How Does Ambrosia artemisiifolia L. Bioaccumulate and Translocate Cr, Cu, Pb, As, Cd, Hg, and Zn in Polluted Soils?
Abstract
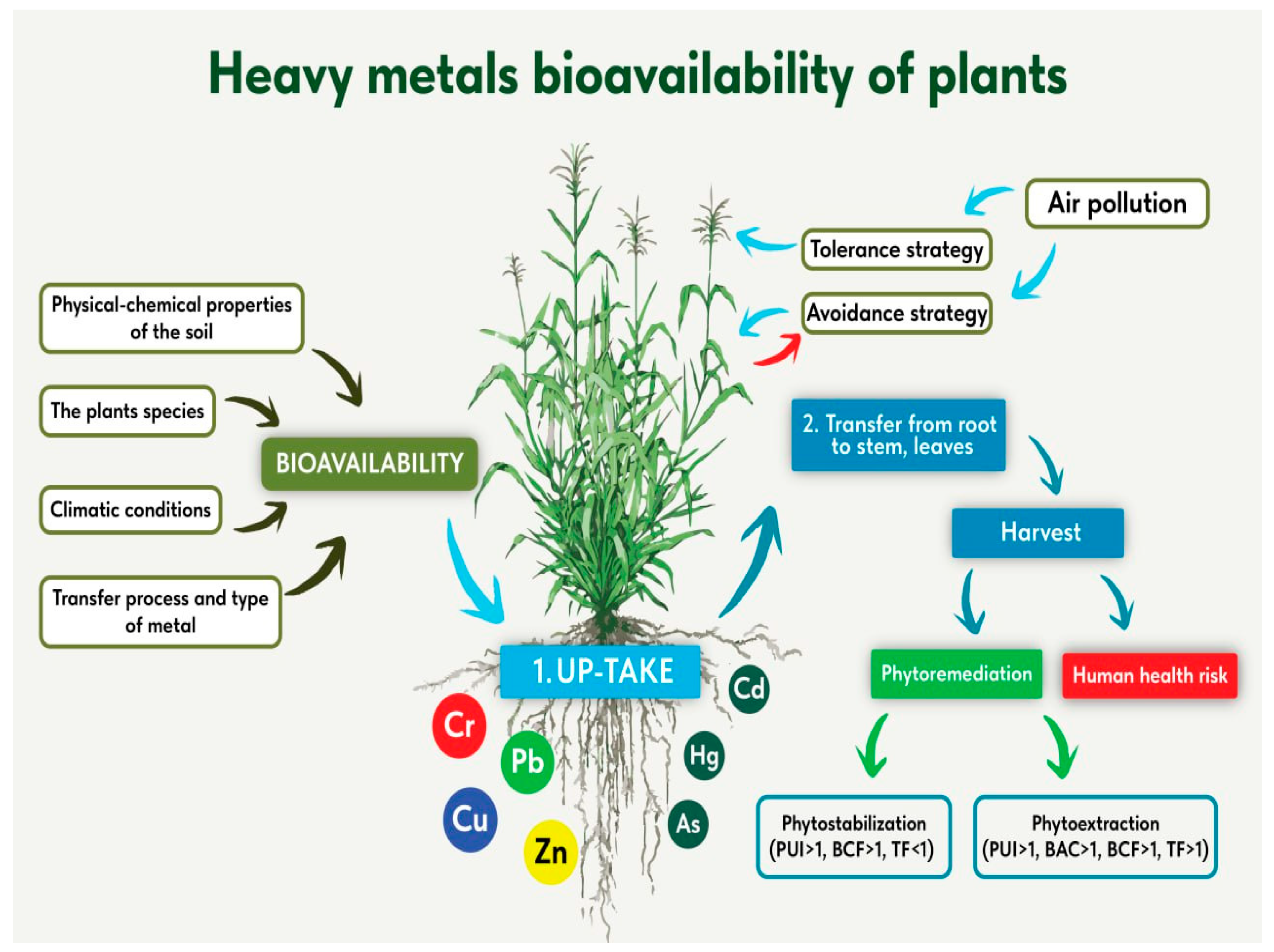
1. Introduction
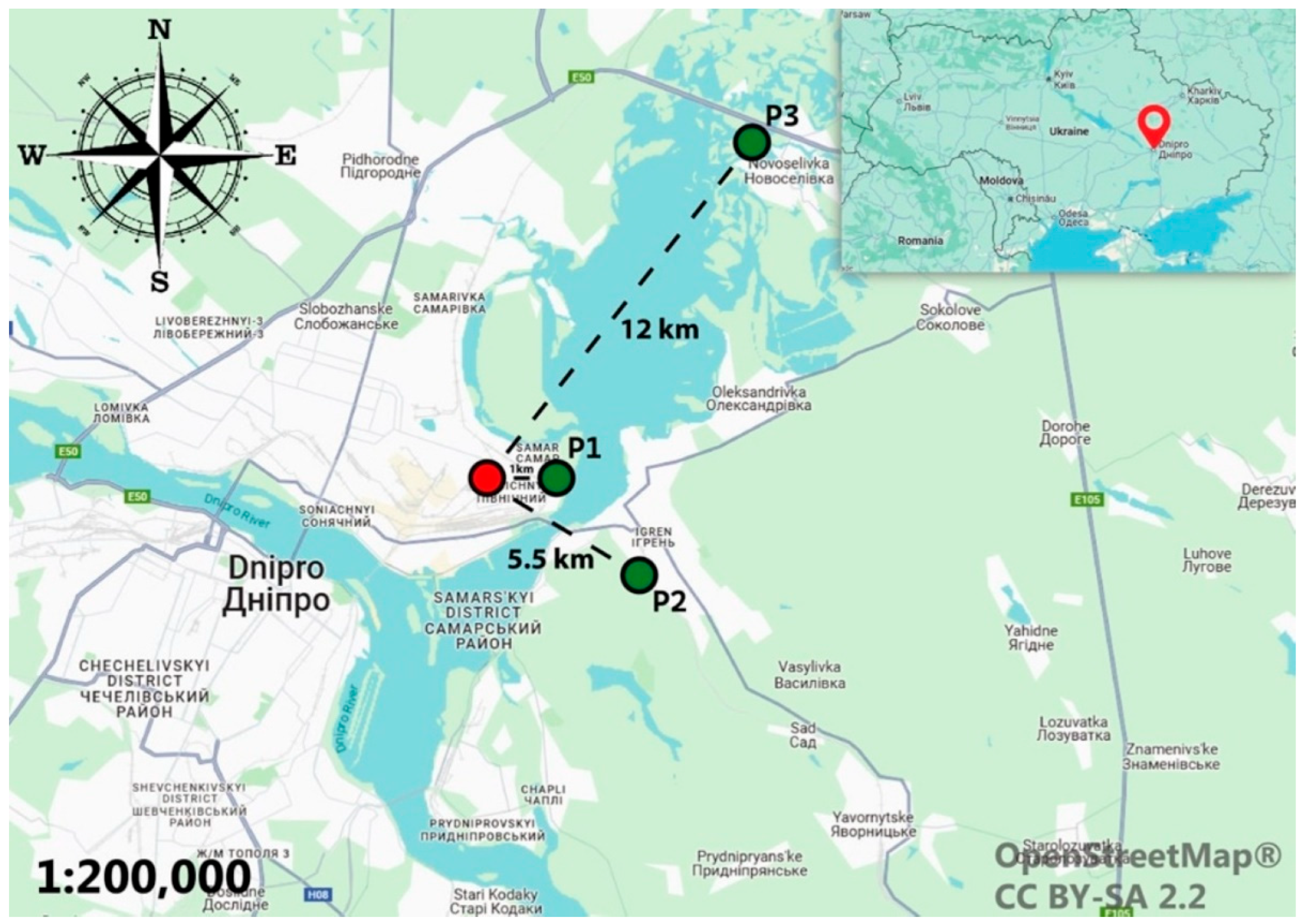
2. Materials and Methods
3. Results and Discussion
3.1. Soil
3.2. Plant, Bioaccumulation and Translocation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMs | Heavy metals |
| As | Arsenic |
| Cd | Cadmium |
| Cr | Chromium |
| Cu | Copper |
| Hg | Mercury |
| Pb | Lead |
| Zn | Zinc |
| PUI | Plant uptake index |
| TF | Translocation factors |
| AR | Availability ratio index |
| Igeo | Geoaccumulation index |
| EF | Enrichment factor |
| CMF | Concentration of mobile forms |
| CTF | Total concentration |
| P1, P2, P3 | Studied plots |
References
- Alloway, B. Heavy metals in soils. In Trace Elements and Metalloids in Soils and Their Bioavailability; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2012; Volume 22, pp. 11–50. [Google Scholar] [CrossRef]
- Hazrat, A.; Ezzat, K.; Ikram, I. Environmental Chemistry and Ecotoxicologyof Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Jadaa, W.; Mohammed, H. Heavy Metals—Definition, Natural and Anthropogenic Sourcesof Releasing into Ecosystems, Toxicity, and Removal Methods—An Overview Study. J. Ecol. Eng. 2023, 24, 249–271. [Google Scholar] [CrossRef]
- Gritsan, N.; Babiy, A. Hazardous materials in the environment of Dnepropetrovsk region (Ukraine). J. Hazard. Mater. 2000, 76, 59–70. [Google Scholar] [CrossRef]
- Kharytonov, M.; Benselhoub, A.; Shupranova, L.; Kryvakovska, R.; Khlopova, V. Environmental assessment of atmospheric pollution in Dnipropetrovsk province (Ukraine). In Studia Universitatis “Vasile Goldis” Arad. Seria Stiintele Vietii (Life Sciences Series); Vasyl Goldis Western University: Arad, Romania, 2015; Volume 25, p. 125. [Google Scholar]
- Shparyk, Y.S.; Parpan, V.I. Heavy metal pollution and forest health in the Ukrainian Carpathians. Environ. Pollut. 2004, 130, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Environmental Passport of the City of Dnipro; Department of Environmental Protection of the Dnipro City Council: Dnipro, Ukraine, 2018; p. 67.
- REGIONAL REPORT on the State of the Environment in the Dnipropetrovska Oblast for 2021; Department of Environment and Natural Resources Dnipropetrovs’k Regional Military Administration: Dnipro, Ukraine, 2022; p. 304.
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S. Soil quality in relation to agricultural uses. In Integrated Soil and Sediment Research: A Basic for Proper Protection; Kluwer Publishers: London, UK; Boston, MA, USA, 1992; pp. 187–198. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–550. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Effects of Heavy Metals on Soil Enzyme Activities. In Soil Heavy Metals; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 19. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Its implications for Phytoremediation. Environ. Rev. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Genton, B.J.; Shykoff, J.A.; Giraud, T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Mol. Ecol. 2005, 14, 4275–4285. [Google Scholar] [CrossRef]
- Fumanal, B.; Gaudot, I.; Bretagnolle, F. Seed-bank dynamics in the invasive plant Ambrosia artemisiifolia in France. Ecol. Model. 2007, 207, 293–306. [Google Scholar] [CrossRef]
- Lommen, S.T.E.; Folkestad, J.; Solhaug, K.A.; Brandsæter, L.O. High plasticity in reproduction and growth in invasive common ragweed (Ambrosia artemisiifolia) in Europe. Weed Res. 2017, 57, 540–550. [Google Scholar] [CrossRef]
- Rybak, A.; Olejniczak, I.; Fajer, M.; Krajewski, P. The potential of common ragweed (Ambrosia artemisiifolia L.) for phytoremediation of soils contaminated with heavy metals. Ecotoxicol. Environ. Saf. 2020, 196, 110507. [Google Scholar]
- Pawłowski, L.; Sowa, I.; Telesiński, A. Accumulation of heavy metals by ragweed (Ambrosia artemisiifolia L.) in urban areas of Poland. Pol. J. Environ. Stud. 2015, 24, 1649–1656. [Google Scholar]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of Soil pH on the Growth, Reproductive Investment and Pollen Allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Bae, J.; Byun, C.; Watson, A.K.; Benoit, D.L. Selection of ground cover species for the management of common ragweed (Ambrosia artemisiifolia L.) on a highway shoulder. Plant Ecol. 2015, 216, 263–271. [Google Scholar] [CrossRef]
- Sajad, M.A.; Khan, M.S.; Ali, H. Lead phytoremediation potential of sixty-one plant species: An open field survey. Pure Appl. Biol. (PAB) 2019, 8, 405–419. [Google Scholar] [CrossRef]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common ragweed: A threat to environmental health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef]
- Ranđelović, D.; Jakovljević, K.; Mišljenović, T.; Savović, J.; Kuzmanović, M.; Mihailović, N.; Jovanović, S. Accumulation of potentially toxic elements in the invasive Ambrosia artemisiifolia in areas with different levels of anthropogenic pollution. Water Air Soil Pollut. 2020, 231, 272. [Google Scholar] [CrossRef]
- Ryzhenko, N.; El Amrani, A.; Giltrap, M.; Furong, T.; Laptiev, V. Bioaccumulation of As, Cd, Cr, Cu, Pb, Zn in Ambrosia artemisiifolia L. in the polluted area by enterprise for the production and processing of batteries. Ann. Civ. Environ. Engeneering 2022, 6, 26–30. [Google Scholar] [CrossRef]
- Laptiev, V.; Apori, S.; Giltrap, M.; Tian, F.; Ryzhenko, N. Bioaccumulation of Cr, Zn, Pb and Cu in Ambrosia artemisiifolia L. and Erigeron canadensis L. Resources 2024, 13, 43. and Erigeron canadensis L. Resources 2024, 13, 43. [Google Scholar] [CrossRef]
- Mihajlovic, D.; Žunic, J.; Kovačević, Z.; Cerekovic, N. Assessment of the potentially toxic elements contamination and their uptake by Ambrosia artemisiifolia L. in soils in the Northern part of Bosnia and Herzegovina. Carpathian J. Earth Environ. Sci. 2024, 19, 25–32. [Google Scholar] [CrossRef]
- Laptiev, V.; Giltrap, M.; Tian, F.; Ryzhenko, N. Assessment of Heavy Metals (Cr, Cu, Pb, and Zn) Bioaccumulation and Translocation by Erigeron canadensis L. in Polluted Soil. Pollutants 2024, 4, 434–451. [Google Scholar] [CrossRef]
- Environmental Passport of the City of Dnipro; Department of Transport and Environmental Protection of the Dnipro City Council: Dnipro, Ukraine, 2016; p. 64.
- DSTU 4287:2004 Soil Quality; Sampling of Samples: Kyiv, Ukraine, 2004.
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2022.
- Amouei, A.; Cherati, A.; Naghipour, D. Heavy Metals Contamination and Risk Assessment of Surface Soils of Babol in Northern Iran. Health Scope 2018, 7, e62423. [Google Scholar] [CrossRef]
- Fateev, A.I.; Pashchenko, Y.V. (Eds.) Background Content of Microelements in Soils Ukraine; Institute of Soil Science and Agrochemistry: Kharkiv, Ukraine, 2003; p. 71. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil polution indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Sutherland, R. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Irfan, A.; Jinxi, S.; Haotian, S.; Sajid, M.; Ping, S.; Asif, A. Contamination Level, Ecological Risk, and Source Identification of Heavy Metals in the Hyporheic Zone of the Weihe River, China. Int. J. Environ. Res. Public Health 2020, 17, 1070. [Google Scholar] [CrossRef]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium stress in plants: Toxicity, tolerance, and phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Rabinowitz, M. Plant uptake of soil and atmospheric lead in Southern California. Chemosphere 1972, 1, 175–180. [Google Scholar] [CrossRef]
- Berthelsen, B.O.; Olsen, R.A.; Steinnes, E. Ectomycorrhizal heavy metal accumulation as a contributing factor to heavy metal levels in organic surface soils. Sci. Total Environ. 1995, 170, 141–149. [Google Scholar] [CrossRef]
- Tomašević, M.; Aničić, M.; Jovanović, L.; Perić-Grujić, A.; Ristić, M. Deciduous tree leaves in trace elements biomonitoring: A contribution to methodology. Ecol. Indic. 2011, 11, 1689–1695. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Xiao, W.; Luo, X.-G.; Shah, S.; Buzdar, M.; Ahmed, I.; Abdullah, S.; Babar, A.; Jakhar, A.M.; Toquier, A. Garlic (Allium sativum) based interplanting alters the heavy metals absorption and bacterial diversity in neighboring plants. Sci. Rep. 2021, 11, 5833. [Google Scholar] [CrossRef]
- Farraji, H.; Aziz, H.; Tajuddin, R.; Mojiri, A. Optimization of Phytoremediation of Lead-contaminated Soil by Spinach (Spinacia oleracea L). Int. J. Sci. Res. Knowl. 2014, 2, 480–486. [Google Scholar] [CrossRef]
- Meharg, A. Marschner’s Mineral Nutrition of Higher Plants. Edited by P. Marschner. Amsterdam, Netherlands: Elsevier/Academic Press (2011), pp. 684, US $124.95. ISBN 978-0-12-384905-2. Exp. Agric. 2011, 48, 305. [Google Scholar] [CrossRef]
- Вondar, O.; Ryzhenko, N.; Laptiev, V.; Makhniuk, V. Bioaccumulation of Hg, Cr, Zn, As, Cd, Pb, Cu in the “soil-plant” system in the rea of influenceof enterprises for the production and processing of batteries. Ecologicalscience 2022, 1, 11–16. [Google Scholar]
- Zunic, J.; Mihajlovic, D.; Kelecevic, B.; Kovačević, Z. Determination of potentially toxic elements in some wild edible and medicinal plants. АГРОЗНАЊЕ 2022, 23, 209–220. [Google Scholar] [CrossRef]
- Lasat, M.M. The Use of Plants for the Removal of Toxic Metals from Contaminated Soil; U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soils; CRC: Boca Raton, FL, USA, 1999; pp. 85–107. [Google Scholar]
- McGrath, S.P.; Zhao, F.-J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Brooks, R.R. Terrestrial Higher Plants which Hyperaccumulate Metallic Elements. A Review of Their Distribution, Ecology and Phytochemistry. Biorecovery 1989, 1, 81. [Google Scholar]
- Purakayastha, T.; Chhonkar, P. Phytoremediation of Heavy Metal Contaminated Soils. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Gladka, G.; Tymoshenko, A.; Kyrylov, S.; Shabliy, O.; Bida, I.; Mariychuk, R.; Tashyrev, O. A noxious weed Ambrosia artemisiifolia L. (Ragweed) as sustainable feedstock for methane production and metals immobilization. Sustainability 2023, 15, 6696. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
| Indicator | Locations | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|
| CTF, mg kg−1 | P1 | 2219.0 ± 177.5 a | 1039.0 ± 98.76 a | 7830.0 ± 720.36 a | 1918.0 ± 230.16 a |
| P2 | 36.51 ± 4.02 bc | 15.42 ± 1.33 bc | 4.53 ± 0.41 bc | 14.29 ± 1.55 bc | |
| P3 | 21.21 ± 2.6 bc | 5.66 ± 0.6 bc | 10.03 ± 1.26 bc | 15.11 ± 1.48 bc | |
| Bi *, mg kg−1 | 49.0 | 19.3 | 10.0 | 53.0 | |
| CMF, mg kg−1 | P1 | 1.65± 0.24 a | 1.80 ± 0.19 ab | 48.96 ± 4.12 bc | 20.67 ± 1.88 a |
| P2 | 0.17 ± 0.02 bc | 0.16 ± 0.01 ab | 0.53 ± 0.05 bc | 0.67 ± 0.06 b | |
| P3 | 0.89 ± 0.17 bc | 0.28 ± 0.05 c | 2.72 ± 0.52 a | 4.73 ± 0.91 c | |
| AR, % | P1 | 0.07 | 0.17 | 0.63 | 1.08 |
| P2 | 0.47 | 1.04 | 11.7 | 4.69 | |
| P3 | 4.2 | 4.95 | 27.12 | 31.3 | |
| Igeo | P1 | 2.78 | 2.88 | 5.73 | 2.95 |
| P2 | −1.33 | −1.33 | −1.73 | −1.95 | |
| P3 | −1.87 | −2.34 | −0.93 | −1.9 | |
| EF | P1 | 2.58 | 3.07 | 44.67 | 2.06 |
| P2 | 0.04 | 0.05 | 0.03 | 0.02 | |
| P3 | 0.03 | 0.02 | 0.06 | 0.02 | |
| Metals | Plots | PUI | BAC | BCF | TF Shoot/Root | TF Inflorescence/Root | TF Leaf/Root | TF Stem/Root |
|---|---|---|---|---|---|---|---|---|
| Cr | P1 | 5.25 | 2.62 | 2.63 | 0.99 | 0.33 | 0.37 | 0.3 |
| P2 | 67.38 | 31.09 | 36.29 | 0.88 | 0.4 | 0.28 | 0.2 | |
| P3 | 21.69 | 12.27 | 9.42 | 1.48 | 0.15 | 1.08 | 0.25 | |
| Cu | P1 | 48.63 | 33.35 | 15.28 | 2.18 | 0.78 | 1.1 | 0.3 |
| P2 | 411.73 | 289.73 | 122.0 | 2.38 | 0.91 | 1.03 | 0.44 | |
| P3 | 216.7 | 125.16 | 91.54 | 1.34 | 0.53 | 0.64 | 0.17 | |
| Pb | P1 | 0.67 | 0.08 | 0.59 | 0.12 | 0.03 | 0.08 | 0.02 |
| P2 | 6.97 | 2.95 | 4.02 | 0.73 | 0.25 | 0.23 | 0.26 | |
| P3 | 1.89 | 0.74 | 1.15 | 0.64 | 0.14 | 0.37 | 0.13 | |
| Zn | P1 | 17.09 | 9.62 | 7.47 | 1.29 | 0.25 | 0.56 | 0.48 |
| P2 | 672.18 | 548.36 | 123.82 | 4.44 | 1.37 | 2.24 | 0.83 | |
| P3 | 106.81 | 75.41 | 31.4 | 2.4 | 0.86 | 1.15 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laptiev, V.; Giltrap, M.; Tian, F.; Ryzhenko, N. How Does Ambrosia artemisiifolia L. Bioaccumulate and Translocate Cr, Cu, Pb, As, Cd, Hg, and Zn in Polluted Soils? Environments 2025, 12, 360. https://doi.org/10.3390/environments12100360
Laptiev V, Giltrap M, Tian F, Ryzhenko N. How Does Ambrosia artemisiifolia L. Bioaccumulate and Translocate Cr, Cu, Pb, As, Cd, Hg, and Zn in Polluted Soils? Environments. 2025; 12(10):360. https://doi.org/10.3390/environments12100360
Chicago/Turabian StyleLaptiev, Volodymyr, Michelle Giltrap, Furong Tian, and Nataliia Ryzhenko. 2025. "How Does Ambrosia artemisiifolia L. Bioaccumulate and Translocate Cr, Cu, Pb, As, Cd, Hg, and Zn in Polluted Soils?" Environments 12, no. 10: 360. https://doi.org/10.3390/environments12100360
APA StyleLaptiev, V., Giltrap, M., Tian, F., & Ryzhenko, N. (2025). How Does Ambrosia artemisiifolia L. Bioaccumulate and Translocate Cr, Cu, Pb, As, Cd, Hg, and Zn in Polluted Soils? Environments, 12(10), 360. https://doi.org/10.3390/environments12100360








