Microplastic Ingestion from Contaminated Prey in the Bearded Fireworm Hermodice carunculata (Pallas, 1766): Evidence for Rapid Excretion and Low Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Acclimation of Bearded Fireworms
2.2. Test Microplastics
2.3. Experimental Set-Up
2.3.1. Exposure of Mussels to Micro-PS
2.3.2. Exposure of Bearded Fireworms to Contaminated Prey
2.4. Processing of Biological Samples
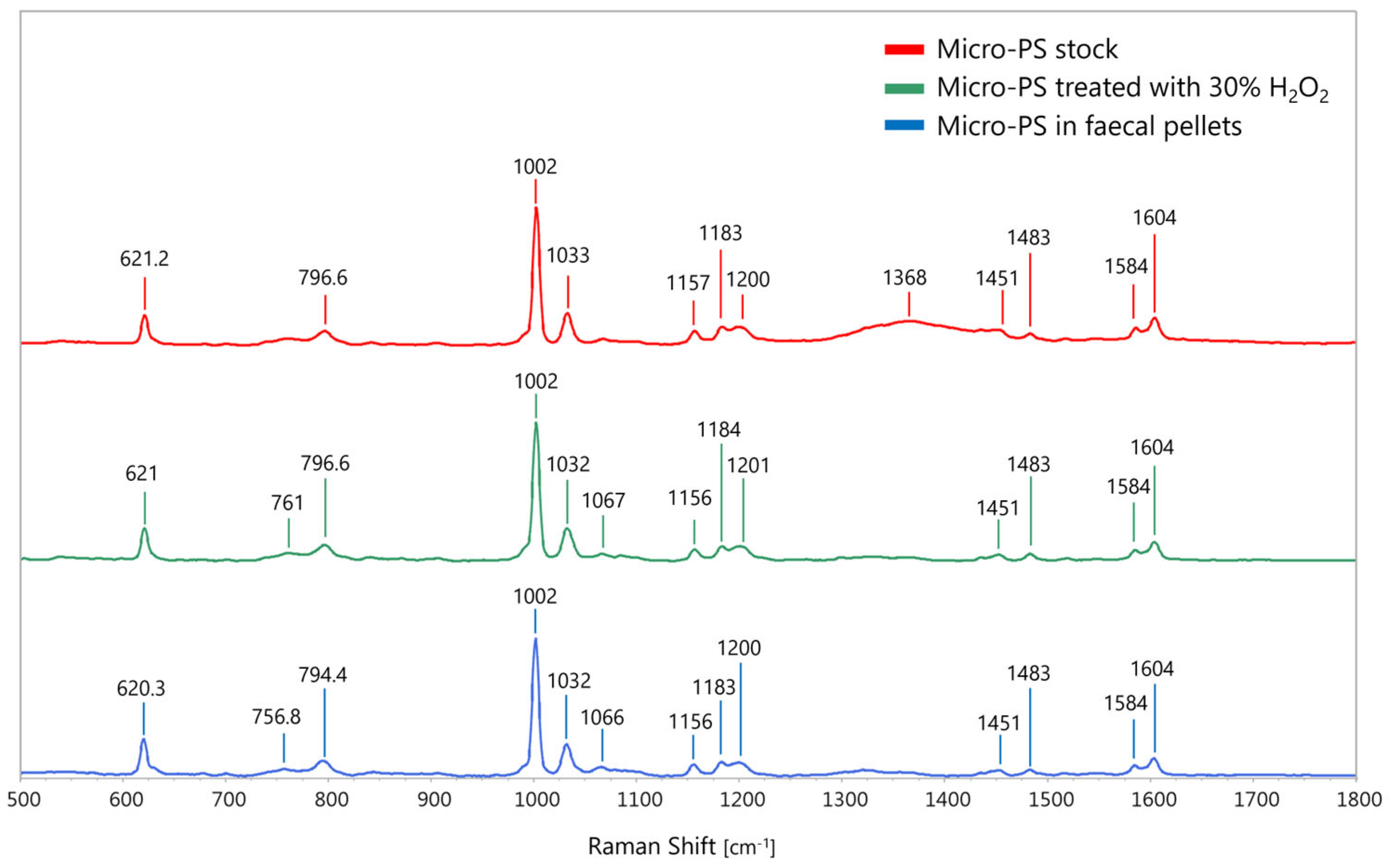
2.5. MP Characterisation
2.6. Data Analysis
3. Results and Discussion
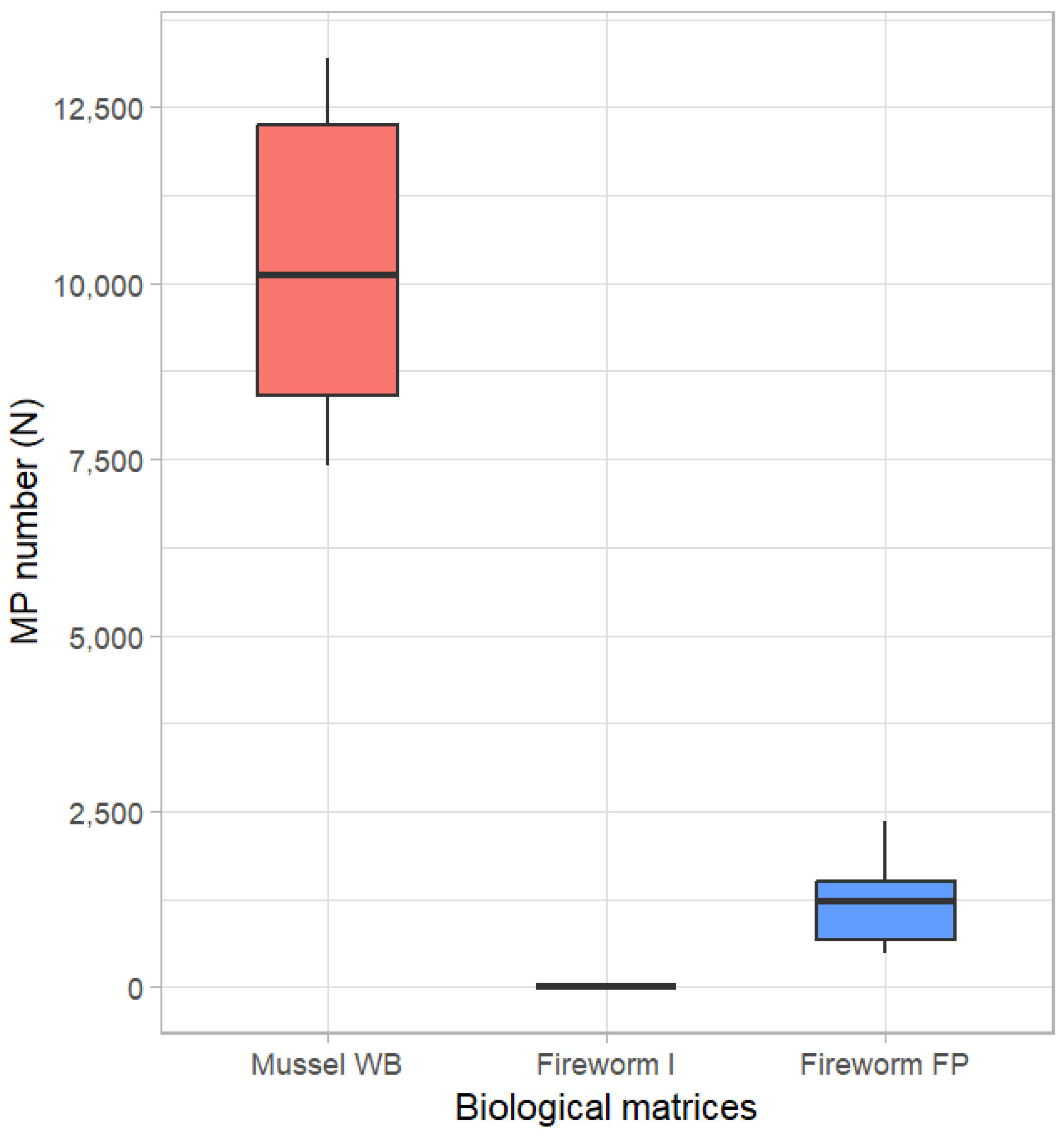
3.1. MP Uptake in Marine Mussels
3.2. MP Transfer to Bearded Fireworms and Rapid Excretion
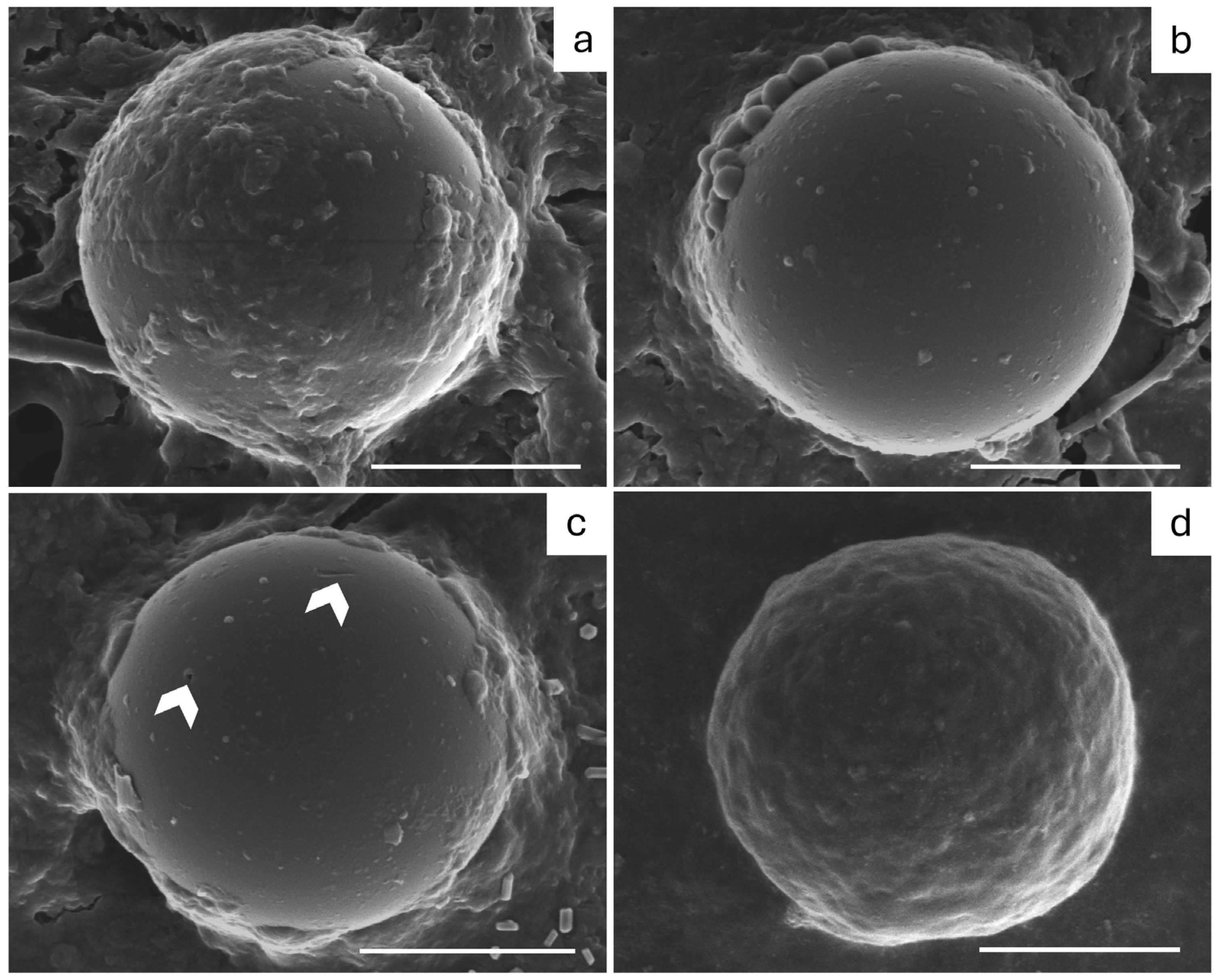
3.3. Limited MP Digestive Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Compa, M.; Alomar, C.; Wilcox, C.; van Sebille, E.; Lebreton, L.; Hardesty, B.D.; Deudero, S. Risk Assessment of Plastic Pollution on Marine Diversity in the Mediterranean Sea. Sci. Tot. Environ. 2019, 678, 188–196. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Thompson, R.C.; Canals, M.; De Haan, W.P. The Imprint of Microfibres in Southern European Deep Seas. PLoS ONE 2018, 13, e0207033. [Google Scholar] [CrossRef]
- Harris, P.T. The Fate of Microplastic in Marine Sedimentary Environments: A Review and Synthesis. Mar. Pollut. Bull. 2020, 158, 111398. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor Microplastic Hotspots Controlle by Deep-Sea Circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of Microplastics in Water and Aquatic Systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic Pollution in the Marine Environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Cássio, F.; Batista, D.; Pradhan, A. Plastic Interactions with Pollutants and Consequences to Aquatic Ecosystems: What We Know and What We Do Not Know. Biomolecules 2022, 12, 798. [Google Scholar] [CrossRef]
- Mason, V.G.; Skov, M.W.; Hiddink, J.G.; Walton, M. Microplastics Alter Multiple Biological Processes of Marine Benthic Fauna. Sci. Tot. Environ. 2022, 845, 157362. [Google Scholar] [CrossRef]
- Sandgaard, M.H.; Palmqvist, A.; Bour, A.; Grønlund, S.N.; Hooge, A.; Selck, H.; Thit, A.; Syberg, K. Sediment Matters as a Route of Microplastic Exposure: A Call for More Research on the Benthic Compartment. Front. Mar. Sci. 2023, 9, 1100567. [Google Scholar] [CrossRef]
- Bonello, G.; Varrella, P.; Pane, L. First Evaluation of Microplastic Content in Benthic Filter-Feeders of the Gulf of La Spezia (Ligurian Sea). J. Aquat. Food Prod. Technol. 2018, 27, 284–291. [Google Scholar] [CrossRef]
- Rubini, S.; Munari, M.; Baldini, E.; Barsi, F.; Meloni, D.; Pussini, N.; Barchiesi, F.; Di Francesco, G.; Losasso, C.; Cocumelli, C.; et al. Microplastics in Mussels (Mytilus galloprovincialis): Understanding Pollution in Italian Seas. Toxics 2025, 13, 144. [Google Scholar] [CrossRef]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from Marine Plastic Debris Induce Food Search Behaviours in a Forage Fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171000. [Google Scholar] [CrossRef]
- Horie, Y.; Mitsunaga, K.; Yamaji, K.; Hirokawa, S.; Uaciquete, D.; Ríos, J.M.; Yap, C.K.; Okamura, H. Variability in Microplastic Color Preference and Intake among Selected Marine and Freshwater Fish and Crustaceans. Discov. Oceans 2024, 1, 5. [Google Scholar] [CrossRef]
- Zantis, L.J.; Bosker, T.; Lawler, F.; Nelms, S.E.; O’Rorke, R.; Constantine, R.; Sewell, M.; Carroll, E.L. Assessing Microplastic Exposure of Large Marine Filter-Feeders. Sci. Tot. Environ. 2022, 818, 151815. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Koelmans, A.A. Accumulation of Plastic Debris and Associated Contaminants in Aquatic Food Webs. Environ. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and Biomagnification of Microplastics in Marine Organisms: A Review and Meta-Analysis of Current Data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in Four Bivalve Species and Basis for Using Bivalves as Bioindicators of Microplastic Pollution. Sci. Tot. Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using Mussel as a Global Bioindicator of Coastal Microplastic Pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Piarulli, S.; Airoldi, L. Mussels Facilitate the Sinking of Microplastics to Bottom Sediments and Their Subsequent Uptake by Detritus-Feeders. Environ. Pollut. 2020, 266, 115151. [Google Scholar] [CrossRef]
- Albignac, M.; Ghiglione, J.F.; Labrune, C.; Ter Halle, A. Determination of the Microplastic Content in Mediterranean Benthic Macrofauna by Pyrolysis-Gas Chromatography-Tandem Mass Spectrometry. Mar. Pollut. Bull. 2022, 181, 113882. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.E.; Fraissinet, S.; Tardio, N.; Rossi, S.; Malitesta, C. Microplastics Determination and Quantification in Two Benthic Filter Feeders Sabella spallanzanii, Polychaeta and Paraleucilla magna, Porifera. Heliyon 2024, 10, e31796. [Google Scholar] [CrossRef]
- Calmão, M.; Blasco, N.; Benito, A.; Thoppil, R.; Torre-Fernandez, I.; Castro, K.; Izagirre, U.; Garcia-Velasco, N.; Soto, M. Time-Course Distribution of Fluorescent Microplastics in Target Tissues of Mussels and Polychaetes. Chemosphere 2023, 311, 137087. [Google Scholar] [CrossRef]
- Pires, A.; Cuccaro, A.; Sole, M.; Freitas, R. Micro(Nano)Plastics and Plastic Additives Effects in Marine Annelids: A Literature Review. Environ. Res. 2022, 214, 113642. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castañeda, V.; Reish, D.J. Polychaetes in environmental studies. In Annelids in Modern Biology; Shain, D.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 203–227. [Google Scholar]
- Rouse, G.; Pleijel, F. Polychaetes; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Giangrande, A.; Licciano, M.; Musco, L. Polychaetes as Environmental Indicators Revisited. Mar. Pollut. Bull. 2005, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Righi, S.; Prevedelli, D.; Simonini, R. Ecology, Distribution and Expansion of a Mediterranean Native Invader, the Fireworm Hermodice carunculata (Annelida). Mediterr. Mar. Sci. 2020, 21, 575–591. [Google Scholar] [CrossRef]
- Krželj, M.; Cerrano, C.; Di Camillo, C.G. Enhancing Diversity Knowledge through Marine Citizen Science and Social Platforms: The Case of Hermodice carunculata (Annelida, Polycheta). Diversity 2020, 12, 311. [Google Scholar] [CrossRef]
- Simonini, R.; Maletti, I.; Righi, S.; Fai, S.; Prevedelli, D. Laboratory Observations on Predator–Prey Interactions between the Bearded Fireworm (Hermodice carunculata) and Mediterranean Benthic Invertebrates. Mar. Freshw. Behav. Physiol. 2018, 51, 145–158. [Google Scholar] [CrossRef]
- Tiralongo, F.; Marino, S.; Ignoto, S.; Martellucci, R.; Lombardo, B.M.; Mancini, E.; Scacco, U. Impact of Hermodice carunculata (Pallas, 1766) (Polychaeta: Amphinomidae) on Artisanal Fishery: A Case Study from the Mediterranean Sea. Mar. Environ. Res. 2023, 192, 106227. [Google Scholar] [CrossRef]
- Vecchi, S.; Bianchi, J.; Scalici, M.; Fabroni, F.; Tomassetti, P. Field Evidence for Microplastic Interactions in Marine Benthic Invertebrates. Sci. Rep. 2021, 11, 292. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An Assessment of the Ability to Ingest and Excrete Microplastics by Filter-Feeders: A Case Study with the Mediterranean Mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Corsi, I.; Bellingeri, A.; Bergami, E. Progress in Selecting Marine Bioindicators for Nanoplastics Ecological Risk Assessment. Ecol. Indic. 2023, 154, 110836. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Smith, K.E.; Lewis, C. The Sea Urchin Paracentrotus lividus as a Bioeroder of Plastic. Sci. Tot. Environ. 2019, 693, 133621. [Google Scholar] [CrossRef] [PubMed]
- Cau, A.; Avio, C.G.; Dessì, C.; Moccia, D.; Pusceddu, A.; Regoli, F.; Cannas, R.; Follesa, M.C. Benthic Crustacean Digestion Can Modulate the Environmental Fate of Microplastics in the Deep Sea. Environ. Sci. Technol. 2020, 54, 4886–4892. [Google Scholar] [CrossRef]
- Babkiewicz, E.; Nowakowska, J.; Zebrowski, M.L.; Kunijappan, S.; Jarosińska, K.; Maciaszek, R.; Zebrowski, J.; Jurek, K.; Maszczyk, P. Microplastic Passage through the Fish and Crayfish Digestive Tract Alters Particle Surface Properties. Environ. Sci. Technol. 2025, 59, 5693–5703. [Google Scholar] [CrossRef]
- Simonini, R.; Righi, S.; Zanetti, F.; Fai, S.; Prevedelli, D. Development and Catch Efficiency of an Attracting Device to Collect and Monitor the Invasive Fireworm Hermodice carunculata in the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 706–714. [Google Scholar] [CrossRef]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How Sea Urchins Face Microplastics: Uptake, Tissue Distribution and Immune System Response. Environ. Pollut. 2020, 264, 114685. [Google Scholar] [CrossRef]
- Murano, C.; Donnarumma, V.; Corsi, I.; Casotti, R.; Palumbo, A. Impact of Microbial Colonization of Polystyrene Microbeads on the Toxicological Responses in the Sea Urchin Paracentrotus lividus. Environ. Sci. Technol. 2021, 55, 7990–8000. [Google Scholar] [CrossRef] [PubMed]
- Righi, S.; Forti, L.; Simonini, R.; Ferrari, V.; Prevedelli, D.; Mucci, A. Novel Natural Compounds and Their Anatomical Distribution in the Stinging Fireworm Hermodice carunculata (Annelida). Mar. Drugs 2022, 20, 585. [Google Scholar] [CrossRef]
- Marsden, J.R. The digestive tract of Hermodice carunculata (Pallas). Polychaeta: Amphinomidae. Can. J. Zool. 1963, 41, 165–184. [Google Scholar] [CrossRef]
- Menges, F. SpectraGryph-Optical Spectroscopy Software, Version 1.2.16.1. 2023. Available online: https://www.effemm2.de/spectragryph/ (accessed on 12 November 2023).
- Munno, K.; De Frond, H.; O’Donnell, B.; Rochman, C.M. Increasing the Accessibility for Characterizing Microplastics: Introducing New Application-Based and Spectral Libraries of Plastic Particles (SLoPP and SLoPP-E). Anal. Chem. 2020, 92, 2443–2451. [Google Scholar] [CrossRef]
- Miller, E.A.; Yamahara, K.M.; French, C.; Spingarn, N.; Birch, J.M.; Van Houtan, K.S. A Raman Spectral Reference Library of Potential Anthropogenic and Biological Ocean Polymers. Sci. Data 2022, 9, 183. [Google Scholar] [CrossRef]
- Cowger, W.; Steinmetz, Z.; Gray, A.; Munno, K.; Lynch, J.; Hapich, H.; Primpke, S.; De Frond, H.; Rochman, C.; Herodotou, O. Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Anal. Chem. 2021, 93, 7543–7548. [Google Scholar] [CrossRef] [PubMed]
- Riisgård, H.U.; Larsen, P.S.; Pleissner, D. Allometric Equations for Maximum Filtration Rate in Blue Mussels Mytilus edulis and Importance of Condition Index. Helgol. Mar. Res. 2014, 68, 193–198. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding Type Affects Microplastic Ingestion in a Coastal Invertebrate Community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef]
- Revel, M.; Yakovenko, N.; Caley, T.; Guillet, C.; Châtel, A.; Mouneyrac, C. Accumulation and Immunotoxicity of Microplastics in the Estuarine Worm Hediste diversicolor in Environmentally Relevant Conditions of Exposure. Environ. Sci. Pollut. Res. 2020, 27, 3574–3583. [Google Scholar] [CrossRef]
- Bour, A.; Sturve, J.; Höjesjö, J.; Carney Almroth, B. Microplastic Vector Effects: Are Fish at Risk When Exposed via the Trophic Chain? Front. Environ. Sci. 2020, 8, 90. [Google Scholar] [CrossRef]
- Piarulli, S.; Vanhove, B.; Comandini, P.; Scapinello, S.; Moens, T.; Vrielinck, H.; Sciutto, G.; Prati, S.; Mazzeo, R.; Booth, A.M.; et al. Do different habits affect microplastics contents in organisms? A trait-based analysis on salt marsh species. Mar. Pollut. Bull. 2020, 153, 110983. [Google Scholar] [CrossRef]
- Keerthika, K.; Padmavathy, P.; Rani, V.; Jeyashakila, R.; Aanand, S.; Kutty, R. Evidence of microplastics in the polychaete worm (capitellids—Capitella capitata) (Fabricicus, 1780) along Thoothukudi region. Environ. Monit. Assess. 2024, 196, 556. [Google Scholar] [CrossRef]
- James, K.; Kripa, V.; Vineetha, G.; Padua, S.; Parvathy, R.; Lavanya, R.; Joseph, R.V.; Abhilash, K.S.; Babu, A.; John, S. Microplastic ingestion by the polychaete community in the coastal waters of Kochi, Southwest coast of India. Reg. Stud. Mar. Sci. 2023, 62, 102948. [Google Scholar] [CrossRef]
- Blasco, N.; Ibeas, M.; Aramendia, J.; Castro, K.; Soto, M.; Izagirre, U.; Garcia-Velasco, N. Depuration kinetics and accumulation of microplastics in tissues of mussel Mytilus galloprovincialis. Mar. Environ. Res. 2024, 202, 106731. [Google Scholar] [CrossRef] [PubMed]
- Hatzonikolakis, Y.; Raitsos, D.E.; Sailley, S.F.; Digka, N.; Theodorou, I.; Tsiaras, K.; Tsangaris, C.; Skia, G.; Ntzouvaras, A.; Triantafyllou, G. Assessing the physiological effects of microplastics on cultured mussels in the Mediterranean Sea. Environ. Pollut. 2024, 363, 125052. [Google Scholar] [CrossRef]
- Musto, P.; Borriello, A.; Agoretti, P.; Napolitano, T.; Di Florio, G.; Mensitieri, G. Selective Surface Modification of Syndiotactic Polystyrene Films: A Study by Fourier Transform- and Confocal-Raman Spectroscopy. Eur. Polym. J. 2010, 46, 1004–1015. [Google Scholar] [CrossRef]
- Gao, H.; Liu, C.; Wang, H.; Shen, H. Raman Spectra Characterization of Size-Dependent Aggregation and Dispersion of Polystyrene Particles in Aquatic Environments. Chemosphere 2023, 333, 138939. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Athalye, S.M.; Boodaghidizaji, M.; Sarathy, N.; Hosseini, M.; Ardekani, A.; Verma, M.S. Limit of Detection of Raman Spectroscopy Using Polystyrene Particles from 25 to 1000 Nm in Aqueous Suspensions. Anal. Chem. 2025, 97, 8908–8914. [Google Scholar] [CrossRef]
- Tian, M.; Morais, C.L.M.; Shen, H.; Pang, W.; Xu, L.; Huang, Q.; Martin, F.L. Direct Identification and Visualisation of Real-World Contaminating Microplastics Using Raman Spectral Mapping with Multivariate Curve Resolution-Alternating Least Squares. J. Hazard. Mater. 2022, 422, 126892. [Google Scholar] [CrossRef] [PubMed]
- Benito-Kaesbach, A.; Amigo, J.M.; Izagirre, U.; Garcia-Velasco, N.; Arévalo, L.; Seifert, A.; Castro, K. Misinterpretation in Microplastic Detection in Biological Tissues: When 2D Imaging Is Not Enough. Sci. Tot. Environ. 2023, 876, 162810. [Google Scholar] [CrossRef]
- Malafaia, G.; da Luz, T.M.; Ahmed, M.A.I.; Karthi, S.; da Costa Araújo, A.P. When Toxicity of Plastic Particles Comes from Their Fluorescent Dye: A Preliminary Study Involving Neotropical Physalaemus cuvieri Tadpoles and Polyethylene Microplastics. J. Hazard. Mater. Adv. 2022, 6, 100054. [Google Scholar] [CrossRef]
- Bergami, E.; Manno, C.; Cappello, S.; Vannuccini, M.L.; Corsi, I. Nanoplastics affect moulting and faecal pellet sinking in Antarctic krill (Euphausia superba) juveniles. Environ. Int. 2020, 143, 105999. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, V.; Simonini, R.; Murano, C.; Prevedelli, D.; Bergami, E. Microplastic Ingestion from Contaminated Prey in the Bearded Fireworm Hermodice carunculata (Pallas, 1766): Evidence for Rapid Excretion and Low Degradation. Environments 2025, 12, 365. https://doi.org/10.3390/environments12100365
Ferrari V, Simonini R, Murano C, Prevedelli D, Bergami E. Microplastic Ingestion from Contaminated Prey in the Bearded Fireworm Hermodice carunculata (Pallas, 1766): Evidence for Rapid Excretion and Low Degradation. Environments. 2025; 12(10):365. https://doi.org/10.3390/environments12100365
Chicago/Turabian StyleFerrari, Valentina, Roberto Simonini, Carola Murano, Daniela Prevedelli, and Elisa Bergami. 2025. "Microplastic Ingestion from Contaminated Prey in the Bearded Fireworm Hermodice carunculata (Pallas, 1766): Evidence for Rapid Excretion and Low Degradation" Environments 12, no. 10: 365. https://doi.org/10.3390/environments12100365
APA StyleFerrari, V., Simonini, R., Murano, C., Prevedelli, D., & Bergami, E. (2025). Microplastic Ingestion from Contaminated Prey in the Bearded Fireworm Hermodice carunculata (Pallas, 1766): Evidence for Rapid Excretion and Low Degradation. Environments, 12(10), 365. https://doi.org/10.3390/environments12100365








