Hydrothermal Versus Physical Mixing: Superior Photocatalytic Activity of TiO2/WO3 Nanocomposites for Water Treatment Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanocomposites
2.3. Preparation of Thin Film
2.4. Experimental Setup and Procedure
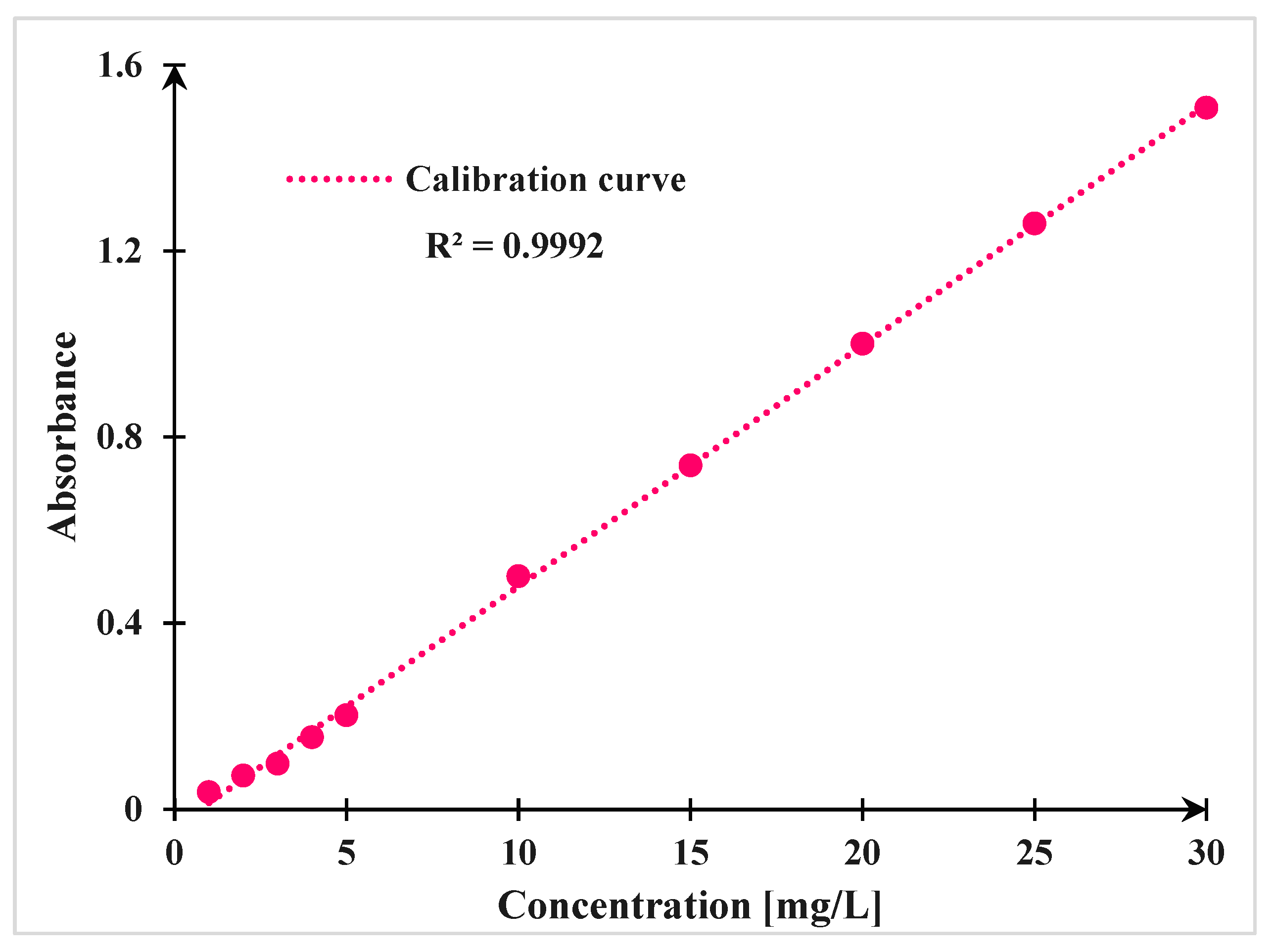
2.5. Analytical Method
3. Results and Discussion
3.1. Characterization of Nanocomposites
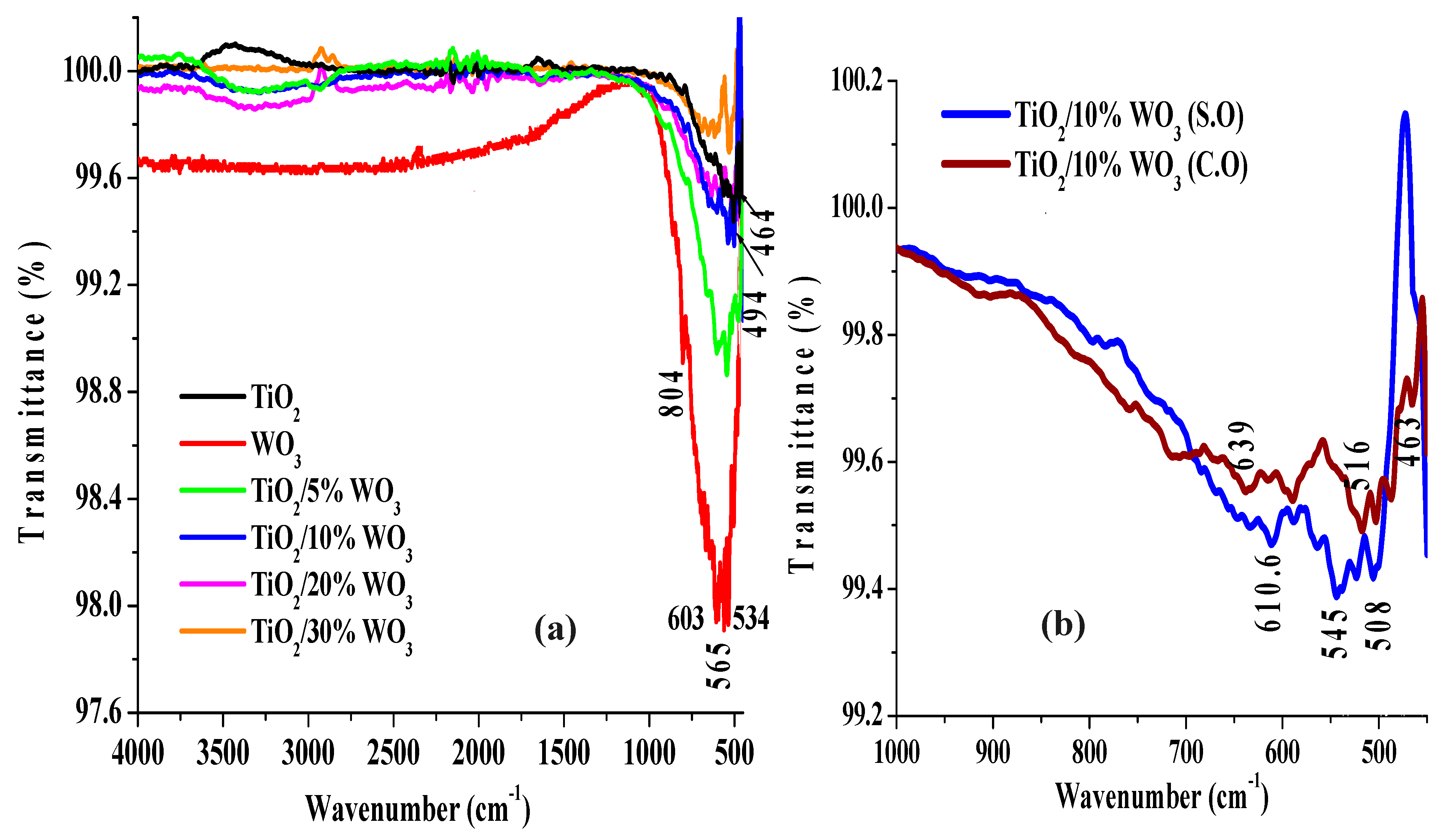
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
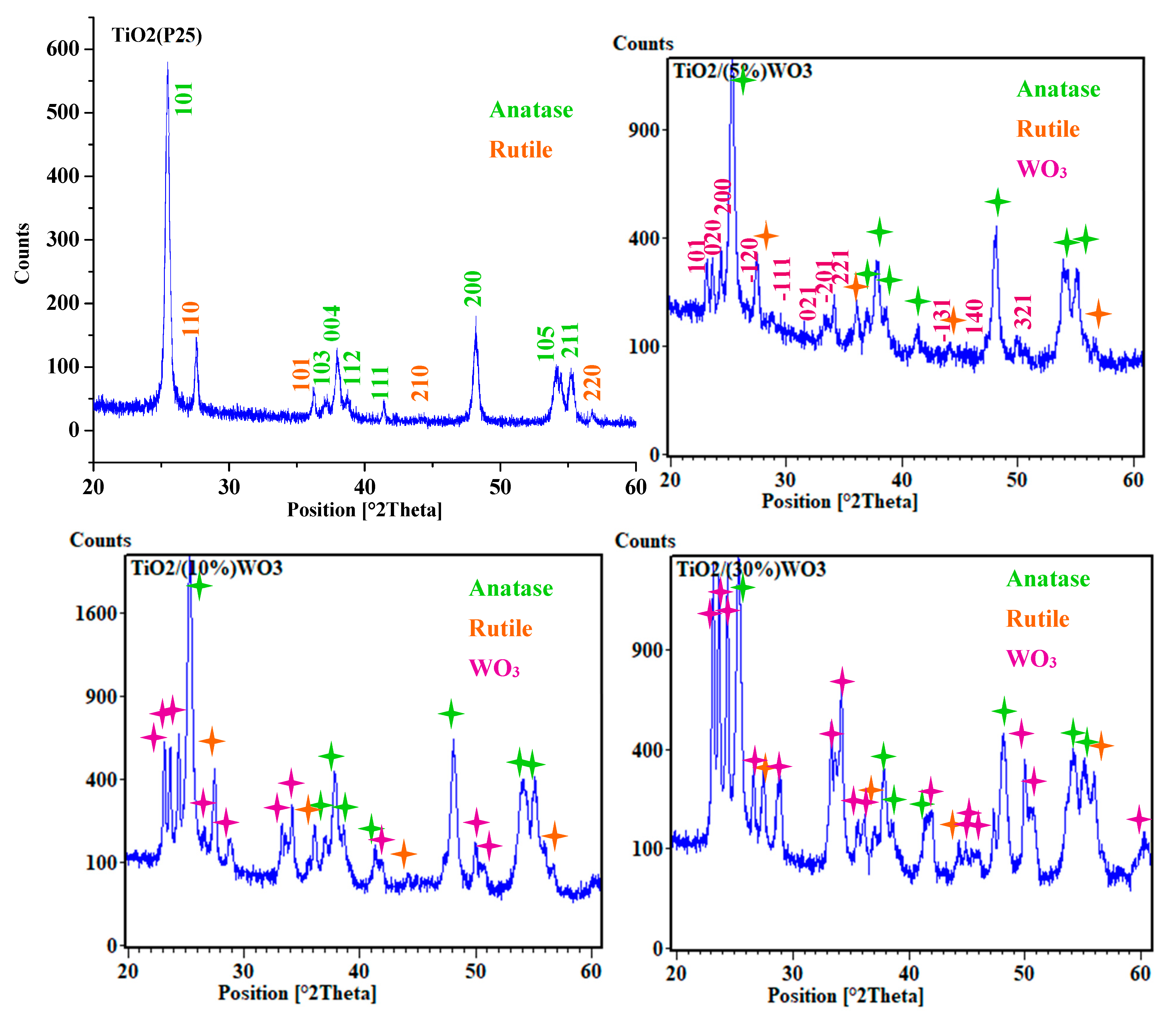
3.1.2. X-Ray Diffraction of Synthesized TiO2/WO3 Nanocomposites
3.1.3. Raman Spectroscopy
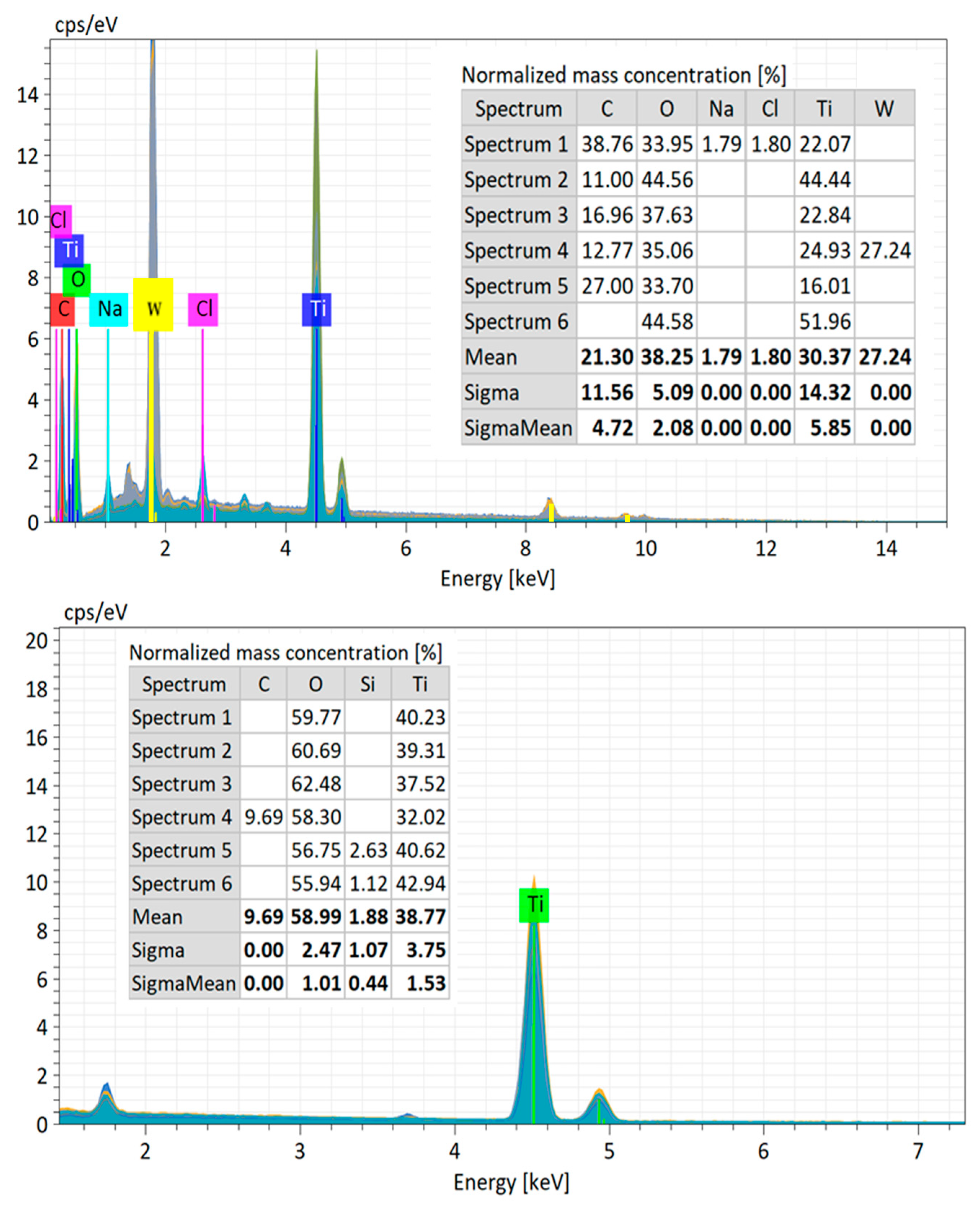
3.1.4. Energy Dispersive X-Ray Spectroscopy (EDX)
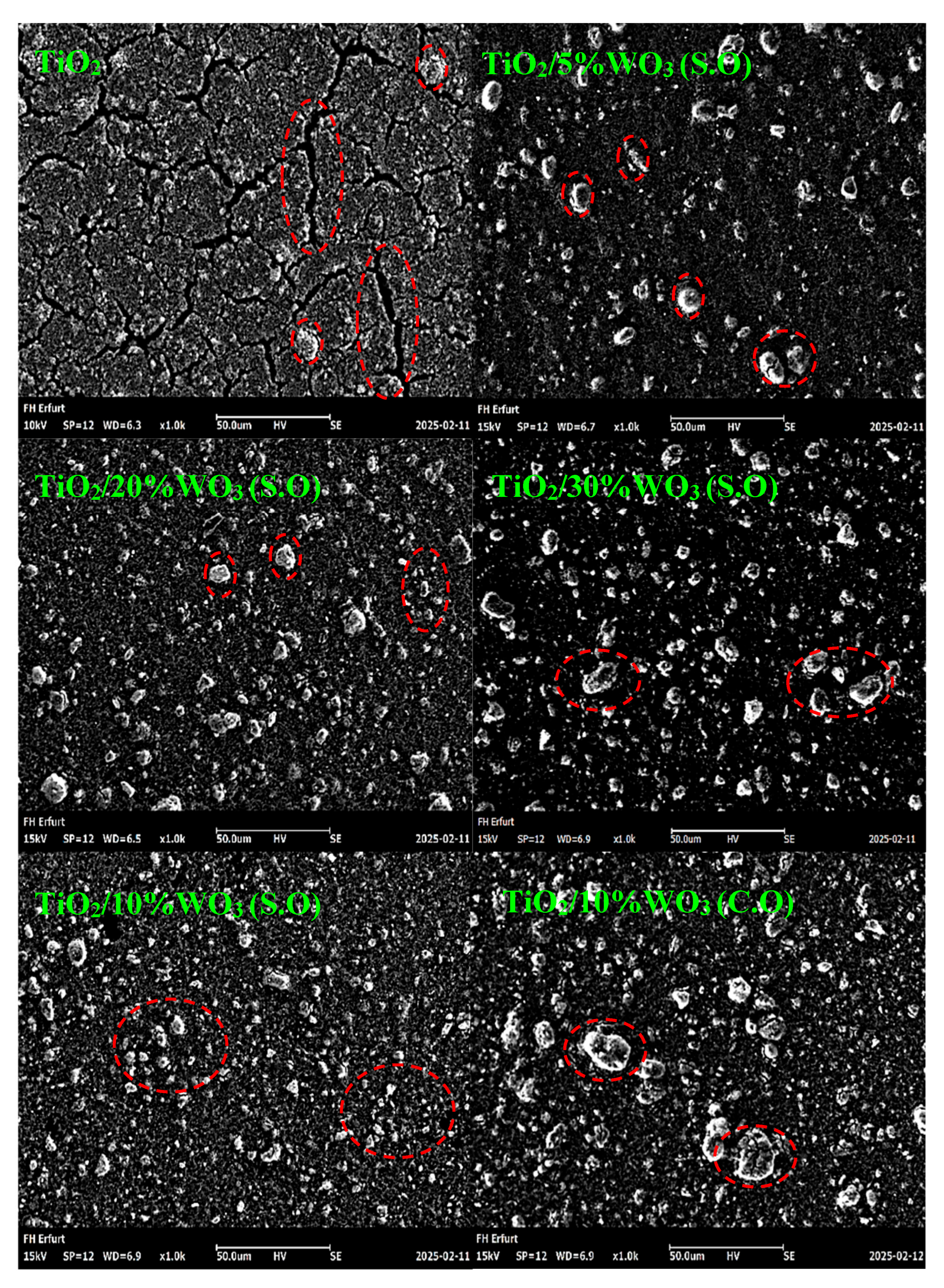
3.1.5. Scanning Electron Microscopy (SEM) of TiO2/WO3 Composites
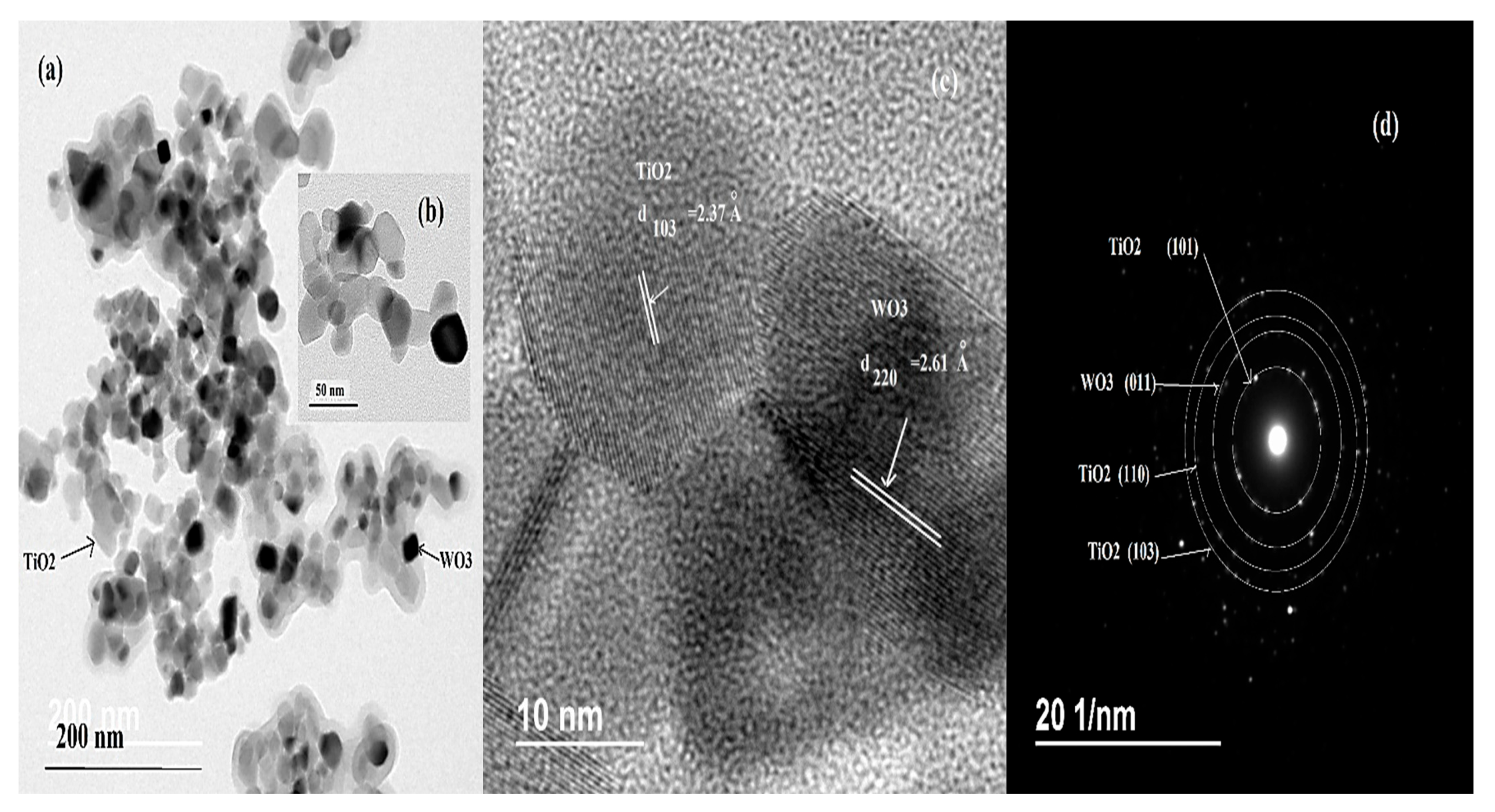
3.1.6. Transmittance Electronic Microscopy (TEM) of TiO2/WO3 Composite
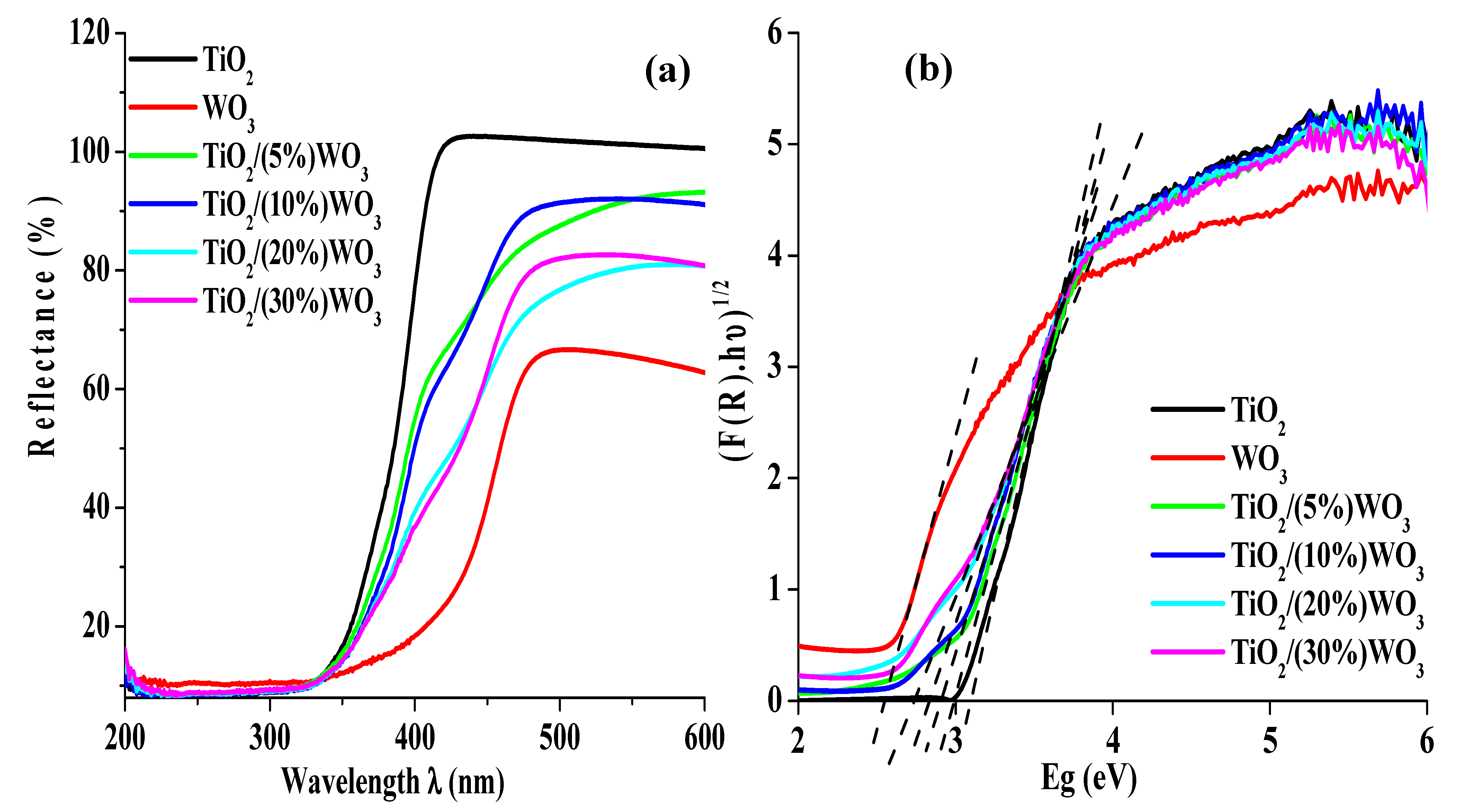
3.1.7. Optical Band Gap (Eg) Estimation via Kubelka-Munk Analysis
3.2. Photocatalytic Activity
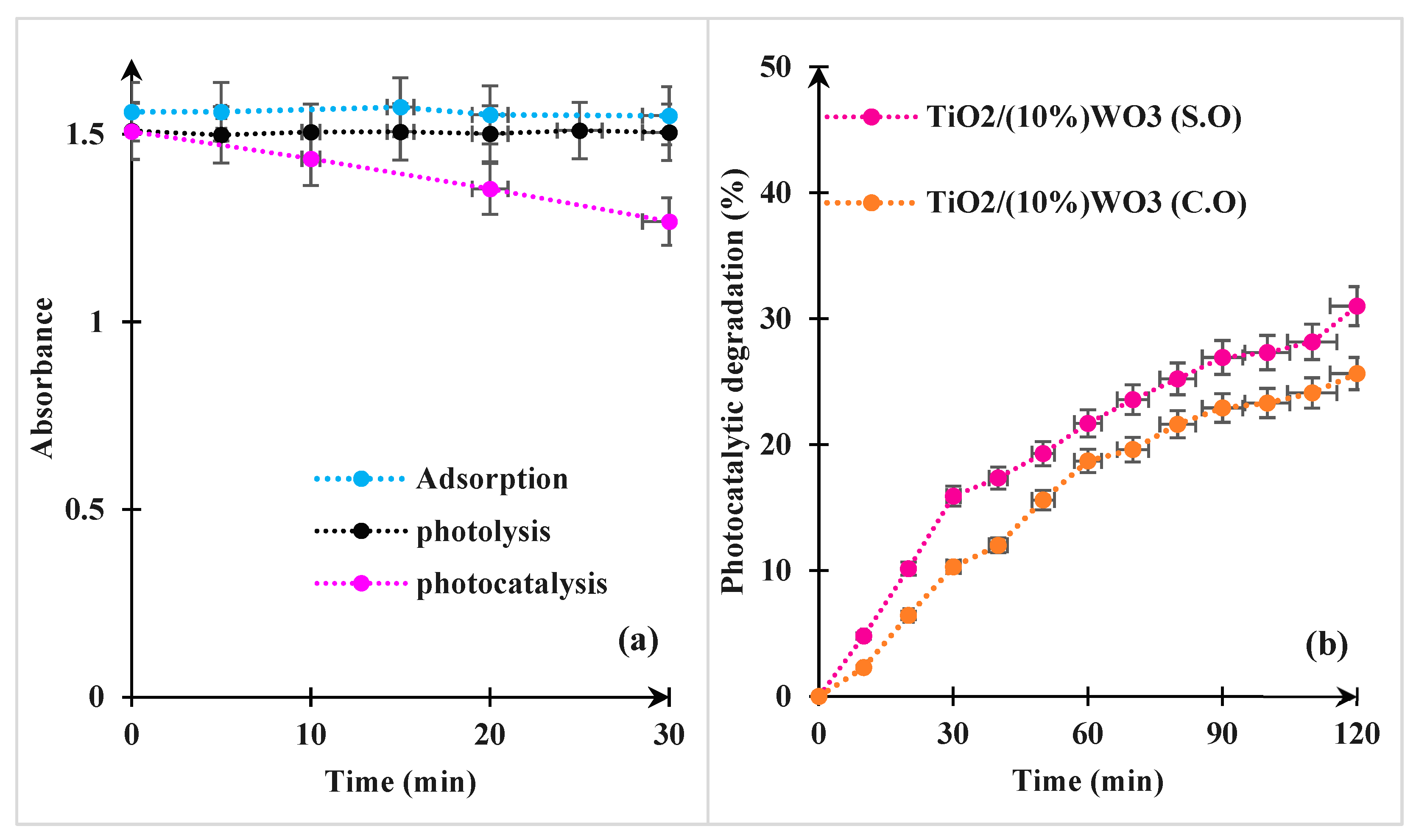
3.2.1. Evaluating Photocatalytic Activity Against Controls
3.2.2. Comparison of the Photocatalytic Performance Between the Synthesized Nanocomposite and That Prepared from Commercial Oxides
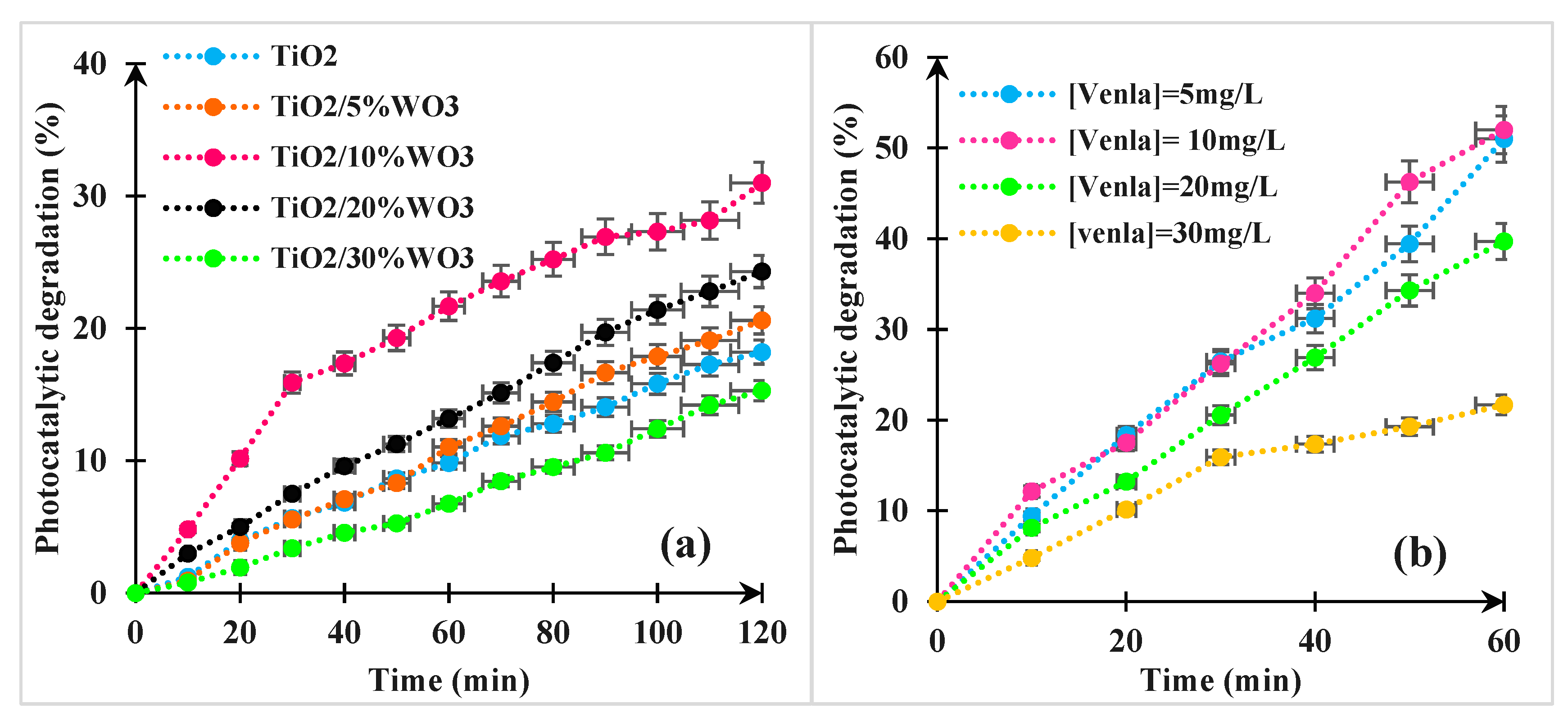
3.2.3. The Effect of WO3 Amount on the Photocatalytic Behavior of TiO2
3.2.4. Effect of Initial Concentration of the Pollutant
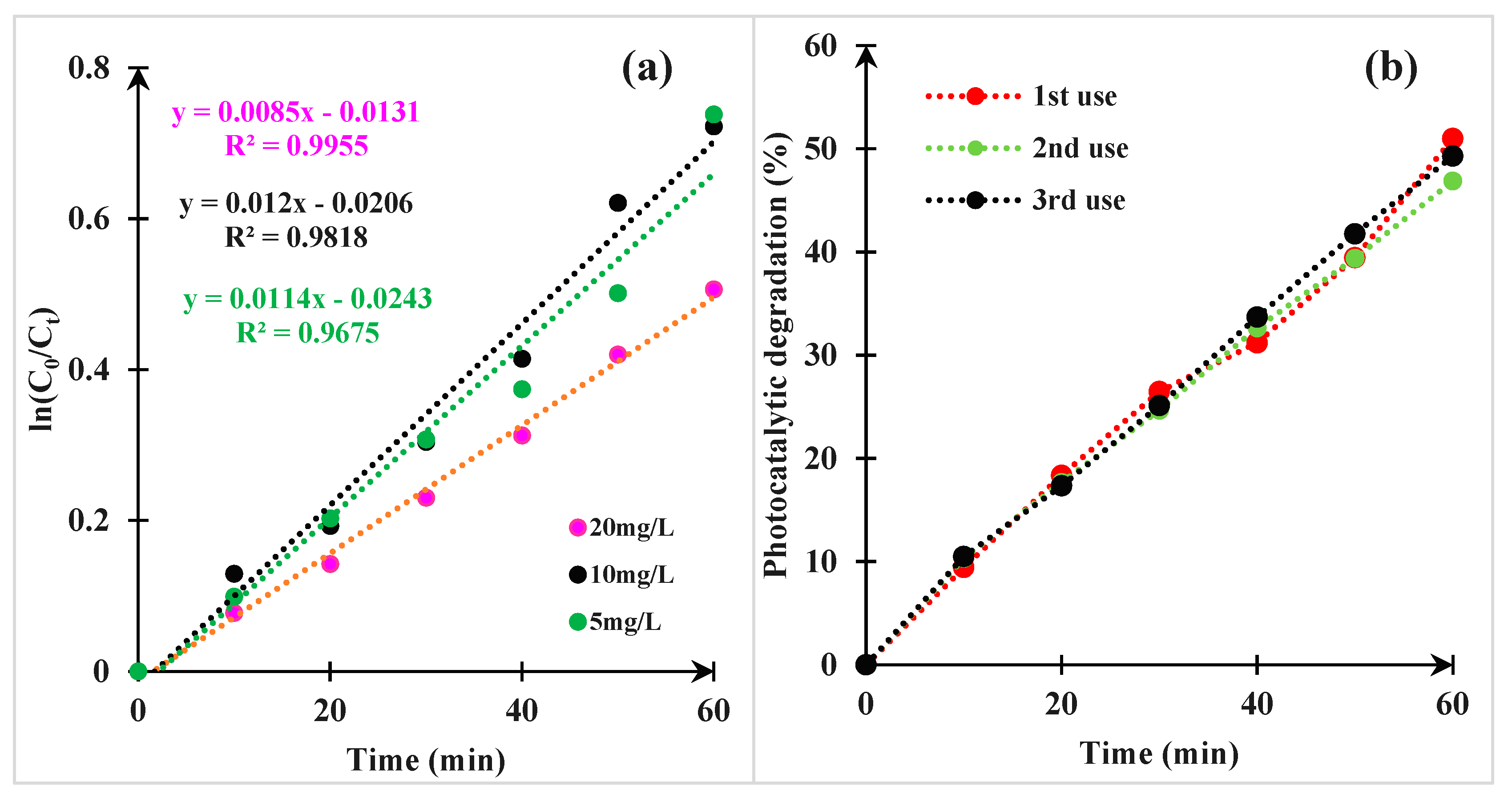
3.2.5. Kinetic Study
3.2.6. Stability of Photocatalyst
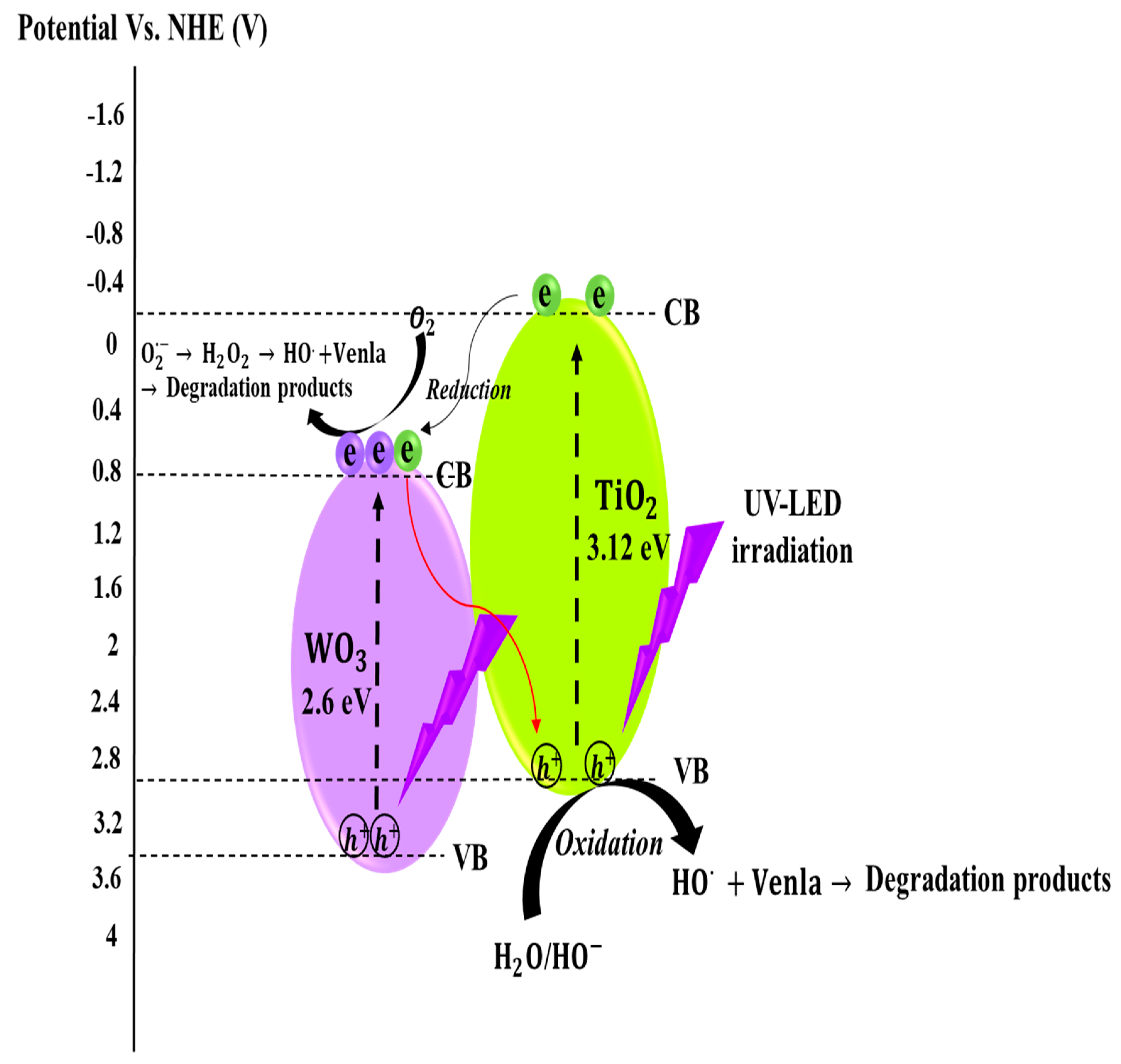
3.2.7. Photocatalytic Charge Transfer Pathways in TiO2/10%WO3 Nanocomposite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bergmann, T.A.D.; Witte, F.-A.; Buser, A.; Heberer, S.; Eberlein, I.; Hildebrandt, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Corcoran, J.; Jacobs, M.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the Aquatic Environment: A Critical Review of the Evidence for Health Effects in Fish. Criti. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Schlich, K.; Hund-Rinke, K. Influence of Soil Properties on the Effect of Silver Nanomaterials on Microbial Activity in Five Soils. Environ. Pollut. 2015, 196, 321–330. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Xu, P.; Xiang, J.; Xu, D.; Cheng, P.; Wang, X.; Wu, L.; Zhang, N.; Chen, Z. Antidepressants as Emerging Contaminants: Occurrence in Wastewater Treatment Plants and Surface Waters in Hangzhou, China. Front. Public Health 2022, 10, 963257. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, C.; Gómez-Motos, I.; Lombraña, J.I.; de Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an Effluent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Cruz-Castillo, L.M.; Martínez-Jerónimo, L.; Martínez-Jerónimo, F. Diclofenac Produces Diverse Toxic Effects on Aquatic Organisms of Different Trophic Levels, Including Microalgae, Cladocerans, and Fish. Water 2025, 17, 1489. [Google Scholar] [CrossRef]
- Fu, L.A.; McCarthy, R.A.; Hawkins, I.J.; West, C.; Koenig, W.; Smith, H.I.; Martin, A.E.; Adams, D.S. Assessing Pharmaceutical Removal and Reduction in Toxicity Provided by Advanced Wastewater Treatment Systems. Environ. Sci.: Water Res. Technol. 2020, 6, 62–77. [Google Scholar] [CrossRef]
- Elskens, C.J.; Van de Meulebroeck, T.V.; Lübberding, T.; Van den Meersche, P.F.; Kraak, M.H.S. Removal of Psychopharmaceuticals from WWTP Effluent by an Algae–Mussel Trophic Cascade: A Potential Nature-Based Solution? Environ. Sci. Water Res. Technol. 2025, 11, 1643–1656. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Gnanasekar, D.; Anandan, S.; Arulraj, R.; Sankaran, A.K.S. Advanced Photocatalytic Materials-Based Degradation of Micropollutants and Their Use in Hydrogen Production—A Review. RSC Adv. 2024, 14, 14392–14424. [Google Scholar] [CrossRef]
- Antonopoulou, M. Homogeneous and Heterogeneous Photocatalysis for the Treatment of Pharmaceutical Industry Wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef]
- Friedmann, D. A General Overview of Heterogeneous Photocatalysis as a Remediation Technology for Wastewaters Containing Pharmaceutical Compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Co-Doped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Huang Kong, E.D.; Lai, C.W.; Juan, J.C.; Pang, Y.L.; Khe, C.S.; Badruddin, I.A.; Gapsari, F.; Anam, K. Recent Advances in Titanium Dioxide Bio-Derived Carbon Photocatalysts for Organic Pollutant Degradation in Wastewater. iScience 2025, 28, 112368. [Google Scholar] [CrossRef]
- Tanos, F.; Razzouk, A.; Lesage, G.; Cretin, M.; Bechelany, M. A Comprehensive Review on Modification of Titanium Dioxide-Based Catalysts in Advanced Oxidation Processes for Water Treatment. Chem. Sus. Chem. 2024, 17, e202301139. [Google Scholar] [CrossRef]
- He, X.; Gong, Y.; Niu, L.; Li, C. Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance. Nanomaterials 2025, 15, 521. [Google Scholar] [CrossRef]
- Levinas, R.; Podlaha, E.; Tsyntsaru, N.; Cesiulis, H. Composites Based on Electrodeposited WO3 and TiO2 Nanoparticles for Photoelectrochemical Water Splitting. Materials 2024, 17, 4914. [Google Scholar] [CrossRef]
- Palma, F.; Baldelli, G.; Schiavano, G.F.; Amagliani, G.; Aliano, M.P.; Brandi, G. Use of Eco-Friendly UV-C LEDs for Indoor Environment Sanitization: A Narrative Review. Atmosphere 2022, 13, 1411. [Google Scholar] [CrossRef]
- Mach, V.; Dvorak, L.; Dvorakova, H.; Mikulikova, L.; Kolarova, K.; Černy, L. Role of Lamp Type in Conventional Batch and Micro-Photoreactor for Photocatalytic Hydrogen Production. Front. Chem. Sec. Photocatal. Photochem. 2023, 11, 1271410. [Google Scholar] [CrossRef]
- Shephered, M.T.; Newman, J.P.; Kubheka, O. Effect of TiO2 Phase on the Photocatalytic Degradation of Methylene Blue Dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Umapathy, M.J.; Magesan, P.; Zhang, X. Biomaterial (Garlic and Chitosan)-Doped WO3-TiO2 Hybrid Nanocomposites: Their Solar Light Photocatalytic and Antibacterial Activities. ACS Omega 2020, 5, 31673–31683. [Google Scholar] [CrossRef]
- Odhiambo, V.O.; Ongarbayeva, A.; Kéri, O.; Simon, L.; Szilágyi, I.M. Synthesis of TiO2/WO3 Composite Nanofibers by a Water-Based Electrospinning Process and Their Application in Photocatalysis. Nanomaterials 2020, 10, 882. [Google Scholar] [CrossRef]
- Dávidné, N.; Tamás, F.; Eszter, D.; Ágnes, S.; Krisztina, L.; Imre, M.S. Photocatalytic WO3/TiO2 Nanowires: WO3 Polymorphs Influencing the Atomic Layer Deposition of TiO2. RSC Adv. 2016, 6, 95369–95377. [Google Scholar] [CrossRef]
- Suvarna, R.; Bathe, P.S.P. Electrochemical Behavior of TiO2 Nanoparticle Doped WO3 Thin Films. Materials 2014, 5, 642069. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, G.; Hu, X.; Wang, W.; Du, Z.; Wang, Y.; Gong, X.Z.; Tan, H.; Guo, F.; Tang, J. Hollow Flower-Like WO3@TiO2 Heterojunction Microspheres for the Photocatalytic Degradation of Rhodamine B and Tetracycline. RSC Adv. 2025, 15, 12629–12644. [Google Scholar] [CrossRef] [PubMed]
- Boga, B.; Székely, I.; Pap, Z.; Baia, L.; Baia, M. Detailed Spectroscopic and Structural Analysis of TiO2/WO3 Composite Semiconductors. J. Spectrosc. 2018, 1–7, 6260458. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Hsu, T.-Y.; Lee, G.-J.; Chen, C.-Y.; Chang, Y.-C.; Chen, J.-H.; Wu, J.J. Hydrothermal Synthesis of Nanocomposites Combining Tungsten Trioxide and Zinc Oxide Nanosheet Arrays for Improved Photocatalytic Degradation of Organic Dye. Nanomaterials 2025, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, C.; Yang, R.; Cheng, S.; Sang, X.; Zhang, M.; Zhang, J.; Wang, Z.; Li, Z. Efficient and Stable Degradation of Triazophos Pesticide by TiO2/WO3 Nanocomposites with S-Scheme Heterojunctions and Oxygen Defects. Catalysts 2023, 13, 1136. [Google Scholar] [CrossRef]
- Lee, W.H.; Lai, C.W.; Hamid, S.B.A. One-Step Formation of WO3-Loaded TiO2 Nanotubes Composite Film for High Photocatalytic Performance. Materials 2015, 8, 2139–2153. [Google Scholar] [CrossRef]
- Paiu, M.; Lutic, D.; Favier, L.; Gavrilescu, M. Heterogeneous Photocatalysis for Advanced Water Treatment: Materials, Mechanisms, Reactor Configurations, and Emerging Applications. Appl. Sci. 2025, 15, 5681. [Google Scholar] [CrossRef]
- Sharma, M.; Mishra, M.K.; Patel, S.; Kumar, R.; Dubey, K. Visible-Light-Driven Photocatalytic Degradation of Tetracycline Using Heterostructured Cu2O–TiO2 Nanotubes, Kinetics, and Toxicity Evaluation of Degraded Products on Cell Lines. ACS Omega 2022, 7, 33572–33586. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiloufi, M.; Schnabel, T.; Springer, C.; Mehling, S.; Wolfram, A.; Touati, F.; Kouass, S. Hydrothermal Versus Physical Mixing: Superior Photocatalytic Activity of TiO2/WO3 Nanocomposites for Water Treatment Applications. Environments 2025, 12, 359. https://doi.org/10.3390/environments12100359
Ghiloufi M, Schnabel T, Springer C, Mehling S, Wolfram A, Touati F, Kouass S. Hydrothermal Versus Physical Mixing: Superior Photocatalytic Activity of TiO2/WO3 Nanocomposites for Water Treatment Applications. Environments. 2025; 12(10):359. https://doi.org/10.3390/environments12100359
Chicago/Turabian StyleGhiloufi, Mabrouka, Tobias Schnabel, Christian Springer, Simon Mehling, Axel Wolfram, Fathi Touati, and Salah Kouass. 2025. "Hydrothermal Versus Physical Mixing: Superior Photocatalytic Activity of TiO2/WO3 Nanocomposites for Water Treatment Applications" Environments 12, no. 10: 359. https://doi.org/10.3390/environments12100359
APA StyleGhiloufi, M., Schnabel, T., Springer, C., Mehling, S., Wolfram, A., Touati, F., & Kouass, S. (2025). Hydrothermal Versus Physical Mixing: Superior Photocatalytic Activity of TiO2/WO3 Nanocomposites for Water Treatment Applications. Environments, 12(10), 359. https://doi.org/10.3390/environments12100359










