lange-Bertalot | 1 | 0 | α | 3 |
| Navicula tripunctata (O.F.Müller) Bory | 1 | 0 | β-α | 2.5 |
| Navicula veneta Kützing | 3 | 0 | α | 3 |
| Nitzschia dissipata (Kützing) Rabenhorst | 3 | 0 | o-β | 1.5 |
| Nitzschia inconspicua Grunow | 2 | 0 | α | 3 |
| Nitzschia palea (Kützing) W.Smith | 6 | 0 | α | 3 |
| Nitzschia sigmoidea (Nitzsch) W.Smith | 2 | 0 | β | 2 |
| Pinnularia subcapitata f. subconstricta A.Cleve | 1 | 0 | o | 1 |
| Reimeria sinuata (W.Gregory) Kociolek & Stoermer | 2 | 0 | β | 2 |
| Surirella angusta Kützing | 9 | 0 | β | 2 |
| Surirella brebissonii Krammer & Lange-Bertalot | 19 | 0 | β-α | 2.5 |
| Total | 428 | |||
| Saprobic index value | 1.79 | |||
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 7.1 | 15.4 | 9.01 | 0.69 | 4.48 | 0.032 | 0.007 | 0.142 |
| Apr. | 7.4 | 16.8 | 8.94 | 1.18 | 5.69 | 0.056 | 0.009 | 0.147 | |
| Jul. | 7.5 | 21.8 | 8.41 | 1.86 | 8.69 | 0.084 | 0.011 | 0.201 | |
| Oct. | 7.2 | 20.4 | 8.82 | 0.89 | 4.39 | 0.039 | 0.005 | 0.114 | |
| 2023 | Jan. | 7.3 | 24.2 | 9.17 | 0.86 | 5.21 | 0.041 | 0.004 | 0.118 |
| Apr. | 7.6 | 25.4 | 9.04 | 1.07 | 6.28 | 0.061 | 0.006 | 0.214 | |
| Jul. | 7.9 | 32.4 | 8.22 | 1.78 | 7.96 | 0.076 | 0.009 | 0.368 | |
| Oct. | 7.2 | 27.4 | 8.69 | 0.58 | 6.24 | 0.039 | 0.005 | 0.120 | |
| 2024 | Jan. | 7.0 | 21.4 | 8.89 | 0.48 | 6.17 | 0.041 | 0.006 | 0.134 |
| Apr. | 7.2 | 18.9 | 8.68 | 0.79 | 6.32 | 0.048 | 0.008 | 0.157 | |
| Jul. | 7.7 | 26.8 | 8.34 | 1.28 | 8.42 | 0.069 | 0.010 | 0.197 | |
| Oct. | 7.5 | 25.6 | 8.79 | 1.18 | 5.68 | 0.054 | 0.0.5 | 0.127 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 267.36 | 136.47 | 21.42 | 9.68 | 1.20 | 47.26 | 3.25 | 22.1 |
| Apr. | 302.14 | 157.48 | 32.06 | 15.62 | 2.14 | 65.34 | 5.24 | 26.3 | |
| Jul. | 420.18 | 215.32 | 45.63 | 25.64 | 3.64 | 89.46 | 7.36 | 35.4 | |
| Oct. | 324.12 | 169.41 | 35.24 | 18.96 | 2.36 | 43.80 | 3.18 | 18.9 | |
| 2023 | Jan. | 189.47 | 126.84 | 19.81 | 14.39 | 1.89 | 35.69 | 2.39 | 29.4 |
| Apr. | 294.62 | 174.24 | 23.68 | 19.37 | 2.39 | 61.14 | 4.36 | 24.8 | |
| Jul. | 352.41 | 189.76 | 39.57 | 31.28 | 4.97 | 77.82 | 6.28 | 41.5 | |
| Oct. | 264.20 | 124.62 | 28.41 | 22.36 | 3.31 | 36.84 | 2.84 | 36.9 | |
| 2024 | Jan. | 221.13 | 112.39 | 26.37 | 16.54 | 2.17 | 35.48 | 4.15 | 31.2 |
| Apr. | 284.63 | 154.63 | 36.41 | 19.37 | 2.36 | 52.14 | 3.98 | 41.6 | |
| Jul. | 362.14 | 241.36 | 56.18 | 36.24 | 4.16 | 77.69 | 5.79 | 54.6 | |
| Oct. | 203.48 | 163.41 | 38.24 | 24.51 | 3.14 | 39.61 | 3.14 | 35.6 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fetotal [mg/L] | Mntotal [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.28 | 0.12 | 0.61 | 0.98 | 56.47 | 0.15 | 0.027 | 0.56 | 18.67 |
| Apr. | 0.62 | 0.36 | 0.58 | 1.14 | 69.47 | 0.29 | 0.031 | 0.75 | 32.41 | |
| Jul. | 1.31 | 0.84 | 0.79 | 2.46 | 114.3 | 0.67 | 0.056 | 1.12 | 45.61 | |
| Oct. | 0.57 | 0.61 | 0.67 | 1.69 | 77.91 | 0.34 | 0.036 | 0.76 | 24.87 | |
| 2023 | Jan. | 0.38 | 0.34 | 0.52 | 1.42 | 63.70 | 0.24 | 0.024 | 0.41 | 24.39 |
| Apr. | 0.49 | 0.27 | 0.68 | 1.25 | 74.51 | 0.37 | 0.038 | 0.69 | 35.69 | |
| Jul. | 1.64 | 0.63 | 0.87 | 3.18 | 154.91 | 0.75 | 0.068 | 0.84 | 48.69 | |
| Oct. | 0.61 | 0.44 | 0.45 | 2.18 | 79.19 | 0.42 | 0.054 | 0.47 | 34.12 | |
| 2024 | Jan. | 0.41 | 0.27 | 0.61 | 1.39 | 73.41 | 0.28 | 0.033 | 0.38 | 19.47 |
| Apr. | 0.39 | 0.36 | 0.54 | 1.54 | 68.49 | 0.34 | 0.047 | 0.57 | 32.45 | |
| Jul. | 0.67 | 0.74 | 0.94 | 2.94 | 164.2 | 0.76 | 0.094 | 0.71 | 65.42 | |
| Oct. | 0.52 | 0.46 | 0.49 | 1.39 | 89.43 | 0.61 | 0.061 | 0.51 | 32.14 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Achnanthidium minutissimum (Kützing) Czarnecki | 237 | 70 | o-β | 1.5 |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 116 | 12 | β | 2 |
| Planothidium rostratoholarcticum Lange-Bertalot & Bak | 8 | 0 | β | 2 |
| Cocconeis pediculus Ehrenberg | 27 | 3 | β | 2 |
| Cymbella ventricosa Agardh | 9 | 0 | o-β | 1.5 |
| Fragilaria capucina Desmazières | 3 | 0 | β | 2 |
| Gomphonema parvulum Kützing | 4 | 0 | β | 2 |
| Navicula tripunctata (O.F.Müller) Bory | 2 | 0 | β-α | 2.5 |
| Nitzschia dissipata (Kützing) Rabenhorst | 30 | 0 | o-β | 1.5 |
| Nitzschia inconspicua Grunow | 41 | 0 | α | 3 |
| Nitzschia sigmoidea (Nitzsch) W.Smith | 2 | 0 | β | 2 |
| Reimeria sinuata (W.Gregory) Kociolek & Stoermer | 2 | 0 | β | 2 |
| Surirella brebissonii Krammer & Lange-Bertalot | 1 | 0 | β-α | 2.5 |
| Total | 567 | |||
| Saprobic index value | 1.58 | |||
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 4.8 | 113 | 10.5 | 3.12 | 42.7 | 0.152 | 0.021 | 0.072 |
| Apr. | 6.8 | 149 | 9.1 | 1.51 | 23.8 | 0.184 | 0.004 | 0.401 | |
| Jul. | 7.1 | 127 | 8.3 | 0.98 | 35.7 | 0.766 | 0.046 | 1.354 | |
| Oct. | 7.4 | 154 | 10.2 | 0.85 | 29.4 | 0.841 | 0.006 | 0.304 | |
| 2023 | Jan. | 5.4 | 96 | 9.8 | 2.41 | 35.7 | 0.187 | 0.014 | 0.068 |
| Apr. | 7.1 | 124 | 9.2 | 1.87 | 28.1 | 0.124 | 0.008 | 0.425 | |
| Jul. | 6.9 | 139 | 8.4 | 1.05 | 42.1 | 0.354 | 0.035 | 0.847 | |
| Oct. | 6.8 | 150 | 9.7 | 0.98 | 38.4 | 0.347 | 0.014 | 0.405 | |
| 2024 | Jan. | 5.5 | 124 | 8.9 | 2.14 | 23.5 | 0.127 | 0.034 | 0.061 |
| Apr. | 6.1 | 189 | 9.4 | 1.74 | 29.4 | 0.367 | 0.008 | 0.352 | |
| Jul. | 6.6 | 143 | 8.7 | 0.76 | 42.1 | 0.424 | 0.057 | 0.874 | |
| Oct. | 7.2 | 106 | 9.7 | 0.96 | 38.7 | 0.204 | 0.025 | 0.632 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 1544 | 569 | 221.4 | 4.11 | 5.62 | 24.0 | 17.32 | 642 |
| Apr. | 1478 | 657 | 175.2 | 5.64 | 4.58 | 32.4 | 15.78 | 547 | |
| Jul. | 2541 | 847 | 552.1 | 5.84 | 8.64 | 42.7 | 22.34 | 614 | |
| Oct. | 1875 | 724 | 153.9 | 2.32 | 7.32 | 24.8 | 23.72 | 487 | |
| 2023 | Jan. | 1237 | 473 | 178.2 | 3.57 | 4.12 | 27.4 | 24.7 | 514 |
| Apr. | 1289 | 541 | 201.7 | 5.38 | 6.14 | 41.5 | 18.7 | 624 | |
| Jul. | 2647 | 947 | 698.3 | 6.23 | 7.48 | 48.1 | 29.4 | 704 | |
| Oct. | 1672 | 842 | 234.1 | 3.04 | 3.81 | 35.2 | 21.7 | 618 | |
| 2024 | Jan. | 1617 | 652 | 245.1 | 4.01 | 3.39 | 18.7 | 22.3 | 446 |
| Apr. | 1784 | 784 | 204.7 | 4.49 | 4.32 | 34.8 | 27.2 | 524 | |
| Jul. | 2631 | 961 | 618.2 | 6.01 | 5.27 | 47.8 | 31.2 | 716 | |
| Oct. | 1327 | 637 | 235.4 | 4.08 | 4.38 | 31.4 | 31.1 | 527 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fetotal [mg/L] | Mntotal [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.74 | 34.01 | 42.17 | 2.37 | 2147.14 | 3.57 | 1.074 | 24.09 | 1587.24 |
| Apr. | 0.49 | 41.69 | 38.74 | 3.60 | 3241.36 | 4.69 | 1.230 | 26.84 | 2145.21 | |
| Jul. | 1.02 | 51.34 | 54.78 | 5.42 | 4162.30 | 6.72 | 2.417 | 42.87 | 3542.17 | |
| Oct. | 0.74 | 38.71 | 42.58 | 4.38 | 1897.41 | 4.29 | 1.687 | 36.01 | 2476.27 | |
| 2023 | Jan. | 0.45 | 28.47 | 39.41 | 1.89 | 1874.35 | 2.89 | 1.339 | 31.74 | 2017.31 |
| Apr. | 0.58 | 38.44 | 49.52 | 2.38 | 2267.09 | 3.67 | 1.876 | 41.58 | 2417.39 | |
| Jul. | 0.79 | 48.71 | 51.07 | 5.07 | 3124.21 | 7.61 | 2.472 | 49.37 | 3247.07 | |
| Oct. | 0.68 | 32.47 | 35.14 | 3.17 | 1567.41 | 5.14 | 1.961 | 28.63 | 2107.28 | |
| 2024 | Jan. | 0.57 | 21.41 | 28.74 | 2.09 | 2146.38 | 3.69 | 0.943 | 24.18 | 1637.28 |
| Apr. | 0.61 | 28.97 | 29.67 | 3.19 | 2643.78 | 4.18 | 1.578 | 29.47 | 1897.34 | |
| Jul. | 0.74 | 35.64 | 44.26 | 4.62 | 2894.20 | 6.81 | 3.214 | 37.19 | 2463.18 | |
| Oct. | 0.61 | 29.37 | 35.26 | 3.47 | 1897.35 | 5.74 | 2.105 | 24.09 | 1894.28 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Achnanthidium minutissimum (Kützing) Czarnecki | 236 | 88 | o-β | 1.5 |
| Fragilaria capucina Desmazières | 6 | 0 | β | 2 |
| Nitzschia palea (Kützing) W. Smith | 2 | 0 | α | 3 |
| Total | 332 | |||
| Saprobic index value | 1.21 | |||
| Parameter | pH | TSS [mg/L] | DO [mgO/L] | BOD5 [mgO/L] | COD-Cr [mgO/L] | N-NH4 [mgN/L] | N-NO2 [mgN/L] | N-NO3 [mgN/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 7.4 | 13.3 | 9.64 | 0.87 | 4.64 | 0.003 | 0.001 | 0.093 |
| Apr. | 7.6 | 19.4 | 9.42 | 0.94 | 6.57 | 0.007 | 0.003 | 0.128 | |
| Jul. | 7.1 | 35.6 | 8.94 | 1.08 | 9.82 | 0.016 | 0.009 | 0.232 | |
| Oct. | 7.3 | 21.4 | 9.14 | 0.74 | 6.33 | 0.011 | 0.005 | 0.186 | |
| 2023 | Jan. | 7.6 | 16.3 | 9.61 | 0.69 | 5.63 | 0.006 | 0.004 | 0.105 |
| Apr. | 7.5 | 21.2 | 9.23 | 0.81 | 7.76 | 0.009 | 0.002 | 0.134 | |
| Jul. | 7.2 | 41.5 | 9.01 | 1.17 | 10.22 | 0.013 | 0.011 | 0.327 | |
| Oct. | 7.5 | 32.1 | 9.34 | 0.84 | 7.51 | 0.008 | 0.006 | 0.214 | |
| 2024 | Jan. | 7.5 | 17.3 | 9.77 | 0.54 | 5.10 | 0.004 | 0.002 | 0.131 |
| Apr. | 7.6 | 21.7 | 9.61 | 0.69 | 6.61 | 0.006 | 0.006 | 0.164 | |
| Jul. | 7.0 | 38.9 | 9.04 | 0.97 | 8.64 | 0.019 | 0.014 | 0.245 | |
| Oct. | 7.3 | 31.4 | 9.34 | 0.62 | 5.34 | 0.005 | 0.008 | 0.171 |
| Parameter | EC [μS/cm] | FR [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | Na+ [mg/L] | HCO3− [mg/L] | Cl− [mg/L] | SO42− [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | |||||||||
| 2022 | Jan. | 214.31 | 134.7 | 16.1 | 5.1 | 3.5 | 81.2 | 6.1 | 28.6 |
| Apr. | 236.87 | 231.0 | 21.3 | 6.2 | 4.5 | 94.1 | 7.6 | 36.7 | |
| Jul. | 297.06 | 324.2 | 36.5 | 14.8 | 8.7 | 135.7 | 11.6 | 49.2 | |
| Oct. | 187.01 | 142.6 | 28.7 | 8.9 | 6.2 | 89.7 | 9.4 | 24.3 | |
| 2023 | Jan. | 196.30 | 156.3 | 19.4 | 7.4 | 4.1 | 91.6 | 6.8 | 31.5 |
| Apr. | 212.42 | 189.4 | 27.6 | 6.8 | 5.6 | 88.6 | 8.7 | 42.9 | |
| Jul. | 234.58 | 268.2 | 45.8 | 9.8 | 9.4 | 143.8 | 14.6 | 57.8 | |
| Oct. | 204.13 | 163.9 | 21.6 | 7.1 | 3.8 | 77.3 | 7.9 | 33.4 | |
| 2024 | Jan. | 223.30 | 162.5 | 18.3 | 6.3 | 4.4 | 79.6 | 7.2 | 31.7 |
| Apr. | 247.98 | 198.1 | 22.5 | 8.1 | 6.3 | 102.3 | 8.6 | 41.6 | |
| Jul. | 256.43 | 284.4 | 41.4 | 11.7 | 8.4 | 144.6 | 15.7 | 56.1 | |
| Oct. | 228.96 | 202.3 | 33.2 | 9.6 | 5.7 | 74.3 | 11.2 | 28.3 |
| Parameter | As2+ [μg/L] | Cd2+ [μg/L] | Cototal (Co2+ + Co3+) [μg/L] | Crtotal (Cr3+ + Cr6+) [μg/L] | Cu2+ [μg/L] | Fetotal [mg/L] | Mntotal [mg/L] | Ni2+ [μg/L] | Zn2+ [μg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time [year/month] | ||||||||||
| 2022 | Jan. | 0.35 | 0.12 | 0.18 | 0.24 | 12.41 | 0.021 | 0.025 | 0.49 | 21.47 |
| Apr. | 0.41 | 0.24 | 0.36 | 0.31 | 18.63 | 0.034 | 0.037 | 0.68 | 34.65 | |
| Jul. | 0.74 | 0.55 | 0.84 | 0.84 | 35.10 | 0.058 | 0.044 | 1.13 | 54.64 | |
| Oct. | 0.44 | 0.41 | 0.52 | 0.42 | 22.42 | 0.025 | 0.032 | 0.74 | 28.30 | |
| 2023 | Jan. | 0.43 | 1.18 | 0.23 | 0.33 | 13.47 | 0.019 | 0.019 | 0.56 | 18.94 |
| Apr. | 0.48 | 0.27 | 0.44 | 0.47 | 21.46 | 0.027 | 0.028 | 0.77 | 24.68 | |
| Jul. | 0.57 | 0.66 | 1.12 | 1.13 | 39.42 | 0.044 | 0.033 | 1.34 | 46.37 | |
| Oct. | 0.39 | 0.32 | 0.67 | 0.65 | 31.22 | 0.026 | 0.024 | 0.71 | 28.71 | |
| 2024 | Jan. | 0.53 | 0.18 | 0.33 | 0.36 | 18.43 | 0.017 | 0.021 | 0.62 | 23.42 |
| Apr. | 0.46 | 0.27 | 0.42 | 0.58 | 24.69 | 0.028 | 0.033 | 0.86 | 33.44 | |
| Jul. | 0.78 | 0.78 | 0.84 | 1.34 | 38.44 | 0.063 | 0.046 | 1.45 | 61.42 | |
| Oct. | 0.47 | 0.41 | 0.50 | 0.61 | 24.81 | 0.030 | 0.022 | 0.60 | 42.13 |
| Scientific Name of a Diatom | Population Size | Degree of Saprobity | Saprobic Indicator Value | |
|---|---|---|---|---|
| Normal | Teratological | |||
| Achnanthidium minutissimum Kützing) Czarnecki | 256 | 61 | o-β | 1.5 |
| Navicula cryptocephala Kützing | 26 | 0 | α | 3 |
| Fragilaria vaucheriae (Kützing) J.B.Petersen | 21 | 0 | β | 2 |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 14 | 0 | β | 2 |
| Amphora pediculus (Kützing) Grunow | 11 | 0 | β | 2 |
| Gomphonella olivacea (Hornemann) Rabenhorst | 8 | 0 | β | 2 |
| Cocconeis pediculus Ehrenberg | 7 | 0 | β | 2 |
| Nitzschia dissipata (Kützing) Rabenhorst | 7 | 0 | o-β | 1.5 |
| Nitzschia inconspicua Grunow | 6 | 0 | α | 3 |
| Navicula lanceolata Ehrenberg | 5 | 0 | α | 3 |
| Cymbella ventricosa Agardh | 4 | 0 | o-β | 1.5 |
| Navicula tripunctata (O.F.Müller) Bory | 3 | 0 | β-α | 2.5 |
| Nitzschia sigmoidea (Nitzsch) W.Smith | 3 | 0 | β | 2 |
| Rhoicosphenia curvata (Kützing) Grunow | 3 | 0 | β | 2 |
| Surirella brebissonii Krammer & Lange-Bertalot | 3 | 0 | β-α | 2.5 |
| Odontidium mesodon (Ehrenberg) Kützing | 2 | 0 | o | 1 |
| Surirella angusta Kützing | 2 | 0 | β | 2 |
| Nitzschia palea (Kützing) W.Smith | 1 | 0 | α | 3 |
| Total | 443 | |||
| Saprobic index value | 1.86 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glevitzky, M.; Corcheş, M.T.; Popa, D.M. Impact of Pollution on Physico-Chemical Parameters and Diatom Communities Diversity in the Main Tributaries of the Arieș River, Romania. Environments 2025, 12, 389. https://doi.org/10.3390/environments12100389
Glevitzky M, Corcheş MT, Popa DM. Impact of Pollution on Physico-Chemical Parameters and Diatom Communities Diversity in the Main Tributaries of the Arieș River, Romania. Environments. 2025; 12(10):389. https://doi.org/10.3390/environments12100389
Chicago/Turabian StyleGlevitzky, Mirel, Mihai Teopent Corcheş, and Doriana Maria Popa. 2025. "Impact of Pollution on Physico-Chemical Parameters and Diatom Communities Diversity in the Main Tributaries of the Arieș River, Romania" Environments 12, no. 10: 389. https://doi.org/10.3390/environments12100389
APA StyleGlevitzky, M., Corcheş, M. T., & Popa, D. M. (2025). Impact of Pollution on Physico-Chemical Parameters and Diatom Communities Diversity in the Main Tributaries of the Arieș River, Romania. Environments, 12(10), 389. https://doi.org/10.3390/environments12100389






