Abstract
Urban expansion alters environmental conditions, influencing tree physiology and performance. Urban trees provide cooling, sequester carbon, support biodiversity, filter contaminants, and enhance human health. This study examines how two common urban trees—Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.)—respond to urban site conditions by assessing leaf morphology, stomatal, and gas exchange traits across street and urban park sites in Chicago, IL. Street trees exhibited structural trait adjustments, including smaller leaf area, reduced specific leaf area, and increased stomatal density, potentially reflecting acclimation to more compact and impervious conditions. Norway Maple showed stable photosynthetic assimilation (A), stomatal conductance (gs), and transpiration (E) across sites, alongside higher intrinsic water-use efficiency (iWUE), indicating a conservative water-use strategy. In contrast, Little-leaved Linden maintained A and gs but showed elevated E and iWUE at street sites, suggesting adaptive shifts in water-use dynamics under street microenvironments. These findings highlight how species-specific physiological strategies and local site conditions interact to shape tree function in cities and underscore the importance of incorporating functional traits into urban forestry planning to improve ecosystem services and climate resilience.
1. Introduction
The ability of urban vegetation to deliver ecosystem services is increasingly constrained by the complex and heterogeneous nature of cities, where plants are exposed to a variety of abiotic stressors. Street-side environments may impose harsh growing conditions for urban trees. These areas are often characterized by extensive impervious and reflective surfaces, compacted soils, restricted rooting space, and increased risk of mechanical damage [1,2]. In contrast, urban parks generally provide more favorable growing conditions, featuring buffered microenvironments, cooler temperatures, higher humidity, and reduced thermal stress [3]. These green spaces also typically feature more permeable soils and lower concentrations of air pollutants [4]. Such site-specific environmental differences create distinct microenvironments across the urban landscape, which can significantly influence tree function, longevity, and stress responses [5]. Moreover, stressed urban trees may emit higher levels of volatile organic compounds, potentially acting as an unintended ecosystem disservice by contributing to secondary ozone formation and deteriorating local air quality—particularly in areas with high temperatures and high anthropogenic emissions of NOx [6,7].
Understanding how trees respond to these distinct urban microenvironments is crucial for informed species selection and management practices, ultimately optimizing ecosystem services to mitigate the effects of climate change and air quality degradation [3,8,9]. In this study, we examine the anatomical and morpho-physiological leaf traits of Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.). Specifically, we analyze morphological traits (leaf area, LA; specific leaf area, SLA), stomatal traits (stomatal density, SD; stomatal area, SA; stomatal length, SL), and gas exchange traits (photosynthetic rate, A; stomatal conductance, gs; transpiration rate, E; intrinsic water-use efficiency, iWUE). These measurements provide insights into how trees manage the challenges of urban environments such as streets and urban parks. Gas exchange traits—A, E, gs, and iWUE—measure a tree’s physiological performance under varying environmental conditions that impact urban ecosystem services [10,11]. Stomatal traits, considered together with morphological traits, indicate how urban trees use water; for example, an increase in SD observed in street trees suggests a structural adjustment to support transpiration and sustain physiological function under drought stress [12].
Norway Maple (Acer platanoides L.) is widely planted in urban areas for its attractive appearance, colorful autumn foliage, and adaptability to city conditions [13]. Little-leaved Linden (Tilia cordata Mill.) also shows strong resilience to climate change and contributes positively to ecosystem services [14]. Both species are valued as street trees because of their resilience to diseases and tolerance of stressors such as heat, compacted soils, and limited rooting space [15,16]. Previous research found that these two species exhibited higher SLA, lower leaf dry matter content, and elevated gas exchange trait values in urban park sites compared to suburban areas, indicating greater exposure to heat stress, as supported by land surface temperature measurements, and possibly to other urban stressors such as airborne pollutants commonly associated with developed environments [8]. However, finer-scale contrasts between specific urban microenvironments—such as street and urban park sites—are particularly important in large metropolitan areas, where substantial variations in environmental conditions can occur within the same city [17]. These microenvironment distinctions are crucial for understanding tree resilience and functionality in different urban settings [18]. Chicago’s urban conditions are particularly distinctive due to its aging infrastructure and the environmental inequities that persist in historically underserved communities. Many of these areas face poor air quality, high pollution, extreme urban heat, degraded soil, and limited green space. These conditions are often more severe in historically disinvested areas, emphasizing the need for nature-based solutions that improve environmental health and build resilience where it is needed most [6,7].
This study aims to examine how urban site conditions influence tree responses by analyzing leaf functional traits across street-side and urban park environments. We hypothesize that street-side conditions, characterized by greater exposure to impervious surfaces, are associated with altered leaf functional traits and tree physiological performance. We analyze morphological, stomatal, and gas exchange traits to assess tree responses of two common urban species—Norway Maple and Little-leaved Linden. We further expect species-specific variation in response to site conditions, reflecting differences in stress tolerance and phenotypic plasticity. To evaluate this, we calculated the phenotypic plasticity index (PPi) for key leaf traits. Additionally, we compared these values with global reference data from the TRY and BIEN plant trait databases to place them in a broader ecological context [19,20]. This approach provides a comprehensive assessment of how urban trees adjust to resource limitations and environmental stressors and inform site-based strategies for urban greening.
2. Materials and Methods
2.1. Characteristics of Sampled Species and Study Locations
Two tree species were selected species for this study, Norway Maple (Acer platanoides L., Sapindaceae) and Little-leaved Linden (Tilia cordata Mill., Malvaceae). These are common urban trees because they are resilient to disease or urban stressors such as heat, compacted soils, and limited rooting space [13,14]. In the Chicago Metropolitan Region, both Norway Maple and Little-leaved Linden are non-native species, representing 11% and 1% of total trees, respectively [15,16]. Individual tree selection was driven by uniformity of individuals by diameter at breast height within a site (Table 1). For each tree, soil moisture was measured on each sampling date at three locations within a 1 m radius as volumetric water content (%) in the top 12 cm of soil using a handheld soil water reflectometer (Hydrosense II, CS659 12 cm rods, Campbell Scientific, Logan, UT, USA), as reported in Table 1. During the growing season (April–October), July recorded the highest precipitation in both 2023 and 2024, with totals of 19.3 cm across 14 rain days in 2023 and 15.0 cm across 11 rain days in 2024. Across the 30-year climate record (1995–2024), July was consistently the hottest month, while May was the wettest, with an average precipitation of 15.5 cm, followed by July at 10.1 cm (Supplementary Figure S1). The study sites did not receive any additional irrigation. Sampling was conducted during the summers of 2023 and 2024 at two distinct sites located at the University of Illinois Chicago (UIC) campus in Chicago, IL. The two sites can be described by the following characteristics:

Table 1.
Species, site, and sampling information for Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) at street-side and urban park sites in 2023 and 2024. The table reports number of individuals (sample sizes) and sampling dates in 2023 and 2024. Diameter at breast height (DBH ± s.d.), distance from impervious surface (± s.d.), and soil moisture (± s.d.) were included.
- Street site (average distance to impervious surface = 0.7 m): Trees located along paved roads and streets. These trees grow in open canopies, without irrigation, surrounded by gray concrete sidewalks and impervious asphalt surfaces, with restricted soil volume for root growth and an increased risk of drought.
- Urban Park site (average distance to impervious surface = 8.0 m): These trees are situated on non-irrigated green yards, surrounded by other trees and shrubs, and not shaded by buildings. They have access to greater soil volume, are located in managed lawns, and represent a more natural habitat compared to street sites.
To account for within-individual variability and ensure robust comparisons, each tree was treated as a biological replicate. From each tree, at least three mature, sun-exposed leaves were collected, resulting in more than 120 leaves analyzed.
2.2. Leaf Morphological Trait Measurements
Tree shoots were collected in July 2023 and July 2024 by cutting branches longer than 50 cm from the southwest-facing outer canopy at mid-height on sunny days at each experimental site using a 4.2 m expandable tree pruner, ensuring consistent exposure to high irradiance [8,21]. Branches were harvested during the day between 10:00 and 14:00 to ensure consistency in environmental conditions. The shoot’s end was cut twice underwater to prevent cavitation and then cut again just before measurement at room temperature in UIC’s Stable Isotope Laboratory. We avoided shoot tips to exclude leaves still unfolding and sampled only fully expanded leaves from main branch shoots, not from elongating nodes. Leaf area (LA) was measured using a leaf area meter (Li-3000, Li-Cor, Lincoln, NE, USA). Individual leaves were placed in aluminum foil, dried at 65 °C until a constant dry mass was achieved, and then weighed. Specific leaf area (SLA), calculated as the projected leaf area per unit dry mass, was determined and reported in standard international units.
The phenotypic plasticity index (PPi) was calculated to assess variation in LA and SLA across sites and years for Norway Maple and Little-leaved Linden. The PPi was calculated by subtracting the minimum trait value from the maximum trait value and dividing the result by the maximum trait value. Global reference values for LA and SLA were obtained from the TRY and BIEN plant trait databases [17,18]. For leaf area, we compiled 113 records for the two study species: Norway Maple (88 records, 22–269 cm2) and Little-leaved Linden (25 records, 9–57 cm2). For SLA, we compiled 117 records: Norway Maple (84 records, 23–345 cm2 g−1) and Little-leaved Linden (33 records, 122–613 cm2 g−1). A complete list of the original publications contributing to the PPi subsets derived from each database is provided in Supplementary Data Sheet S1. This provides a unitless value ranging from 0 (no plasticity) to 1 (maximum plasticity).
2.3. Gas Exchange Measurements
Gas exchange traits, including photosynthetic assimilation rate (A), transpiration rate (E), and stomatal conductance (gs), were measured under controlled conditions using a Li−6400 instrument equipped with 6 cm2 cuvettes and a 6400−02B red–blue light source (Li−Cor, Lincoln, NE, USA) with an 80:20 red-to-blue ratio. Measurements were taken on the sampling dates in July 2023 between 10:00 and 14:00.
To assess gas exchange, three leaves per tree were randomly selected and prepared using a method similar to that employed for leaf morphological trait analysis. Measurements were conducted under a photosynthetic photon flux density (PPFD) of 1200 µmol m−2 s−1 using the 6400-02B LED light source, which provides a broader spectral output. Environmental conditions in the leaf chamber were maintained at a vapor pressure deficit of 1.9 ± 0.5 kPa, relative humidity between 45 and 60%, temperature of 28 °C, reference CO2 concentration of 400 µmol mol−1, and a flow rate of 500 μmol s−1. Gas exchange measurements were recorded once CO2 concentrations in the sample cell stabilized, typically within 10 min of exposure to 1200 µmol m−2 s−1 PPFD. Intrinsic water-use efficiency (iWUE) was calculated as the ratio of A to gs.
2.4. Stomatal Density and Aperture Measurements
To obtain stomatal impressions, a thin layer of clear nail polish was applied to the upper surface of each leaf and allowed to dry for 20–30 min [22]. Once dried, the polish was carefully peeled off using clear tape and mounted onto a microscope slide, creating an impression of the leaf’s epidermal cells for subsequent analysis.
Stomatal density (SD) was determined from the leaf impressions. Three fields of view on each glass slide were analyzed. High-resolution images were captured using a Samsung Galaxy Note S21 smartphone mounted on an Olympus CX31 microscope (Tokyo, Japan) with an adapter at 400× magnification. Camera settings were adjusted for optimal focus and exposure, and images were saved in JPEG format with a resolution of 3000 × 4000 pixels. To ensure consistency, each image was cropped to a square size of 3000 × 3000 pixels before annotation and stomatal counting. SD was calculated as the total number of stomata per mm2.
Stomatal area (SA) and stomatal length (SL) were measured using ImageJ (version 1.54n, Wayne Rasband/NIH, Bethesda, MD, USA). Each image was calibrated using a microscale ruler (μm units) captured under the same magnification to ensure accurate scaling prior to measurement. SA was manually traced around the perimeter of both the guard cells and subsidiary cells using the polygon tool in ImageJ, while SL was measured as the distance from the tip to the base of the guard cells. A scale bar was included in each image to ensure accurate scaling during analysis.
2.5. Statistical Analysis
All statistical analyses and visualizations were conducted in R (version 4.4.3). Descriptive statistics, including means and standard deviations, were calculated for all measured variables. Morphological and gas exchange traits were analyzed at the tree level, whereas stomatal traits were analyzed at the leaf level. To meet assumptions of normality, log transformations were applied to variables including LA, SLA, SL, and gs. iWUE was transformed using the Yeo-Johnson method. Normality was assessed using the Shapiro–Wilk test (stats package), and homogeneity of variances was evaluated using Levene’s test (car package). Differences between tree species, site type (street vs. urban park), and year for LA and SLA were assessed using three-way ANOVAs (Supplementary Table S1). For gas exchange traits (A, gs, E, iWUE), two-way ANOVAs were conducted to assess differences between species and site type. For stomatal traits (SD, SA, and SL), two-way ANOVAs were conducted with species and site as fixed factors (including their interaction) and soil moisture (log-transformed) included as a covariate. Post hoc Tukey HSD tests (stats package) were conducted for morphological (LA and SLA) and gas exchange traits (A, E, gs, and iWUE), and letters indicating significant differences among groups were added to the plots. While some statistical tests were conducted on transformed data, the corresponding mean values reported in the Section 3 reflect the original (untransformed) scale to aid interpretation. Statistical significance was determined at p < 0.05 for all tests. Data visualizations were created using the ggplot2 package to illustrate traits differences and differences across species, site type, and year.
3. Results
3.1. Leaf Morphological Trait Responses to Urban Site Conditions
Log-transformed leaf area (LA) was lower at street sites compared to urban parks, with a more pronounced reduction in Norway Maple in 2023 compared to Little-leaved Linden (Figure 1). For reference, the average LA (original values) in 2023 was 65 cm2 at street sites and 77 cm2 at urban parks for Norway Maple and 34 cm2 (street) vs. 43 cm2 (park) for Little-leaved Linden. In 2024, Norway Maple showed higher LA values (83 cm2 at street, 89 cm2 at park), while Little-leaved Linden remained relatively unchanged (43 cm2 at street, 42 cm2 at park). Log-transformed specific leaf area (SLA) was also reduced at street sites compared to urban parks across both species and years. Significant effects of year, site, and species on LA were detected, along with a site × species interaction (p = 0.029, p < 0.001, and p = 0.020, respectively; Supplementary Table S1). SLA was significantly affected by site (p < 0.001), showing consistently lower values at street sites. For reference, average SLA (original values) in 2023 was 156 cm2 g−1 (street) vs. 312 cm2 g−1 (park) for Norway Maple, and 127 cm2 g−1 (street) vs. 187 cm2 g−1 (park) for Little-leaved Linden. In 2024, SLA values were 142 cm2 g−1 (street) and 308 cm2 g−1 (park) for Norway Maple, and 121 cm2/g (street) and 192 cm2 g−1 (park) for Little-leaved Linden.
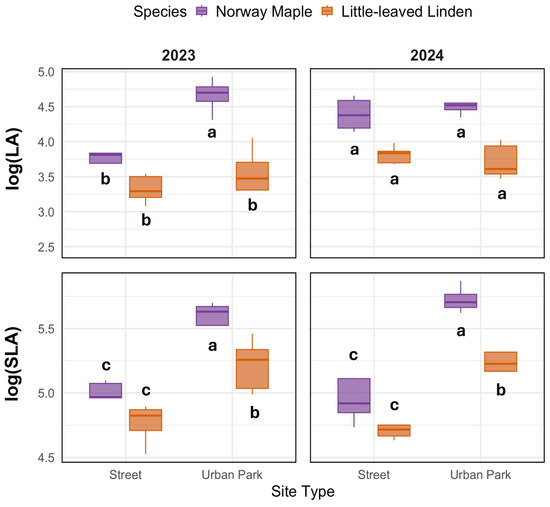
Figure 1.
Log-transformed leaf area (LA, originally measured in cm2) and log-transformed specific leaf area (SLA, originally measured in cm2 g−1) of Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) at street and urban park sites in 2023 and 2024. Different letters indicate significant differences within each species and site based on Tukey’s HSD test (p < 0.05); identical letters denote no significant difference. The highest value within each group is labeled ‘a’.
To evaluate trait responsiveness to urban microenvironment variation, we calculated the phenotypic plasticity index (PPi) for LA and SLA (Table 2). Norway Maple exhibited higher plasticity than Little-leaved Linden under street conditions. At the street site, PPi values for LA and SLA in Norway Maple were 0.82 and 0.54, respectively, while values at the urban park site were lower (0.56 and 0.40). In contrast, Little-leaved Linden showed more consistent SLA plasticity across site types (0.50–0.53) compared to Norway Maple (0.40–0.54). When compared with global trait datasets, both LA and SLA plasticity were lower in this study; global PPi values ranged from 0.92 to 0.93 in Norway maple and 0.80–0.84 in Little-leaved Linden.

Table 2.
Phenotypic Plasticity Index (PPi) values for leaf area (LA) and specific leaf area (SLA) of Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) sampled in 2023 and 2024 at the street and urban park sites, compared with the global dataset.
3.2. Leaf Gas Exchange Traits Exhibit Site Differences
The photosynthetic assimilation rate (A) and log-transformed stomatal conductance (gs) were relatively consistent across street and urban park sites, with no significant site effects (p = 0.632 and p = 0.861, respectively; Figure 2). The original gs values were 0.04 mol m−2 s−1 at both street and park sites in Norway Maple and 0.15 and 0.14 mol m−2 s−1 in Little-leaved Linden. In contrast, the transpiration rate (E) and intrinsic water-use efficiency (iWUE) showed notable variation across sites, with significantly higher values at street locations (p = 0.031 and p < 0.001, respectively), along with significant site × species interactions (p = 0.031 and p < 0.001). For reference, the original iWUE values were 5.1 and 5.3 µmol mol−1 in Norway Maple and 3.9 and 5.4 µmol mol−1 in Little-leaved Linden at street and park sites, respectively. Trait responses to site conditions differed between species. Norway Maple exhibited consistent values for all gas exchange traits across sites, while Little-leaved Linden maintained stable A and gs but showed greater variation in E and iWUE.
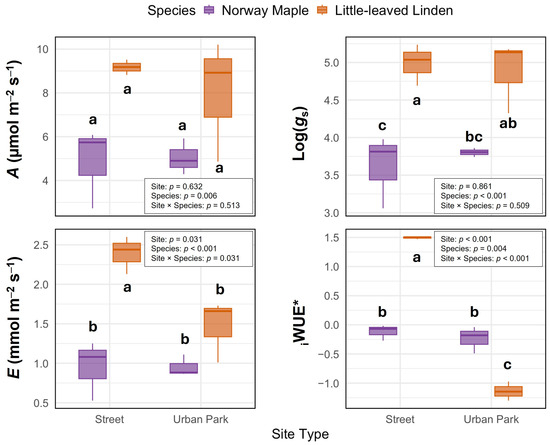
Figure 2.
Leaf gas exchange responses of Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) across street-side and urban park sites in 2023. Traits include photosynthetic assimilation rate (A, µmol m−2 s−1), log-transformed stomatal conductance (gs, originally measured in mol m−2 s−1), and transpiration rate (E, mmol m−2 s−1). Intrinsic water-use efficiency (iWUE) was calculated as A/gs and expressed in µmol mol−1, then transformed using the Yeo-Johnson method to meet normality assumptions; transformed values are indicated with an asterisk (*). Boxplots display the median, interquartile range, and species-specific variation by site type. Statistical results from two-way ANOVAs are shown within each panel, testing for main effects of site, species, and their interaction. Different letters indicate significant differences within each species and site based on Tukey’s HSD test (p < 0.05); identical letters denote no significant difference. The highest value within each group is labeled ‘a’.
3.3. Stomatal Traits Vary by Urban Site Conditions
Stomatal density (SD) was significantly higher at street sites than at urban parks (Table 3). This pattern was consistent across both tree species. While species and site × species interaction effects were significant (p < 0.001 and p = 0.013, respectively), the dominant trend was an overall increase in SD at street sites. Stomatal area (SA) showed a significant site effect (p < 0.001) but no significant species effect. Stomatal length (SL, log-transformed) was also significantly affected by site (p < 0.001), with no significant species or interaction effects. For reference, the average SL (original values) at street and urban park sites was 21.5 and 21.2 µm for Norway Maple and 26.3 and 27.6 µm for Little-leaved Linden.

Table 3.
Stomatal traits of Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) at street-side and urban park sites. ANOVA p-values indicate the effects of site, species, and their interaction. SD: stomatal density (Total number of stomata mm−2); SA: stomatal area (µm2); SL: stomatal length (µm), log transformed. Values are presented as mean ± standard error.
4. Discussion
Urban trees face a complex suite of environmental stressors, including restricted rooting space near impervious surfaces and elevated surface temperatures from adjacent roads. These stressors vary considerably across the urban landscape, particularly between street-side and park environments [1,2]. In this study, we observed that both Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) exhibited distinct structural and physiological adjustments when growing in street environments. These adjustments—including reduced specific leaf area (SLA) and increased stomatal density (SD)—reflect plastic strategies to cope with elevated heat, compacted soils, and limited water availability. Such trait shifts help maintain photosynthetic function and water regulation under contrasting environmental conditions, supporting tree survival in densely built environments. These findings highlight the importance of linking plant functional traits with site-specific microenvironments to inform species selection and urban forestry planning.
Street trees exhibited structural adjustments (reduced LA and SLA) in both Norway Maple and when compared to urban park trees. Lower SLA, reflecting thicker and/or denser leaves, promotes longer leaf lifespan, greater nutrient retention, and enhanced protection against desiccation [23]. Little-leaved Linden trees in high-traffic and industrial areas showed a clear decrease in SLA, indicating an adaptive plastic response in which leaves become smaller and denser under stress [24]. This is consistent with the phenotypic plasticity index (PPi), which was higher in street trees than in urban park trees for both species (Table 2), reflecting greater trait variability under environmental stressors. Compared to Norway Maple, Little-leaved Linden produced significantly smaller leaves (species effect p < 0.001; Supplementary Table S1). Smaller leaves reduce the boundary layer, facilitating convective cooling and heat dissipation, consistent with acclimation to warmer conditions [25] and may contribute to higher intrinsic water-use efficiency (iWUE) in street environments. At the same time, reduced SLA can decrease mesophyll diffusion conductance because denser leaf tissue—with tightly packed mesophyll cells and thicker cell walls—increases resistance to CO2 diffusion from intercellular air spaces to chloroplasts [26]. This increased resistance may lead to larger internal CO2 drawdown, potential constraining photosynthesis.
Norway Maple and Little-leaved Linden maintained relatively similar photosynthetic assimilation rates (A) and stomatal conductance (gs) across street and park environments (Figure 2), indicating sustained photosynthetic function despite differences in site conditions. This stability suggests that both species can maintain leaf carbon gain under varying urban site conditions, reflecting physiological resilience [27]. Notably, Little-leaved Linden exhibited elevated transpiration rates (E) and iWUE at the street site, suggesting physiological adjustments in water-use strategy that may enhance evaporative cooling under increased pavement temperatures and soil compaction [28]. In contrast, Norway Maple showed consistent E and higher iWUE across both street and urban park sites, reflecting a conservative water-use strategy that prioritizes carbon gain while minimizing water loss under varying urban conditions [29]. The elevated SD observed in both species at street sites may serve as a compensatory adaptation, increasing total evaporative surface area to mitigate the effects of limited soil moisture and elevated vapor pressure deficit [30], thereby supporting evaporative cooling under compacted, heat-prone urban conditions. These findings reinforce that structural adjustments (e.g., SD) and physiological homeostasis (A and gs) enable urban trees to maintain function under diverse site conditions.
Leaf anatomical traits, including stomatal patterning and vein distribution, co-vary with leaf size to regulate water transport and evaporative cooling, thereby lowering leaf temperature and mitigating heat stress [24]. In our study, stomatal parameters were influenced by both sites and species, with SD being significantly higher at street sites (Table 3). Stomatal area (SA) and length (SL) varied primarily by species, with Little-leaved Linden exhibiting larger SA and longer SL than Norway Maple. In Little-leaved Linden, elevated SD at street sites coincided with higher E and iWUE, while A remained stable across sites. These results suggest a coordinated anatomical and morphological response that allows street trees to maintain gas exchange at levels comparable to urban park trees, consistent with observations in other species that sustain stable gas exchange traits despite urban environmental constraints [30]. Differences in hydraulic conductivity related to tree size (DBH) may also play a role, as street Little-leaved Linden had smaller DBH (13–15 cm) compared to urban park trees (48 cm), whereas Norway Maple exhibited similar DBH across both locations. Smaller trees may allocate resources differently to leaf structure, which could explain the lower SLA and higher SD observed in street Little-leaved Lindens [31]. Combied with gas exchange patterns in street Lindens, these results suggest that smaller street trees may be acclimated to greater environmental stress. Although gs showed little site variation, the increased stomatal number per unit area likely expanded the total evaporative surface, contributing to elevated E. These structural and physiological adjustments—particularly increased SD and reduced SLA—have also been highlighted as reliable indicators of tree responses to urban environmental stressors [12,32].
Differences in morphological and stomatal traits between sites indicate that microenvironmental variation within the urban landscape—such as between street-side and park sites—can significantly influence plant physiological performance. This emphasizes the importance of carefully matching tree species to specific site conditions in urban green infrastructure planning to improve tree health, ecosystem services, and climate resilience. Street sites often present greater environmental challenges—such as compacted soils, reduced rooting volume, and elevated heat loads [2,11]—whereas urban parks provide more buffered microenvironments through increased vegetation cover, shading, cooler conditions, and improved air quality [3,4]. In Chicago, tree placement relative to micro-environmental conditions has been shown to influence summer cooling potential [18]. Integrating knowledge of species-specific functional traits, physiological strategies, and local environmental variation into urban forestry planning can enhance ecosystem services, reduce tree mortality, and promote more resilient and equitable green spaces [8,33]. Selecting highly plastic species (Table 2) can help create sustainable urban spaces. Future studies should incorporate finer-scale environmental metrics—such as air temperature, humidity, soil moisture, temperature, pH, air quality, vapor pressure deficit, and leaf wetness—to better elucidate the drivers of tree performance across urban sites. The consistent physiological function of Norway Maple in street environments, along with the elevated gas exchange traits of Little-leaved Linden in urban park settings, highlights the importance of selecting species that align with site-specific constraints. Urban greening efforts should prioritize species with functional traits adapted to local environmental conditions to enhance biodiversity, mitigate urban heat islands, improve air quality, and support human health and well-being [34].
5. Conclusions
Functional traits offer valuable insight into how trees respond to urban microenvironments and can guide strategies to enhance ecosystem services and resilience. This study highlights that site conditions—including the contrasting environments of street and urban park settings—play a critical role in shaping leaf functional traits in cities. Both Norway Maple (Acer platanoides L.) and Little-leaved Linden (Tilia cordata Mill.) showed structural adjustments in street environments, with reduced specific leaf area and increased stomatal density. Norway Maple maintained stable gas exchange traits across sites, exhibiting a conservative water-use strategy that minimizes water loss while sustaining carbon gain. Little-leaved Linden exhibited trait plasticity in street environments, with elevated transpiration and intrinsic water-use efficiency, accompanied with marked variation in tree size. Taken together, morphological and stomatal adjustments, along with stable gas exchange traits, indicate species-specific strategies that enable urban trees to sustain physiological function across contrasting urban microenvironments. Integrating leaf-level traits into urban forestry planning can promote adaptive, climate-resilient greening strategies that optimize tree performance and enhance well-being in urban communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12100361/s1, Figure S1: Growing-season (April-October) climate summary for 1995–2024: monthly mean temperature and monthly total precipitation, representing the 30-year period ending in the last sampled year (2024); Table S1: Results of three-way ANOVA testing effects of year, species, and site on leaf area (LA, cm²) and specific leaf area (SLA, cm² g−¹), including F-values and p-values for main effects and interactions; Data Sheet S1: A complete list of the original publications contributing to the phenotypic plasticity index subsets derived from the TRY and BIEN plant trait databases.
Author Contributions
N.D.: Conceptualization, Formal Analysis, Investigation, Validation, Methodology, Statistical Analysis, Visualization, Writing—original draft, review and editing. M.A.G.-M.: Conceptualization, Funding acquisition, Project administration, Methodology, Resources, Writing—review and editing, Supervision. A.C.: Conceptualization, Formal analysis, Investigation, Validation, Methodology, Supervision, Writing—original draft, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research’s Urban Integrated Field Laboratories CROCUS project research activity, under Award Number DE-SC0023226.
Data Availability Statement
The data used in this study originate from the TRY and BIEN plant trait databases. Additional details and source information are provided in the Supplementary Materials.
Acknowledgments
We thank Max Berkelhammer for his support of this research and his contribution of scientific insights. We are grateful to Gabriela C. Nunez Mir for her guidance on plant functional traits and statistical advice, and to Roser Matamala for her expertise on stomatal impression methodologies. We also thank Aria Davis and Cindy Hanson for their assistance with field and laboratory work in 2023, and Maya Jimenez, Sarah Dietzen, Bárbara Ramaldes, Asdaq Bakhshi, and Aysha Zakaria for their help in 2024. Special thanks to Umer Khan for capturing the stomatal images used in this study, and to Brenda Arrez and Arlina Ramirez for their support with manual stomatal measurements, which were essential to completing the analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Watson, G.; Hewitt, A.; Custic, M.; Lo, M. The Management of Tree Root Systems in Urban and Suburban Settings: A Review of Soil Influence on Root Growth. Arboric. Urban For. 2014, 40, 193–217. [Google Scholar] [CrossRef]
- Kjelgren, R.; Montague, T. Urban tree transpiration over turf and asphalt surfaces. Atmos. Environ. 1998, 32, 35–41. [Google Scholar] [CrossRef]
- Georgi, N.J.; Zafiriadis, K. The Impact of Park Trees on Microclimate in Urban Areas. Urban Ecosyst. 2006, 9, 195–209. [Google Scholar] [CrossRef]
- Setälä, H.; Francini, G.; Allen, J.A.; Jumpponen, A.; Hui, N.; Kotze, D.J. Urban Parks Provide Ecosystem Services by Retaining Metals and Nutrients in Soils. Environ. Pollut. 2017, 231, 451–461. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Marshall, K.A.; Gonzalez-Meler, M.A. Can Ecosystem Services Be Part of the Solution to Environmental Justice? Ecosyst. Serv. 2016, 22, 202–203. [Google Scholar] [CrossRef]
- Cho, A.; Love, N.; Cintron, R.; Nicholson, J.; Xu, L.; Nunez-Mir, G.C.; Lee, J.; Berkelhammer, M.; Gonzalez-Meler, M.A. Plant Species Selection and Participatory Community Co-Design Are Essential in Balancing Ecosystem Services and Disservices in Urban Areas. Environ. Res. Lett. 2025, 20, 051003. [Google Scholar] [CrossRef]
- Cho, A.; Dziedzic, N.; Davis, A.; Hanson, C.; Lee, J.; Nunez-Mir, G.C.; Gonzalez-Meler, M.A. Leaf Functional Traits Highlight Phenotypic Variation of Two Tree Species in the Urban Environment. Front. Plant Sci. 2024, 15, 1450723. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, N. Can Plant Functional Traits Be Used as Integrators of Environmental Stressors in Urban Areas? Master’s Thesis, University of Illinois Chicago, Chicago, IL, USA, 2024. [Google Scholar] [CrossRef]
- Horike, H.; Kinoshita, T.; Kume, A.; Hanba, Y.T. Responses of Leaf Photosynthetic Traits, Water Use Efficiency, and Water Relations in Five Urban Shrub Tree Species under Drought Stress and Recovery. Trees 2023, 37, 53–67. [Google Scholar] [CrossRef]
- Wang, X.-M.; Wang, X.-K.; Su, Y.-B.; Zhang, H.-X. Land Pavement Depresses Photosynthesis in Urban Trees Especially under Drought Stress. Sci. Total Environ. 2019, 653, 120–130. [Google Scholar] [CrossRef]
- Huang, S.; Knight, C.A.; Hoover, B.K.; Ritter, M. Leaf functional traits as predictors of drought tolerance in urban trees. Urban For. Urban Green. 2020, 48, 126577. [Google Scholar] [CrossRef]
- Kunakh, O.; Zhukov, O. The Norway maple (Acer platanoides) population space location and vital state in the urban park. Agrology 2025, 8, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Latte, N.; Taverniers, P.; de Jaegere, T.; Claessens, H. Dendroecological assessment of climate resilience of the rare and scattered forest tree species Tilia cordata Mill. in northwestern Europe. For. Int. J. Forest Res. 2020, 93, 675–684. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hoehn, R.E.I.; Bodine, A.R.; Crane, D.E.; Dwyer, J.F.; Bonnewell, V.; Watson, G. Urban Trees and Forests of the Chicago Region; Resource Bulletin, NRS-84, U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2013; Volume 84, 106p. [Google Scholar] [CrossRef]
- Morton Arboretum. Chicago Region Tree Census Executive Summary. Available online: https://mortonarb.org/app/uploads/2021/05/2020-Chicago-Region-Tree-Census-Executive-Summary__FINAL.pdf (accessed on 10 June 2025).
- Li, P.; Sharma, A. Detailed Height Mapping of Trees and Buildings (HiTAB) in Chicago and Its Implications to Urban Climate Studies. Environ. Res. Lett. 2024, 19, 094013. [Google Scholar] [CrossRef]
- Petri, A.C.; Wilson, B.; Koeser, A. Planning the Urban Forest: Adding Microclimate Simulation to the Planner’s Toolkit. Land Polic. 2019, 88, 104117. [Google Scholar] [CrossRef]
- Botanical Information and Ecology Network (BIEN). Available online: https://bien.nceas.ucsb.edu/bien/biendata/ (accessed on 8 November 2023).
- TRY Plant Trait Database. Available online: https://www.try-db.org (accessed on 21 November 2023).
- Takagi, M.; Gyokusen, K. Light and Atmospheric Pollution Affect Photosynthesis of Street Trees in Urban Environments. Urban For. Urban Green. 2004, 2, 167–171. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, B. Using Clear Nail Polish to Make Arabidopsis Epidermal Impressions for Measuring the Change of Stomatal Aperture Size in Immune Response. Methods Mol. Biol. 2017, 1578, 243–248. [Google Scholar] [CrossRef]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Bierza, K.; Bierza, W. The effect of industrial and urban dust pollution on the ecophysiology and leaf element concentration of Tilia cordata Mill. Environ. Sci. Poll. Res. 2024, 31, 58413–58429. [Google Scholar] [CrossRef]
- Evans, J.R.; Loreto, F. Acquisition and diffusion of CO2 in higher plant leaves. In Photosynthesis: Physiology and Metabolism; Springer: Dordrecht, The Netherlands, 2000; pp. 321–351. ISBN 978-0-306-48137-6. [Google Scholar]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmes, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 60, 2249–2270. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Z.; Xu, H.; Kong, Z.; Xu, Z.; Liu, Q.; Liu, P.; Zhang, Z. Biophysical regulations of transpiration and water use strategy in a mature Chinese pine (Pinus tabulaeformis) forest in a semiarid urban environment. Hydrol. Process. 2022, 36, e14485. [Google Scholar] [CrossRef]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Noriega, J.E.; Deva, C.; Beebe, S.E.; Polanía, J.A.; Casanoves, F.; Rao, I.M. Water use, leaf cooling and carbon assimilation efficiency of heat resistant common beans evaluated in Western Amazonia. Front. Plant Sci. 2021, 12, 644010. [Google Scholar] [CrossRef]
- Ding, J.; Jiao, X.; Bai, P.; Hu, Y.; Zhang, J.; Li, J. Effect of vapor pressure deficit on the photosynthesis, growth, and nutrient absorption of tomato seedlings. Sci. Hort. 2022, 293, 110736. [Google Scholar] [CrossRef]
- Gillner, S.; Korn, S.; Roloff, A. Leaf-gas exchange of five tree species at urban street sites. Arboric. Urban For. 2015, 41, 113–124. [Google Scholar] [CrossRef]
- Ferdous, J.; Islam, M.; Rahman, M. The role of tree size, wood anatomical and leaf stomatal traits in shaping tree hydraulic efficiency and safety in a South Asian tropical moist forest. Glob. Ecol. Conser. 2023, 43, e02453. [Google Scholar] [CrossRef]
- Balasooriya, B.L.W.K.; Samson, R.; Mbikwa, F.; Vitharana, U.W.A.; Boeckx, P.; Meirvenne, M.V. Biomonitoring of Urban Habitat Quality by Anatomical and Chemical Leaf Characteristics. Environ. Exp. Bot. 2009, 65, 386–394. [Google Scholar] [CrossRef]
- Endreny, T.A. Strategically Growing the Urban Forest Will Improve Our World. Nat. Commun. 2018, 9, 1160. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).