Electric Field Effects on Microbial Cell Properties: Implications for Detection and Control in Wastewater Systems
Abstract
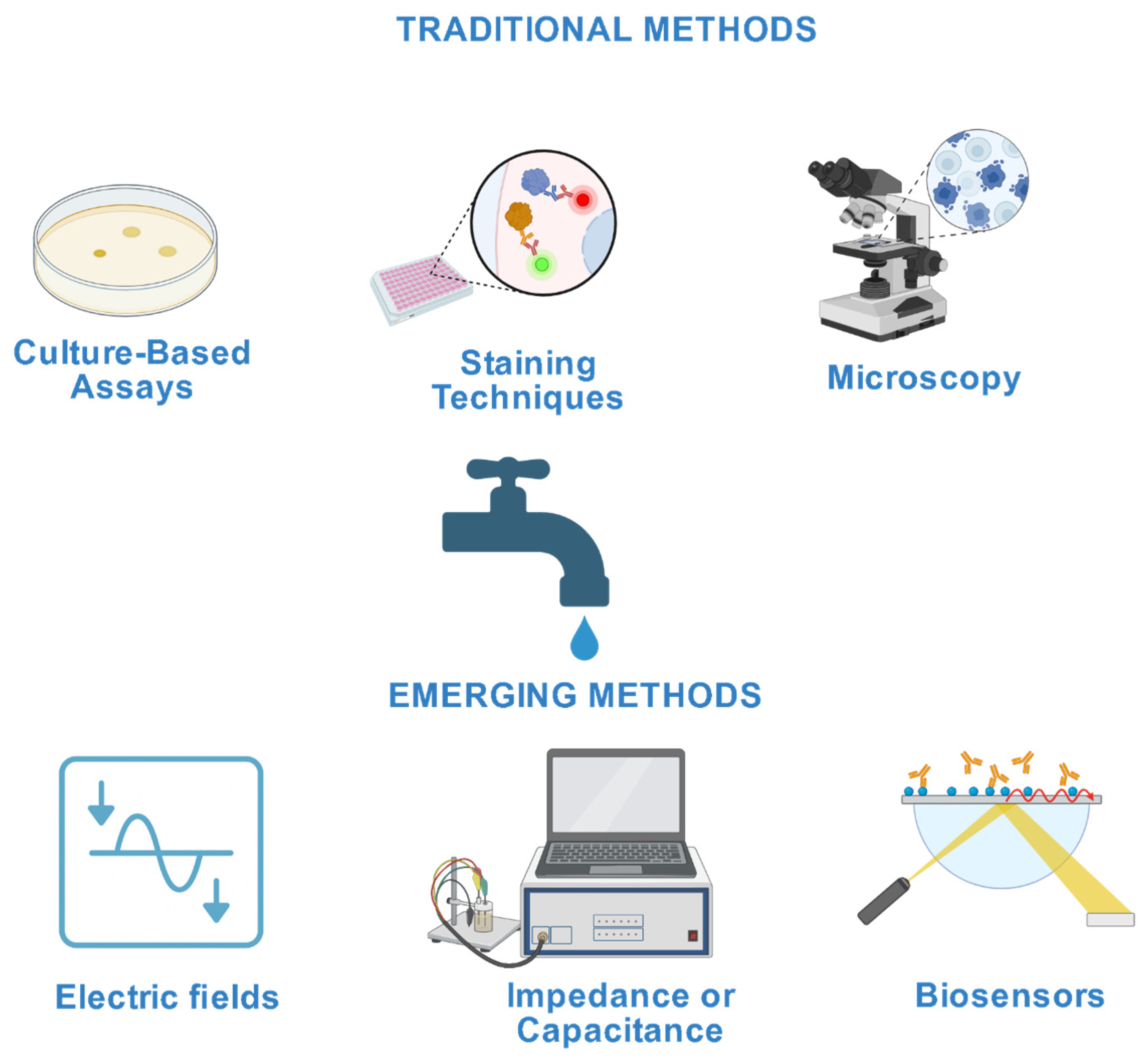
1. Introduction
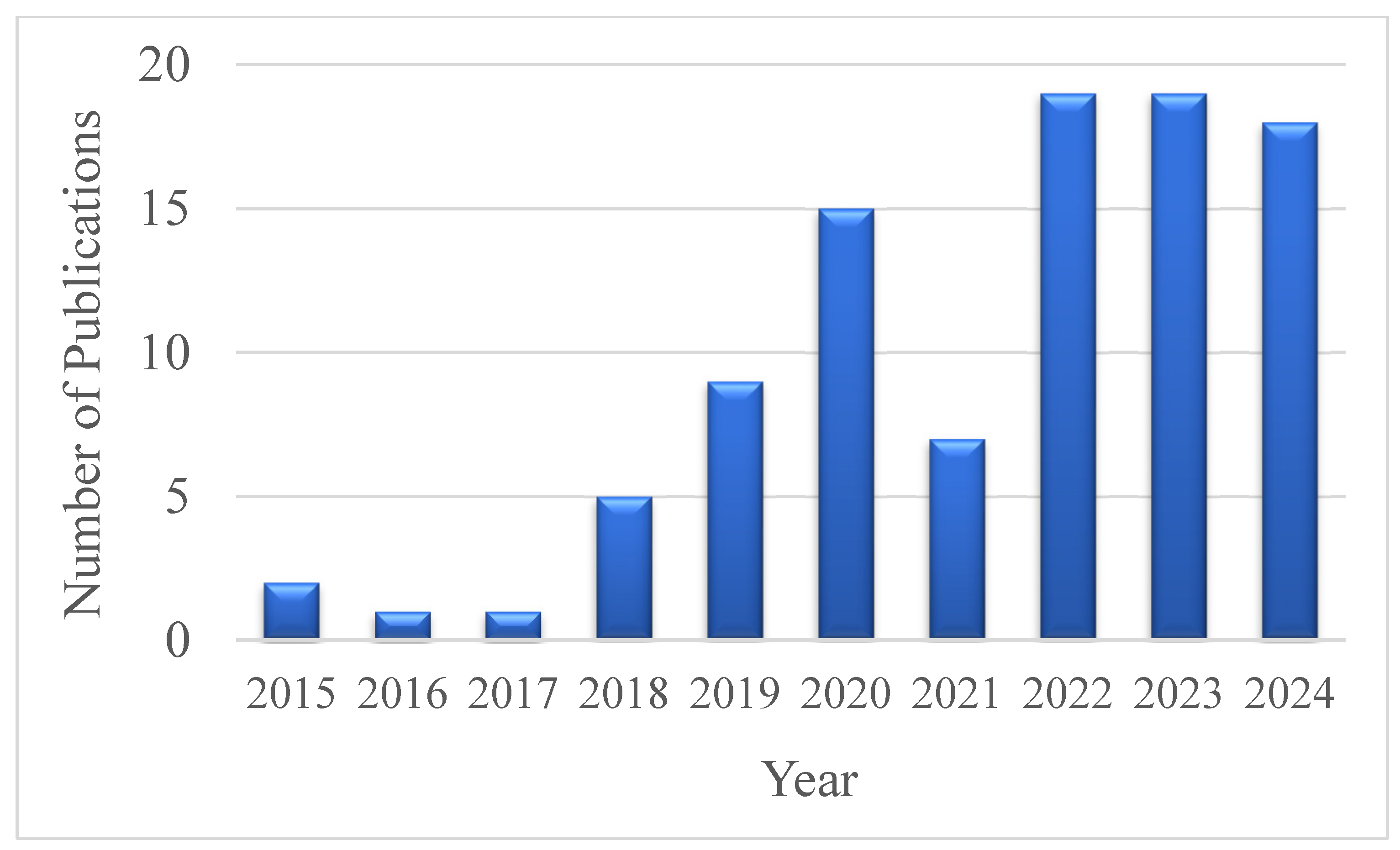
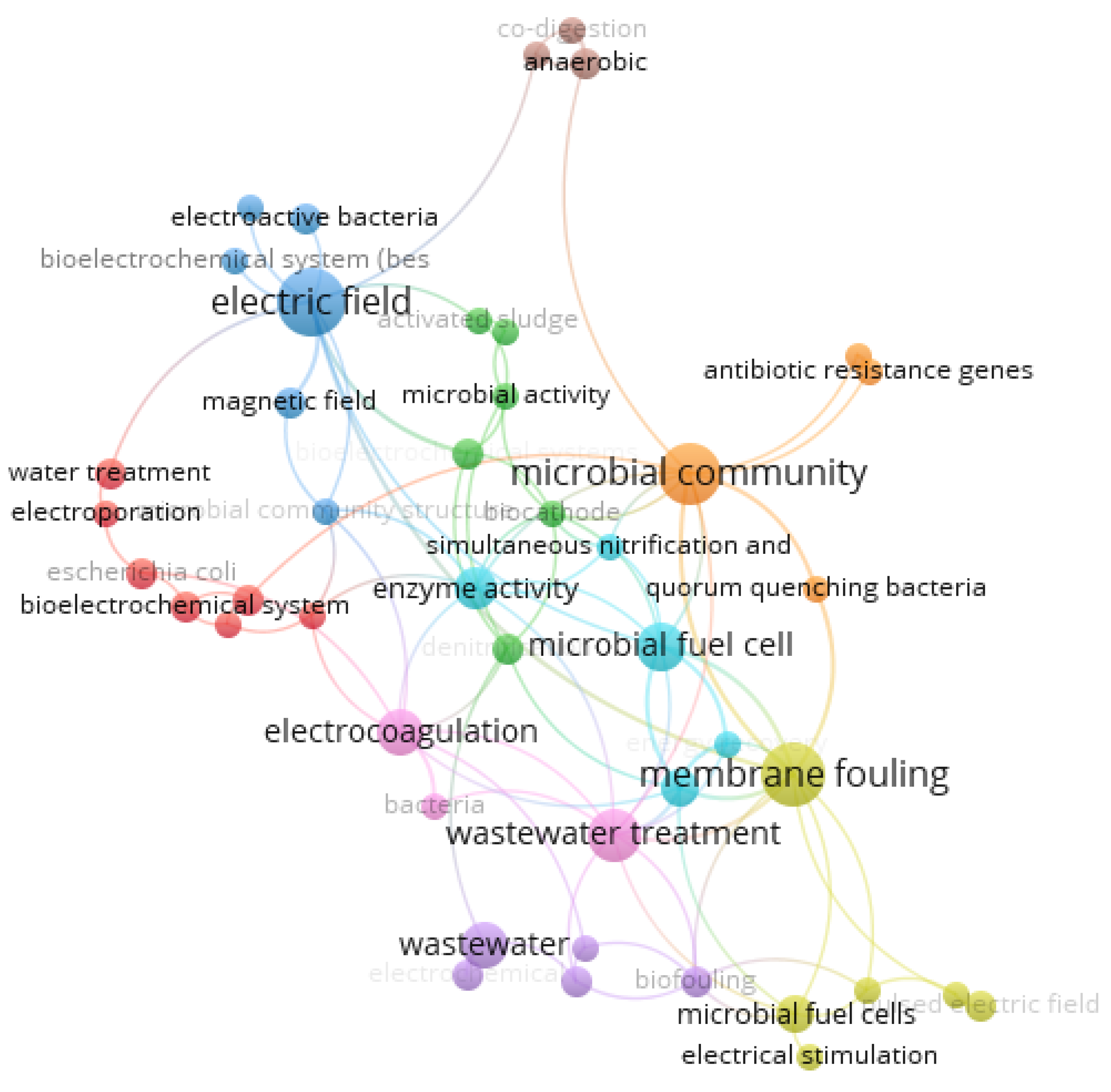
Research Trends and Bibliometric Insights
2. Basics of Electric Fields and Microbial Systems
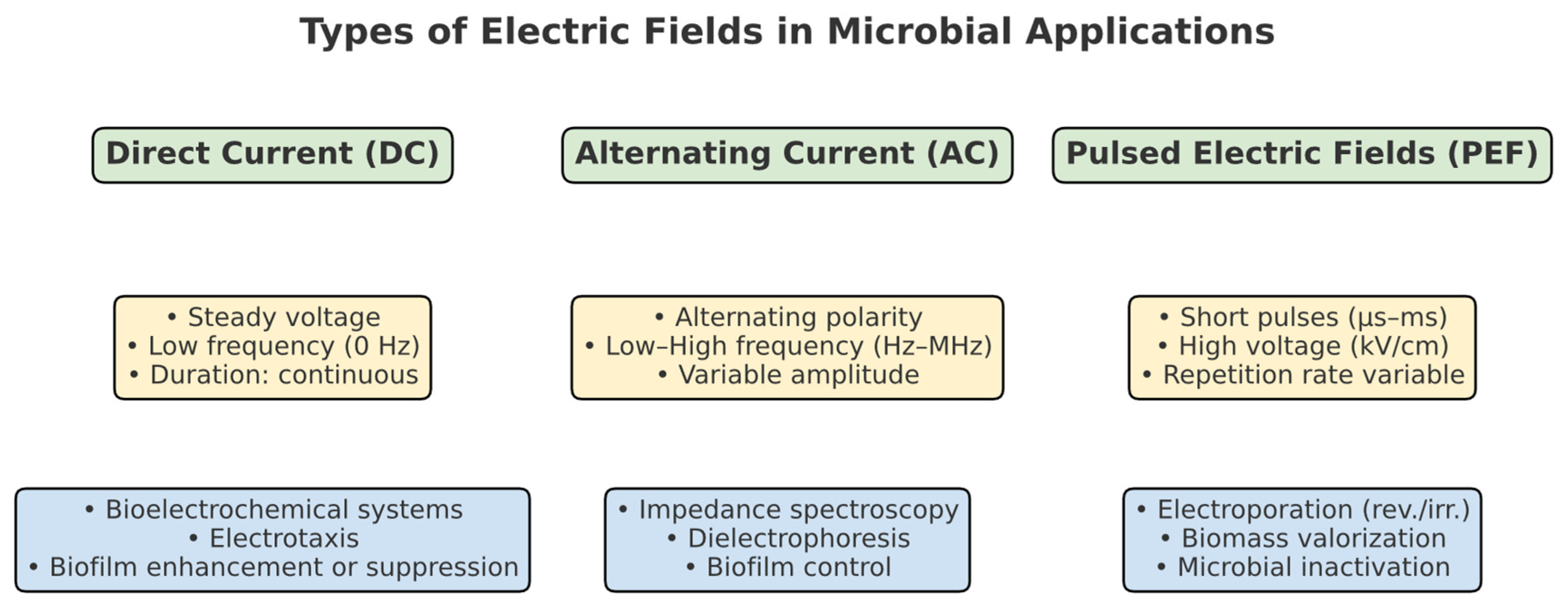
2.1. Types of Electric Fields
- (a)
- Direct Current (DC) Electric Fields
- (b)
- Alternating Current (AC) Electric Fields
- (c)
- Pulsed Electric Fields (PEFs)
2.2. Microbial Structures Relevant to Electrical Interaction
2.2.1. Cell Membrane and Cell Wall
2.2.2. Cytoplasm
2.2.3. Spores and Dormant Forms
2.2.4. Surface Structures (e.g., Pili, Flagella, Biofilms)
3. Membrane-Level Bioelectrical and Dielectric Properties
3.1. Membrane Thickness
3.2. Membrane Potential (ΔΨ)
3.3. Membrane Electrical Conductivity
3.4. Dielectric Permeability of the Membrane
4. Cytoplasmic Bioelectrical and Dielectric Properties
4.1. Cytoplasmic Conductivity
4.2. Dielectric Permeability of the Cytoplasm
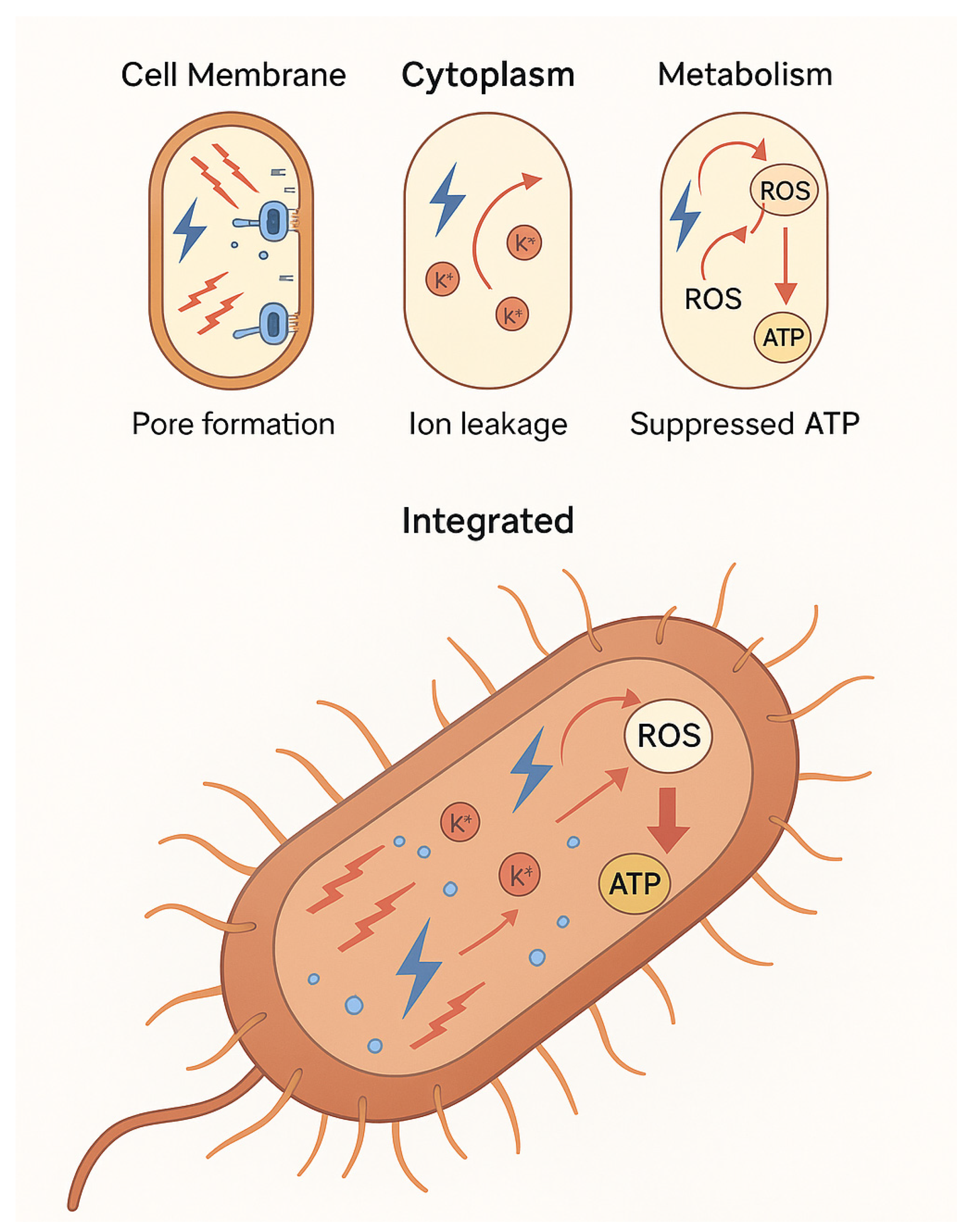
5. Electric Field Effects on Microbial Physiology
5.1. Vegetative Cells
5.2. Spores
5.3. Broader Metabolic Effects
5.4. Electrical Impedance Spectroscopy (EIS)
5.5. Dielectrophoresis
5.6. Field-Assisted Biosensors
6. Challenges and Research Opportunities
7. Outlook: Electric Field-Based Wastewater Monitoring and Control
7.1. Smart Biosensing: Integrating AI with EF-Based Readouts
7.2. Targeted Microbial Control in Decentralized Wastewater Systems
7.3. Hybrid Biotechnological–Electrical Approaches
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| ATP | Adenosine Triphosphate |
| B. subtilis | Bacillus subtilis |
| C. botulinum | Clostridium botulinum |
| C. sporogenes | Clostridium sporogenes |
| DC | Direct Current |
| DEP | Dielectrophoresis |
| DFE | Differential Field Excitation |
| DNA | Deoxyribonucleic Acid |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| EIS | Electrical Impedance Spectroscopy |
| EPS | Extracellular Polymeric Substances |
| GHz | Gigahertz |
| HPT | High-Pressure Thermal |
| Hz | Hertz |
| kHz | Kilohertz |
| LPS | Lipopolysaccharide |
| MHz | Megahertz |
| mM | Millimolar |
| nm | Nanometer |
| pH | Potential of Hydrogen |
| PEF | Pulsed Electric Field |
| PHB | Polyhydroxybutyrate |
| ROS | Reactive Oxygen Species |
| SFE | Specific Field Energy |
| SHE | Standard Hydrogen Electrode |
| TDDS | Time-Domain Dielectric Spectroscopy |
| UV-VIS-NIR | Ultraviolet–Visible–Near-Infrared |
| WHO | World Health Organization |
| µm | Micrometer |
| µA | Microampere |
References
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater: Significance and Implications for Treatment and Disinfection Processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef]
- López, A.; Rodríguez-Chueca, J.; Mosteo, R.; Gómez, J.; Rubio, E.; Goñi, P.; Ormad, M.P. How Does Urban Wastewater Treatment Affect the Microbial Quality of Treated Wastewater? Process Saf. Environ. Prot. 2019, 130, 22–30. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Wu, J.; Coin, L.; O’Brien, J.; Hai, F.; Jiang, G. Molecular Methods for Pathogenic Bacteria Detection and Recent Advances in Wastewater Analysis. Water 2021, 13, 3551. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The Importance of the Viable but Non-Culturable State in Human Bacterial Pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Saaed, F.M.A.; Ongerth, J.E. Giardia and Cryptosporidium in Children with Diarrhea, Kufra, Libya, a North African Migration Route City. Int. J. Hyg. Environ. Health 2019, 222, 840–846. [Google Scholar] [CrossRef]
- Sanseverino, I.; Gómez, L.; Navarro, A.; Cappelli, F.; Niegowska, M.; Lahm, A.; Barbiere, M.; Porcel-Rodríguez, E.; Valsecchi, S.; Pedraccini, R.; et al. Holistic Approach to Chemical and Microbiological Quality of Aquatic Ecosystems Impacted by Wastewater Effluent Discharges. Sci. Total Environ. 2022, 835, 155388. [Google Scholar] [CrossRef] [PubMed]
- Elfadadny, A.; Ragab, R.F.; AlHarbi, M.; Badshah, F.; Ibáñez-Arancibia, E.; Farag, A.; Hendawy, A.O.; De Los Ríos-Escalante, P.R.; Aboubakr, M.; Zakai, S.A.; et al. Antimicrobial Resistance of Pseudomonas aeruginosa: Navigating Clinical Impacts, Current Resistance Trends, and Innovations in Breaking Therapies. Front. Microbiol. 2024, 15, 1374466. [Google Scholar] [CrossRef]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide Regulations and Guidelines for Agricultural Water Reuse: A Critical Review. Water 2020, 12, 971. [Google Scholar] [CrossRef]
- Zamorska, J.; Karwowska, E.; Przystaś, W. Assessment of Microbiological Quality of Water Using Culture Methods, Flow Cytometry and Luminometry. Water 2023, 15, 4077. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Bernárdez-Rodas, N.; Rosales, E.; Pazos, M.; González-Romero, E.; Sanromán, M.Á. Biosensor Technologies for Water Quality: Detection of Emerging Contaminants and Pathogens. Biosensors 2025, 15, 189. [Google Scholar] [CrossRef]
- Ahmad, S.; Lohiya, S.; Taksande, A.; Meshram, R.J.; Varma, A.; Vagha, K. A Comprehensive Review of Innovative Paradigms in Microbial Detection and Antimicrobial Resistance: Beyond Traditional Cultural Methods. Cureus 2024, 16, e61476. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Gonçalves, J.; Pequeno, J.; Diaz, I.; Kržišnik, D.; Žigon, J.; Koritnik, T. Killing Two Crises with One Spark: Cold Plasma for Antimicrobial Resistance Mitigation and Wastewater Reuse. Water 2025, 17, 1218. [Google Scholar] [CrossRef]
- González, Y.; Gómez, G.; Moeller-Chávez, G.E.; Vidal, G. UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms. Sustainability 2023, 15, 11262. [Google Scholar] [CrossRef]
- Badarne-Abbasi, S.; Armon, R.; Nasser, A. Reduction of Infectious Cryptosporidium and Microbial Indicators in Wastewater Effluents by Disinfection with UV Irradiation or Chlorine. J. Water Resour. Prot. 2022, 14, 407–418. [Google Scholar] [CrossRef]
- Delgado-Viscogliosi, P.; Solignac, L.; Delattre, J.M. Viability PCR, a Culture-Independent Method for Rapid and Selective Quantification of Viable Legionella pneumophila Cells in Environmental Water Samples. Appl. Environ. Microbiol. 2009, 75, 3502–3512. [Google Scholar] [CrossRef] [PubMed]
- Jütte, M.; Abdighahroudi, M.S.; Waldminghaus, T.; Lackner, S.; Lutze, H.V. Bacterial Inactivation Processes in Water Disinfection—Mechanistic Aspects of Primary and Secondary Oxidants—A Critical Review. Water Res. 2023, 231, 119626. [Google Scholar] [CrossRef]
- Madadelahi, M.; Agarwal, R.; Martinez-Chapa, S.O.; Madou, M.J. A Roadmap to High-Speed Polymerase Chain Reaction (PCR): COVID-19 as a Technology Accelerator. Biosens. Bioelectron. 2024, 246, 115830. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, F.E.; Singh, G.; Reddy, P.; Bux, F.; Stenström, T.A. Efficiency of Chlorine and UV in the Inactivation of Cryptosporidium and Giardia in Wastewater. PLoS ONE 2019, 14, e0216040. [Google Scholar] [CrossRef]
- Pasciucco, F.; Pecorini, I.; Iannelli, R. Planning the Centralization Level in Wastewater Collection and Treatment: A Review of Assessment Methods. J. Clean. Prod. 2022, 375, 134092. [Google Scholar] [CrossRef]
- Olvera, D.; Monaghan, M.G. Electroactive Material-Based Biosensors for Detection and Drug Delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef]
- Bartha, C.; Jipa, M.; Caramitu, A.-R.; Voina, A.; Tókos, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Behavior of Microorganisms from Wastewater Treatments in Extremely Low-Frequency Electric Field. Biointerface Res. Appl. Chem. 2022, 12, 5071–5080. [Google Scholar] [CrossRef]
- Emanuel, E.; Dubrovin, I.; Pogreb, R.; Pinhasi, G.A.; Cahan, R. Resuscitation of Pulsed Electric Field-Treated Staphylococcus aureus and Pseudomonas putida in a Rich Nutrient Medium. Foods 2021, 10, 660. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality Aspects and Safety of Pulsed Electric Field (PEF) Processing on Dairy Products: A Comprehensive Review. Food Rev. Int. 2020, 38 (Suppl. S1), 96–117. [Google Scholar] [CrossRef]
- Kotnik, T. Transmembrane Voltage Induced by Applied Electric Fields. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Rockenbach, A.; Sudarsan, S.; Berens, J.; Kosubek, M.; Lazar, J.; Demling, P.; Hanke, R.; Mennicken, P.; Ebert, B.E.; Blank, L.M.; et al. Microfluidic Irreversible Electroporation—A Versatile Tool to Extract Intracellular Contents of Bacteria and Yeast. Metabolites 2019, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Pluhackova, K.; Horner, A. Native-Like Membrane Models of E. coli Polar Lipid Extract Shed Light on the Importance of Lipid Composition Complexity. BMC Biol. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Lyon, R.; Jones, R.A.; Shropshire, H.; Aberdeen, I.; Scanlan, D.J.; Millard, A. Membrane Lipid Renovation in Pseudomonas aeruginosa—Implications for Phage Therapy? Environ. Microbiol. 2022, 24, 4533–4546. [Google Scholar] [CrossRef] [PubMed]
- Haberl Meglic, S.; Marolt, T.; Miklavcic, D. Protein Extraction by Means of Electroporation from E. coli with Preserved Viability. J. Membr. Biol. 2015, 248, 893–901. [Google Scholar] [CrossRef]
- Kunlasubpreedee, P.; Tobino, T.; Nakajima, F. Influence of High-Frequency, Low-Voltage Alternating Electric Fields on Biofilm Development Processes of Escherichia coli and Pseudomonas aeruginosa. Water 2023, 15, 3055. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Haberl Meglič, S.; Peterka, M.; Miklavčič, D. Electroporation-Based Applications in Biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Rosenzweig, Z.; Garcia, J.; Thompson, G.L.; Perez, L.J. Inactivation of Bacteria Using Synergistic Hydrogen Peroxide with Split-Dose Nanosecond Pulsed Electric Field Exposures. PLoS ONE 2024, 19, e0311232. [Google Scholar] [CrossRef]
- Turcan, I.; Caras, I.; Schreiner, T.G.; Tucureanu, C.; Salageanu, A.; Vasile, V.; Avram, M.; Tincu, B.; Olariu, M.A. Dielectrophoretic and Electrical Impedance Differentiation of Cancerous Cells Based on Biophysical Phenotype. Biosensors 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Riccò, B. Electrical Impedance Spectroscopy (EIS) for Biological Analysis and Food Characterization: A Review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef]
- Gutsul, O.; Rutherford, D.; Barinkova, M.; Slobodyan, V.; Rezek, B. The Detection of E. coli and S. aureus on Sensors without Immobilization by Using Impedance Spectroscopy. Eng. Proc. 2023, 58, 79. [Google Scholar] [CrossRef]
- Perianes-Rodriguez, A.; Waltman, L.; van Eck, N.J. Constructing Bibliometric Networks: A Comparison between Full and Fractional Counting. J. Informetr. 2016, 10, 1178–1195. [Google Scholar] [CrossRef]
- Beretta, G.; Mastorgio, A.F.; Pedrali, L.; Saponaro, S.; Sezenna, E. The Effects of Electric, Magnetic and Electromagnetic Fields on Microorganisms in the Perspective of Bioremediation. Rev. Environ. Sci. Biotechnol. 2019, 18, 29–75. [Google Scholar] [CrossRef]
- Chong, P.; Erable, B.; Bergel, A. How Bacteria Use Electric Fields to Reach Surfaces. Biofilm 2021, 3, 100048. [Google Scholar] [CrossRef]
- Hirsch, L.O.; Gandu, B.; Chiliveru, A.; Dubrovin, I.A.; Jukanti, A.; Schechter, A.; Cahan, R. Hydrogen Production in Microbial Electrolysis Cells Using an Alginate Hydrogel Bioanode Encapsulated with a Filter Bag. Polymers 2024, 16, 1996. [Google Scholar] [CrossRef]
- Pethig, R. Review Article—Dielectrophoresis: Status of the Theory, Technology, and Applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [PubMed]
- Asami, K.; Hanai, T.; Koizumi, N. Dielectric Analysis of Escherichia coli Suspensions in the Light of the Theory of Interfacial Polarization. Biophys. J. 1980, 31, 215–228. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive Review on Pulsed Electric Field in Food Preservation: Gaps in Current Studies for Potential Future Research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Pulse Electric Field Technology for Wastewater and Biomass Residues’ Improved Valorization. Processes 2021, 9, 736. [Google Scholar] [CrossRef]
- Tongdonyod, S.; Yossanun, Y.; Kunma, N.; Klanreung, A.; Kammeekum, P.; Klangpetch, W. Inactivation of Escherichia coli in Coconut Water Using Pulsed Electric Field. In Proceedings of the 25th Food Innovation Asia Conference, Bangkok, Thailand, 15–17 June 2023. [Google Scholar]
- Wang, F.; Li, L.; Li, X.; Hu, X.; Zhang, B. Pulsed Electric Field Promotes the Growth Metabolism of Aerobic Denitrifying Bacteria Pseudomonas putida W207-14 by Improving Cell Membrane Permeability. Environ. Technol. 2023, 44, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Z.; Cuvillier, L.; Dobhal, G.; Goreham, R.V. Electroporation of Outer Membrane Vesicles Derived from Pseudomonas aeruginosa with Gold Nanoparticles. SN Appl. Sci. 2019, 1, 1600. [Google Scholar] [CrossRef]
- Cifra, M.; Fields, J.Z.; Farhadi, A. Electromagnetic Cellular Interactions. Prog. Biophys. Mol. Biol. 2011, 105, 223–246. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; The Lipid Bilayer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26871 (accessed on 5 April 2025).
- El-Enshasy, H.A. Filamentous Fungal Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 9; pp. 225–261. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J. Detection of Fungal and Bacterial Carbohydrates: Do the Similar Structures of Chitin and Peptidoglycan Play a Role in Immune Dysfunction? PLoS Pathog. 2018, 14, e1007271. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar] [CrossRef]
- Beeby, M.; Gumbart, J.C.; Roux, B.; Jensen, G.J. Gram-Positive Peptidoglycan Structure. Mol. Microbiol. 2013, 88, 664–672. [Google Scholar] [CrossRef]
- Schwan, H.P.; Takashima, S. Dielectric Behavior of Biological Cells and Membranes (Commemoration Issue Dedicated to Professor Tetsuya Hanai on the Occasion of His Retirement). Bull. Inst. Chem. Res. Kyoto Univ. 1991, 69, 459–475. [Google Scholar]
- Mosgaard, L.D.; Zecchi, K.A.; Heimburg, T. Mechano-capacitive Properties of Polarized Membranes. Soft Matter 2015, 11, 7899–7910. [Google Scholar] [CrossRef]
- BioNumbers—The Database of Useful Biological Numbers. Available online: http://www.bionumbers.hms.harvard.edu/ (accessed on 15 July 2025).
- Sundararaj, S.; Guo, A.; Habibi-Nazhad, B.; Rouani, M.; Stothard, P.; Ellison, M.; Wishart, D.S. The CyberCell Database (CCDB): A Comprehensive, Self-Updating, Relational Database to Coordinate and Facilitate In Silico Modeling of Escherichia coli. Nucleic Acids Res. 2004, 32, D293–D295. [Google Scholar] [CrossRef]
- Theillet, F.X.; Binolfi, A.; Frembgen-Kesner, T.; Hingorani, K.; Sarkar, M.; Kyne, C.; Li, C.; Crowley, P.B.; Gierasch, L.; Pielak, G.J.; et al. Physicochemical Properties of Cells and Their Effects on Intrinsically Disordered Proteins (IDPs). Chem. Rev. 2014, 114, 6661–6714. [Google Scholar] [CrossRef]
- Romero-González, L.E.; Montelongo-Martínez, L.F.; González-Valdez, A.; Quiroz-Morales, S.E.; Cocotl-Yañez, M.; Franco-Cendejas, R.; Soberón-Chávez, G.; Pardo-López, L.; Bustamante, V.H.; Drlica, K. Pseudomonas aeruginosa Isolates from Water Samples of the Gulf of Mexico Show Similar Virulence Properties but Different Antibiotic Susceptibility Profiles than Clinical Isolates. Int. J. Microbiol. 2024, 2024, 6959403. [Google Scholar] [CrossRef] [PubMed]
- Tolouei Gilani, J.; Goudarztalejerdi, A.; Yavari, M.; Nouri Kalourazi, M. Isolation and Identification of Aeromonas hydrophila from Cyprinidae Suspected with Hemorrhagic Septicemia in Pools of Warm Water Fishes in Gilan Province. Int. J. Nutr. Sci. 2021, 6, 52–58. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, Function and Regulation of Pseudomonas aeruginosa Porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Maeda, Y.; Fujita, T.; Sugiura, Y.; Koga, S. Physical Properties of Water in Spores of Bacillus megaterium. J. Gen. Appl. Microbiol. 1968, 14, 217–226. Available online: https://www.jstage.jst.go.jp/article/jgam1955/14/3/14_3_217/_pdf (accessed on 15 July 2025). [CrossRef]
- Wu, W.J.; Chang, J. Inactivation of Vegetative Cells, Germinated Spores, and Dormant Spores of Bacillus atrophaeus by Pulsed Electric Field with Fixed Energy Input. J. Food Process Eng. 2022, 45, e13959. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Q.H.; Sastry, S.K. High Voltage Pulsed Electric Field Treatment Chambers for the Preservation of Liquid Food Products. Patent EP1028635B1, 25 November 1997. [Google Scholar]
- Sunde, E.P.; Setlow, P.; Hederstedt, L.; Halle, B. The Physical State of Water in Bacterial Spores. Proc. Natl. Acad. Sci. USA 2009, 106, 19334–19339. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.H.; Ali, M.I.; Korza, G.; Setlow, P.; Sastry, S. Accelerated Inactivation of Bacterial Spores by Interaction of Electric Fields with Key Spore Components. In Proceedings of the 14th International Conference on Engineering and Food (ICEF14), Nantes, France, 20–23 June 2023; Available online: https://www.icef14.com/abstracts/export/export-abstract-pdf-A5897SS.pdf (accessed on 15 July 2025).
- Lenz, C.A.; Reineke, K.; Knorr, D.; Vogel, R.F. High-Pressure Thermal Inactivation of Clostridium botulinum Type E Endospores—Kinetic Modeling and Mechanistic Insights. Front. Microbiol. 2015, 6, 652. [Google Scholar] [CrossRef]
- Talà, L.; Fineberg, A.; Kukura, P.; Persat, A. Pseudomonas aeruginosa Orchestrates Twitching Motility by Sequential Control of Type IV Pili Movements. Nat. Microbiol. 2019, 4, 774–780. [Google Scholar] [CrossRef]
- David, A.; Tahrioui, A.; Tareau, A.-S.; Forge, A.; Gonzalez, M.; Bouffartigues, E.; Lesouhaitier, O.; Chevalier, S. Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics 2024, 13, 688. [Google Scholar] [CrossRef]
- Charles-Orszag, A.; van Wolferen, M.; Lord, S.J.; Albers, S.-V.; Mullins, R.D. Adhesion Pilus Retraction Powers Twitching Motility in the Thermoacidophilic Crenarchaeon Sulfolobus acidocaldarius. Nat. Commun. 2024, 15, 5051. [Google Scholar] [CrossRef]
- Gall, I.; Herzberg, M.; Oren, Y. The effect of electric fields on bacterial attachment to conductive surfaces. Soft Matter 2013, 9, 2443–2452. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Czerwińska-Główka, D.; Krukiewicz, K. A journey in the complex interactions between electrochemistry and bacteriology: From electroactivity to electromodulation of bacterial biofilms. Bioelectrochemistry 2020, 131, 107401. [Google Scholar] [CrossRef] [PubMed]
- Matuła, K.; Richter, Ł.; Janczuk-Richter, M.; Nogala, W.; Grzeszkowiak, M.; Peplińska, B.; Jurga, S.; Wyroba, E.; Suski, S.; Bilski, H.; et al. Phenotypic Plasticity of Escherichia coli upon Exposure to Physical Stress Induced by ZnO Nanorods. Sci. Rep. 2019, 9, 8575. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.L.; Beveridge, T.J. Structural Differentiation of the Bacillus subtilis 168 Cell Wall. J. Bacteriol. 1994, 176, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.L.; Stinear, T.P.; Monk, I.R. Barriers to Genetic Manipulation of Enterococci: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2022, 46, fuac036. [Google Scholar] [CrossRef]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan Equation and Transmembrane Potential Induced by Alternating Electric Field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U. Electrical Breakdown, Electropermeabilization and Electrofusion. Rev. Physiol. Biochem. Pharmacol. 1986, 105, 176–256. [Google Scholar]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Zilberstein, D.; Schuldiner, S. pH Homeostasis in Bacteria. Biochim. Biophys. Acta Rev. Biomembr. 1981, 650, 151–166. [Google Scholar] [CrossRef]
- Krulwich, T.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef]
- Poole, R.J. Energy Coupling for Membrane Transport. Annu. Rev. Plant Physiol. 1978, 29, 437–460. [Google Scholar] [CrossRef]
- Miller, J.B.; Koshland, D.E., Jr. Sensory Electrophysiology of Bacteria: Relationship of the Membrane Potential to Motility and Chemotaxis in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1977, 74, 4752–4756. [Google Scholar] [CrossRef] [PubMed]
- Damper, P.D.; Epstein, W. Role of the Membrane Potential in Bacterial Resistance to Aminoglycoside Antibiotics. Antimicrob. Agents Chemother. 1981, 20, 803–808. [Google Scholar] [CrossRef]
- Bot, C.T.; Prodan, C. Quantifying the Membrane Potential during E. coli Growth Stages. Biophys. Chem. 2010, 146, 133–137. [Google Scholar] [CrossRef]
- Stratford, J.P.; Edwards, C.L.A.; Ghanshyam, M.J.; Malyshev, D.; Delise, M.A.; Hayashi, Y.; Asally, M. Electrically Induced Bacterial Membrane-Potential Dynamics Correspond to Cellular Proliferation Capacity. Proc. Natl. Acad. Sci. USA 2019, 116, 9552–9557. [Google Scholar] [CrossRef]
- Barauskaitė, N.; Visockis, M.; Bubnytė, D.; Meldaikytė, I.; Gelažunaitė, S.; Šarkinas, A.; Rafanavičius, A.; Ruzgys, P. Using Pulse Electric Fields (PEF) for Selective Inactivation of Coliform Bacteria. Biologija 2022, 68, 240–247. [Google Scholar] [CrossRef]
- Chen, C.; Smye, S.W.; Robinson, M.P.; Evans, J.A. Membrane Electroporation Theories: A Review. Med. Biol. Eng. Comput. 2006, 44, 5–14. [Google Scholar] [CrossRef]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef]
- Pucihar, G.; Mir, L.M.; Miklavčič, D. The Effect of Pulse Repetition Frequency on the Uptake into Electropermeabilized Cells in vitro with Possible Applications in Electrochemotherapy. Bioelectrochemistry 2002, 57, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane Thickness, Lipid Phase and Sterol Type Are Determining Factors in the Permeability of Membranes to Small Solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Pethig, R. Dielectrophoresis: Theory, Methodology and Biological Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Carstensen, E.L.; Cox, H.A., Jr.; Mercer, W.B.; Natale, L.A. Passive electrical properties of microorganisms. I. Conductivity of Escherichia coli and Micrococcus lysodeikticus. Biophys. J. 1965, 5, 289–300. [Google Scholar] [CrossRef]
- González-Cuevas, J.A.; Argüello, R.; Florentin, M.; André, F.M.; Mir, L.M. Experimental and Theoretical Brownian Dynamics Analysis of Ion Transport during Cellular Electroporation of E. coli Bacteria. Ann. Biomed. Eng. 2024, 52, 103–123. [Google Scholar] [CrossRef]
- Benz, R.; Hancock, R.E. Properties of the Large Ion-Permeable Pores Formed from Protein F of Pseudomonas aeruginosa in Lipid Bilayer Membranes. Biochim. Biophys. Acta Biomembr. 1981, 646, 298–308. [Google Scholar] [CrossRef]
- Ameer, S.; Ibrahim, H.; Yaseen, M.U.; Kulsoom, F.; Cinti, S.; Sher, M. Electrochemical Impedance Spectroscopy-Based Sensing of Biofilms: A Comprehensive Review. Biosensors 2023, 13, 777. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Burnet, J.B.; Demeter, K.; Dorner, S.; Farnleitner, A.H.; Hammes, F.; Pinto, A.J.; Prest, E.I.; Prévost, M.; Stott, R.; van Bel, N. Automation of on-site microbial water quality monitoring from source to tap: Challenges and perspectives. Water Res. 2025, 274, 123121. [Google Scholar] [CrossRef]
- Pudasaini, S.; Perera, A.T.K.; Ahmed, S.S.U.; Chong, Y.B.; Ng, S.H.; Yang, C. An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation. Inventions 2020, 5, 2. [Google Scholar] [CrossRef]
- El-Hag, A.H.; Jayaram, S.H.; Rodriguez Gonzalez, O.; Griffiths, M.W. The Influence of Size and Shape of Microorganism on Pulsed Electric Field Inactivation. IEEE Trans. Nanobiosci. 2011, 10, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, A.; Zulkarnain, A.; Zin, N.M.; Abdulhameed, A.; Kayani, A.A.; Buyong, R. Experimentally Profiling Dielectric Properties of Escherichia coli and Staphylococcus aureus by Movement Velocity and Force. Sci. Rep. 2025, 15, 22079. [Google Scholar] [CrossRef] [PubMed]
- Checa, M.; Millan-Solsona, R.; Blanco, N.; Torrents, E.; Fabregas, R.; Gomila, G. Mapping the Dielectric Constant of a Single Bacterial Cell at the Nanoscale with Scanning Dielectric Force Volume Microscopy. Nanoscale 2019, 11, 20809–20819. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Nikaido, H. Permeability of Pseudomonas aeruginosa Outer Membrane to Hydrophilic Solutes. J. Bacteriol. 1982, 152, 636–642. [Google Scholar] [CrossRef]
- Manrique, P.D.; Leus, I.V.; López, C.A.; Mehla, J.; Malloci, G.; Gervasoni, S.; Vargiu, A.V.; Kinthada, R.K.; Herndon, L.; Hengartner, N.W.; et al. Predicting Permeation of Compounds across the Outer Membrane of Pseudomonas aeruginosa Using Molecular Descriptors. Commun. Chem. 2024, 7, 84. [Google Scholar] [CrossRef]
- Russel, M.; Sophocleous, M.; JiaJia, S.; Xu, W.; Xiao, L.; Maskow, T.; Alam, M.; Georgiou, J. High-Frequency, Dielectric Spectroscopy for the Detection of Electrophysiological/Biophysical Differences in Different Bacteria Types and Concentrations. Anal. Chim. Acta 2018, 1028, 86–95. [Google Scholar] [CrossRef]
- Carstensen, E.L.; Marquis, R.E.; Gerhardt, P. Dielectric Study of the Physical State of Electrolytes and Water within Bacillus cereus Spores. J. Bacteriol. 1971, 107, 106–113. [Google Scholar] [CrossRef]
- Irimajiri, A.; Asami, K.; Ichinowatari, T.; Kinoshita, Y. Passive Electrical Properties of the Membrane and Cytoplasm of Cultured Rat Basophil Leukemia Cells. I. Dielectric Behavior of Cell Suspensions in 0.01–500 MHz and Its Simulation with a Single-Shell Model. Biochim. Biophys. Acta 1987, 896, 203–213. [Google Scholar] [CrossRef]
- Giannoukos, G.; Min, M.; Rang, T. Relative Complex Permittivity and Its Dependence on Frequency. World J. Eng. 2017, 14, 532–537. [Google Scholar] [CrossRef]
- Asami, K. Low-Frequency Dielectric Dispersion of Bacterial Cell Suspensions. Colloids Surf. B Biointerfaces 2014, 119, 1–5. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Morgan, H. Analytical and Numerical Modeling Methods for Impedance Analysis of Single Cells On-Chip. Nano 2008, 3, 55–63. [Google Scholar] [CrossRef]
- Houssin, T.; Bridle, H.; Senez, V. Electrochemical Detection. In Waterborne Pathogens, 2nd ed.; Bridle, H., Ed.; Academic Press: Cambridge, MA, USA, 2021; Chapter 6; pp. 147–187. [Google Scholar] [CrossRef]
- Castellví, Q.; Mercadal, B.; Ivorra, A. Assessment of Electroporation by Electrical Impedance Methods. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Zarrinkhat, F.; Jofre-Roca, L.; Jofre, M.; Rius, J.M.; Romeu, J. Experimental Verification of Dielectric Models with a Capacitive Wheatstone Bridge Biosensor for Living Cells: E. coli. Sensors 2022, 22, 2441. [Google Scholar] [CrossRef]
- Sohrabi, H.; Hemmati, A.; Majidi, M.R.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Adlpour-Azar, R.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. TrAC Trends Anal. Chem. 2021, 143, 116344. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Liu, K.; Lan, T.; Wang, Z.; Zhu, Z. Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors 2021, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhao, K.S.; Asami, K. Dielectric properties of E. coli cell as simulated by the three-shell spheroidal model. Biophys. Chem. 2006, 122, 136–142. [Google Scholar] [CrossRef]
- Tomaś, N.; Myszka, K.; Wolko, Ł.; Juzwa, W. Global transcriptome analysis of Pseudomonas aeruginosa NT06 response to potassium chloride, sodium lactate, sodium citrate, and microaerophilic conditions in a fish ecosystem. FEMS Microbiol. Lett. 2024, 371, fnae043. [Google Scholar] [CrossRef]
- Tokonami, A.; Kawanaka, M.; Ikeda, H.; Nishii, S.; Kamegawa, T.; Yamamoto, Y.; Sadanaga, Y.; Shiigi, H. Monitoring the Metabolic Activity of a Single Bacterial Cell Based on Scattering Intensity. Anal. Chem. 2025, 97, 8293–8300. [Google Scholar] [CrossRef]
- Goldsmith, D.J.A.; Hilton, P.J. Relationship between intracellular proton buffering capacity and intracellular pH. Kidney Int. 1992, 41, 43–49. [Google Scholar] [CrossRef][Green Version]
- Shen, A.; Edwards, A.N.; Sarker, M.R.; Paredes-Sabja, D. Sporulation and germination in Clostridial pathogens. Microbiol. Spectr. 2019, 7, 903–926. [Google Scholar] [CrossRef]
- Molines, A.T.; Lemière, J.; Gazzola, M.; Steinmark, I.E.; Edrington, C.H.; Hsu, C.T.; Real-Calderon, P.; Suhling, K.; Goshima, G.; Holt, L.J.; et al. Physical properties of the cytoplasm modulate the rates of microtubule polymerization and depolymerization. Dev. Cell 2022, 57, 466–479.e6. [Google Scholar] [CrossRef]
- Myers, B.; Agus, R.; Bouharrak, B.; Avino, F.; Furno, I. Investigating the effects of plasma-activated water on E. coli with the novel application of single-cell impedance flow cytometry. Sci. Rep. 2025, 15, 24064. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Multari, C.; Palego, C.; Ma, X.; Du, X.; Ning, Y.; Buceta, J.; Hwang, J.C.M.; Cheng, X. Differentiation of live and heat-killed E. coli by microwave impedance spectroscopy. Sens. Actuators B Chem. 2018, 255 Pt 2, 1614–1622. [Google Scholar] [CrossRef]
- Jamroskovic, J.; Chromikova, Z.; List, C.; Bartova, B.; Barak, I.; Bernier-Latmani, R. Variability in DPA and Calcium Content in the Spores of Clostridium Species. Front. Microbiol. 2016, 7, 1791. [Google Scholar] [CrossRef]
- Fu, P.; Ramchandran, R.; Sudhadevi, T.; Kumar, P.P.K.; Krishnan, Y.; Liu, Y.; Zhao, Y.; Parinandi, N.L.; Harijith, A.; Sadoshima, J.; et al. NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium. Antioxidants 2021, 10, 477. [Google Scholar] [CrossRef]
- Garcia, P.A.; Ge, Z.; Moran, J.L.; Buie, C.R. Microfluidic Screening of Electric Fields for Electroporation. Sci. Rep. 2016, 6, 21238. [Google Scholar] [CrossRef]
- Saraeva, I.; Zayarny, D.; Tolordava, E.; Nastulyavichus, A.; Khmelnitsky, R.; Khmelenin, D.; Shelygina, S.; Kudryashov, S. Locally Enhanced Electric Field Treatment of E. coli: TEM, FT-IR and Raman Spectrometry Study. Chemosensors 2023, 11, 361. [Google Scholar] [CrossRef]
- Novickij, V.; Zinkevičienė, A.; Stanevičienė, R.; Gruškienė, R.; Servienė, E.; Vepštaitė-Monstavičė, I.; Krivorotova, T.; Lastauskienė, E.; Sereikaitė, J.; Girkontaitė, I.; et al. Inactivation of Escherichia coli Using Nanosecond Electric Fields and Nisin Nanoparticles: A Kinetics Study. Front. Microbiol. 2018, 9, 3006. [Google Scholar] [CrossRef]
- Zand, E.; Schottroff, F.; Steinacker, E.; Mae-Gano, J.; Schoenher, C.; Wimberger, T.; Wassermann, K.J.; Jaeger, H. Advantages and Limitations of Various Treatment Chamber Designs for Reversible and Irreversible Electroporation in Life Sciences. Bioelectrochemistry 2021, 141, 107841. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Mañas, P.; Gómez, N.; Raso, J.; Pagán, R. Biosynthetic Requirements for the Repair of Sublethal Membrane Damage in Escherichia coli Cells after Pulsed Electric Fields. J. Appl. Microbiol. 2006, 100, 428–435. [Google Scholar] [CrossRef]
- Berthelot, R.; Doxsee, K.; Neethirajan, S. Electroceutical Approach for Impairing the Motility of Pathogenic Bacterium Using a Microfluidic Platform. Micromachines 2017, 8, 207. [Google Scholar] [CrossRef]
- Jakstys, B.; Jakutaviciute, M.; Uzdavinyte, D.; Satkauskiene, I.; Satkauskas, S. Correlation between the Loss of Intracellular Molecules and Cell Viability after Cell Electroporation. Bioelectrochemistry 2020, 135, 107550. [Google Scholar] [CrossRef]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M.-P. Cell Wall as a Target for Bacteria Inactivation by Pulsed Electric Fields. Sci. Rep. 2016, 6, 19778. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chang, J.; Jin, Y.; Wu, W.J. Pulsed Electric Field Treatments with Nonlethal Field Strength Alter the Properties of Bacterial Spores. J. Food Prot. 2022, 85, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Siemer, C.; Toepfl, S.; Heinz, V. Inactivation of Bacillus subtilis Spores by Pulsed Electric Fields (PEF) in Combination with Thermal Energy—I. Influence of Process- and Product Parameters. Food Control 2014, 39, 163–171. [Google Scholar] [CrossRef]
- Al-Sharify, Z.T.; Al-Najjar, S.Z.; Anumudu, C.K.; Hart, A.; Miri, T.; Onyeaka, H. Non-Thermal Technologies in Food Processing: Implications for Food Quality and Rheology. Appl. Sci. 2025, 15, 3049. [Google Scholar] [CrossRef]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue Light Treatment of Pseudomonas aeruginosa: Strong Bactericidal Activity, Synergism with Antibiotics and Inactivation of Virulence Factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Adams, M.E.; Allison, K.N.; Montgomery, M.C.; Mosher, H.; Cassol, E.; Overhage, J. Oxidative Stress Responses in Biofilms. Biofilm 2024, 7, 100203. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Liu, L.; Qiu, C.; Xiao, S.; Ouyang, Q.; Ji, M. Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm. Water 2025, 17, 1944. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Sun, K.; Liu, Q.; Chu, W.; Fu, L.; Dai, D.; Liang, Z.; Lin, C.-T. Electrochemical Impedance Spectroscopy-Based Biosensors for Label-Free Detection of Pathogens. Biosensors 2025, 15, 443. [Google Scholar] [CrossRef]
- Hargol Zadeh, S.; Kashanian, S.; Nazari, M. A Label-Free Carbohydrate-Based Electrochemical Sensor to Detect Escherichia coli Pathogenic Bacteria Using D-mannose on a Glassy Carbon Electrode. Biosensors 2023, 13, 619. [Google Scholar] [CrossRef]
- Razmi, N.; Lazouskaya, M.; Pajcin, I.; Petrovic, B.; Grahovac, J.; Simic, M.; Willander, M.; Nur, O.; Stojanovic, G.M. Monitoring the Effect of pH on the Growth of Pathogenic Bacteria Using Electrical Impedance Spectroscopy. Results Eng. 2023, 20, 101425. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Review: Microbial Analysis in Dielectrophoretic Microfluidic Systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Xiao, M.; Xu, P.; Xu, Y.-C.; Du, W. An Integrated Microfluidic Device Utilizing Dielectrophoresis and Multiplex Array PCR for Point-of-Care Detection of Pathogens. Lab Chip 2014, 14, 3917–3924. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.; Sun, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Lu, N. Electrochemical Impedance Spectroscopy Applied to Microbial Fuel Cells: A Review. Front. Microbiol. 2022, 13, 973501. [Google Scholar] [CrossRef]
- You, E.; Sarmadi, M.; Sangree, C. Cutting-Edge Advancements in EIS Technologies for Rapid Detection of Pathogenic Bacteria in Water. J. Stud. Res. 2023, 12, 1–10. [Google Scholar] [CrossRef]
- Song, X.; Fredj, Z.; Zheng, Y.; Zhang, H.; Rong, G.; Bian, S.; Sawan, M. Biosensors for Waterborne Virus Detection: Challenges and Strategies. J. Pharm. Anal. 2023, 13, 1252–1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kivirand, K.; Min, M.; Rinken, T. Challenges and Applications of Impedance-Based Biosensors in Water Analysis. In Biosensing Technologies for the Detection of Pathogens—A Prospective Way for Rapid Analysis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Ojha, R.; Dash, J.; Satpathy, S.S.; Singh, V.; Kumar, A.; Mohanty, P.; Sethi, J.K.; Kim, H. A Brief Review on Factors Affecting the Performance of Microbial Fuel Cell and Integration of Artificial Intelligence. Discov. Sustain. 2025, 6, 702. [Google Scholar] [CrossRef]
- Sreelakshmi, C.S.; Kini, V.; Singh, M.; Mukhopadhyay, C.; Nag, P.; Sadani, K. Disposable Electrochemical Biosensors for the Detection of Bacteria in the Light of Antimicrobial Resistance. Biotechnol. Bioeng. 2024, 121, 1351–1366. [Google Scholar] [CrossRef]
- McDaniel, E.A.; Wahl, S.A.; Ishii, S.; Pinto, A.; Ziels, R.; Nielsen, P.H.; McMahon, K.D.; Williams, R.B.H. Prospects for Multi-omics in the Microbial Ecology of Water Engineering. arXiv 2021, arXiv:2105.08856. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, T.; Mthunzi-Kufa, P. Recent Advances in Artificial Intelligence and Machine Learning Based Biosensing Technologies. In IntechOpen; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Hanifa, R.; Cha, M.; Kang, W.; Yu, J.; Kim, K.-J.; Yun, Y.-M.; Kim, S. Development of Water Quality Analysis for Anomaly Detection and Correlation with Case Studies in Water Supply Systems. Electronics 2025, 14, 1933. [Google Scholar] [CrossRef]
- The Potential of Artificial Intelligence in Water Quality Monitoring. Available online: https://www.waterandwastewater.com/the-potential-of-artificial-intelligence-in-water-quality-monitoring/ (accessed on 15 July 2025).
- Kumar, S.; Kaushal, J.B.; Lee, H.P. Sustainable Sensing with Paper Microfluidics: Applications in Health, Environment, and Food Safety. Biosensors 2024, 14, 300. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.-S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 202400011. [Google Scholar] [CrossRef]
- Yousfi, N.; Merbahi, N.; Bouajila, J.; Taillandier, P.; Debouba, M. Microbial Fermentation Assisted by Pulsed Electric Fields, Magnetic Fields and Cold Atmospheric Plasma: State of the Art. Fermentation 2025, 11, 417. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rezaee, A. Environmental Pollution Removal Using Electrostimulation of Microorganisms by Alternative Current. Enzyme Microb. Technol. 2024, 174, 110369. [Google Scholar] [CrossRef]
- Cavazza, A.; Molina-Estévez, F.J.; Plaza Reyes, Á.; Ronco, V.; Naseem, A.; Malenšek, Š.; Pečar, P.; Santini, A.; Heredia, P.; Aguilar-González, A.; et al. Advanced Delivery Systems for Gene Editing: A Comprehensive Review from the GenE-HumDi COST Action Working Group. Mol. Ther. Nucleic Acids 2025, 36, 102457. [Google Scholar] [CrossRef] [PubMed]
- Buica, G.-O.; Birzan, L.; Tecuceanu, V.; Razus, A.C.; Arnold, G.-L.; Ungureanu, E.-M. Modified Electrodes Based on Poly[(2E)-2-(Azulen-1-ylmethylidene)hydrazinecarbothioamide] for Heavy Metal Ions Complexation. Electroanalysis 2017, 29, 244–252. [Google Scholar] [CrossRef]
- Tenea, A.-G.; Dinu, C.; Buica, G.-O.; Vasile, G.-G. Electrochemical System for Field Control of Hg2+ Concentration in Wastewater Samples. Sensors 2023, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
| Microorganism/Structure | Relevant Structural Characteristics | Electrical Parameters/Response to EF | Refs. |
|---|---|---|---|
| Gram-negative bacteria (E. coli, P. aeruginosa) | Membrane thickness 4–6 nm; thin peptidoglycan layer; P. aeruginosa has asymmetric outer membrane with LPS; porins for ionic exchange | Reversible electroporation at ~10 kV/cm; membrane capacitance 10−6–10−4 S/m; cytoplasmic conductivity 0.1–1 S/m; detectable via EIS and DEP | [26,27,28,29,30,53,59] |
| Gram-positive bacteria (B. subtilis, E. faecalis) | Thick peptidoglycan wall (20–30 nm); multilayered envelope | Higher electroporation threshold than Gram-negatives; lower membrane conductivity (~0.5–1 × 10−6 S/m) | [53,54] |
| Yeast (S. cerevisiae) | Thick wall with glucans and mannans | Electroporation at ~7.5 kV/cm; used for intracellular extraction | [26,30] |
| Spores (Bacillus, Clostridium spp.) | Multilayered structure; low water content (<30%); highly resistant wall | Activation/inactivation threshold >30 kV/cm; low dielectric permittivity; high PEF resistance | [63,64,65,66,67,68] |
| Surface structures (pili, flagella, biofilm) | Type IV pili in P. aeruginosa responsive to EF; biofilm thickness 1–50 μm, high mechanical resistance | EF can induce electrotaxis, modify adhesion, detach biofilm (AC 0.1–2 Hz, DC 50–250 μA); affects local field distribution | [69,70,71,72,73,74] |
| Cytoplasmic properties | Main ions: K+, Na+; cytoplasmic conductivity ~0.1–1 S/m; dielectric constant ~25–30 (hydrated) | EF influences ion distribution and polarization at mid–high frequencies; measurable via impedance | [57,58,59] |
| Property | Microorganism/Example | Reported Values/Characteristics | Notes on Electric Field Interaction | Refs. |
|---|---|---|---|---|
| Membrane Thickness | E. coli (Gram-negative) | Inner membrane ~4 nm; peptidoglycan ~3 nm; total ~15 nm | Thin membranes polarize faster; lower electroporation threshold (~10 kV/cm) | [75,78] |
| B. subtilis (Gram-positive) | Peptidoglycan layer 25–40 nm | Thicker wall increases mechanical resistance; higher electroporation threshold | [76,79] | |
| E. faecalis (Gram-positive) | Cell wall ~40 nm with teichoic acids | High structural robustness under EF | [77] | |
| Membrane Potential | General bacteria | Resting potential −100 to −200 mV; E. coli: −220 mV (early exponential) to −140 mV (late exponential) | Drives ion transport, motility; EF causes depolarization/hyperpolarization depending on orientation | [80,81,82,83,84,85,86,87] |
| Membrane Conductivity | E. coli | 1 × 10−6 to 3 × 10−6 S/m; increases to ~10−4 S/m after electroporation | Higher conductivity → faster polarization, lower EF threshold for breakdown | [95,98] |
| P. aeruginosa | ~5 × 10−6 S/m | Linked to fluid membrane and protein P channels | [97] | |
| B. subtilis | 0.5 × 10−6 to 1 × 10−6 S/m | Thick wall, fewer ion channels → lower conductivity | [28] | |
| Dielectric Permittivity | E. coli | εr ≈ 5–9 | Fluid-phase lipid bilayer; strong β-polarization at kHz | [103,111] |
| B. subtilis | Lower εr than E. coli (exact value NA) | Reduced dielectric response at GHz | [107] | |
| Spores (Bacillus, Clostridium) | <4 (dehydrated core) | Low water content → high EF resistance (>30 kV/cm); distinct DEP separation | [108,113] |
| Property | Microorganism/State | Value/Range | Notes | Refs. |
|---|---|---|---|---|
| Cytoplasmic Conductivity | E. coli (viable) | 0.22 S/m | Typical intact membrane; pronounced β-dispersion at 1–10 MHz | [117,118] |
| E. coli (post-PEF) | ↓ significantly | Reduction due to ion leakage and metabolic arrest | [32] | |
| P. aeruginosa (active) | Higher than E. coli | Linked to high intracellular K+, Na+, Cl−, and organic acids | [119,120] | |
| Low pH (~6.5) | ↓ ~20% | Reduced protein protonation and buffering capacity | [121] | |
| Clostridium spores | <0.05 S/m | Dehydration and ionic shielding; low impedance response | [122] | |
| Dielectric Permittivity | E. coli (hydrated) | εr ~100 | High polarizability; β-dispersion around 1–10 MHz | [115,118] |
| E. coli (dry core model) | 5–6.5 | Represents proteins and nucleic acids without hydration | [125] | |
| E. coli (ambient hydrated) | 25–30 | Strong hydration effects | [125] | |
| Clostridium spores | <30 | Dense dipicolinic acid–calcium complexes; low polarizability | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, C.; Răileanu, S.; Ștefan, D.S.; Lingvay, I.; Tokos, A.; Ștefan, M. Electric Field Effects on Microbial Cell Properties: Implications for Detection and Control in Wastewater Systems. Environments 2025, 12, 343. https://doi.org/10.3390/environments12100343
Ungureanu C, Răileanu S, Ștefan DS, Lingvay I, Tokos A, Ștefan M. Electric Field Effects on Microbial Cell Properties: Implications for Detection and Control in Wastewater Systems. Environments. 2025; 12(10):343. https://doi.org/10.3390/environments12100343
Chicago/Turabian StyleUngureanu, Camelia, Silviu Răileanu, Daniela Simina Ștefan, Iosif Lingvay, Attila Tokos, and Mircea Ștefan. 2025. "Electric Field Effects on Microbial Cell Properties: Implications for Detection and Control in Wastewater Systems" Environments 12, no. 10: 343. https://doi.org/10.3390/environments12100343
APA StyleUngureanu, C., Răileanu, S., Ștefan, D. S., Lingvay, I., Tokos, A., & Ștefan, M. (2025). Electric Field Effects on Microbial Cell Properties: Implications for Detection and Control in Wastewater Systems. Environments, 12(10), 343. https://doi.org/10.3390/environments12100343









