Sediment Microplastic Pollution in Contrasting Estuarine Environments of the Biobío Region South-Central Chile
Abstract
1. Introduction
2. Materials and Methods
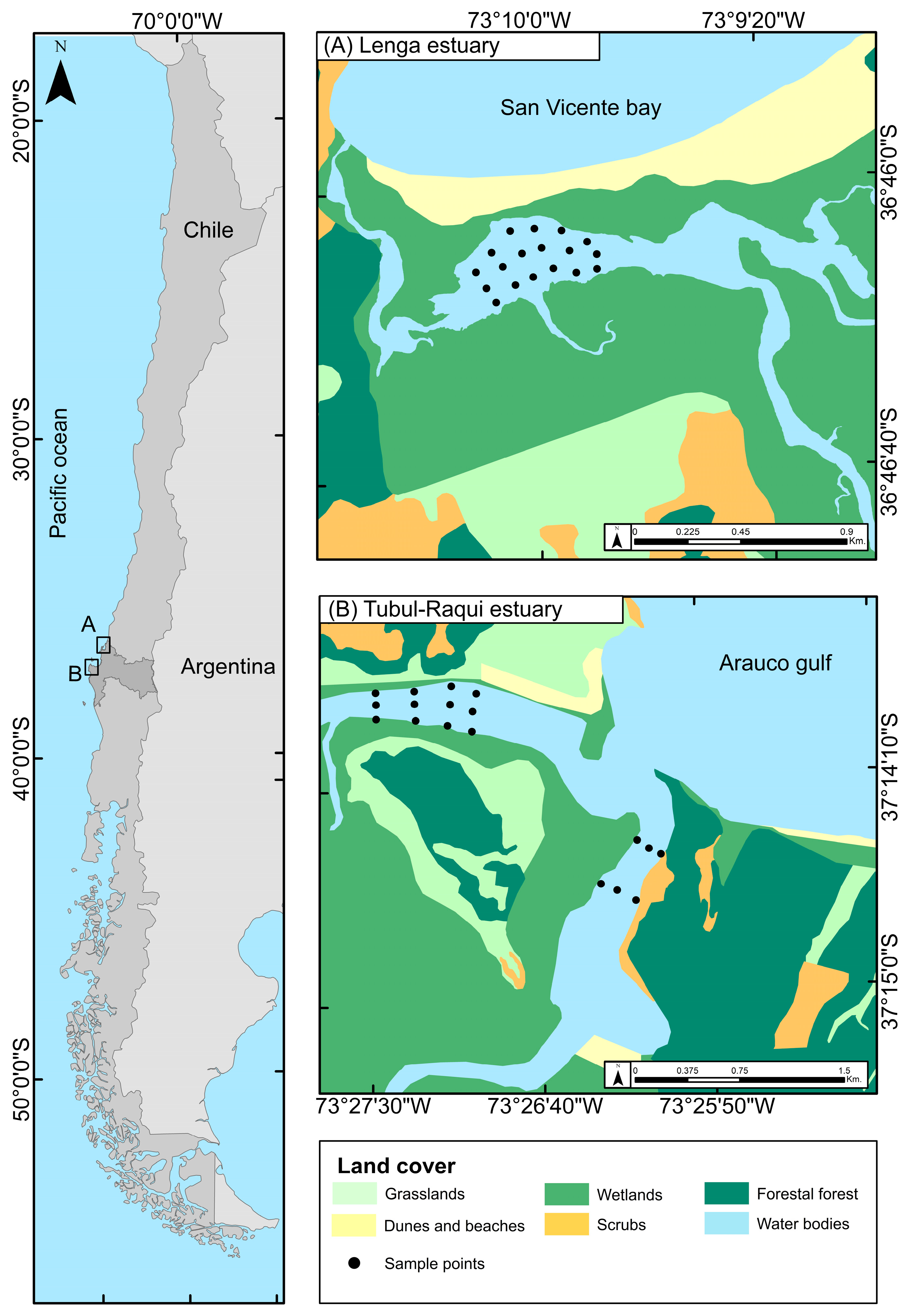
2.1. Study Area
2.2. Sample Collection
2.3. Microplastic Extraction
2.4. Physicochemical Parameter Analysis
2.4.1. Particle Size
2.4.2. Organic Matter
2.4.3. pH
2.5. Statistical Analysis
3. Results
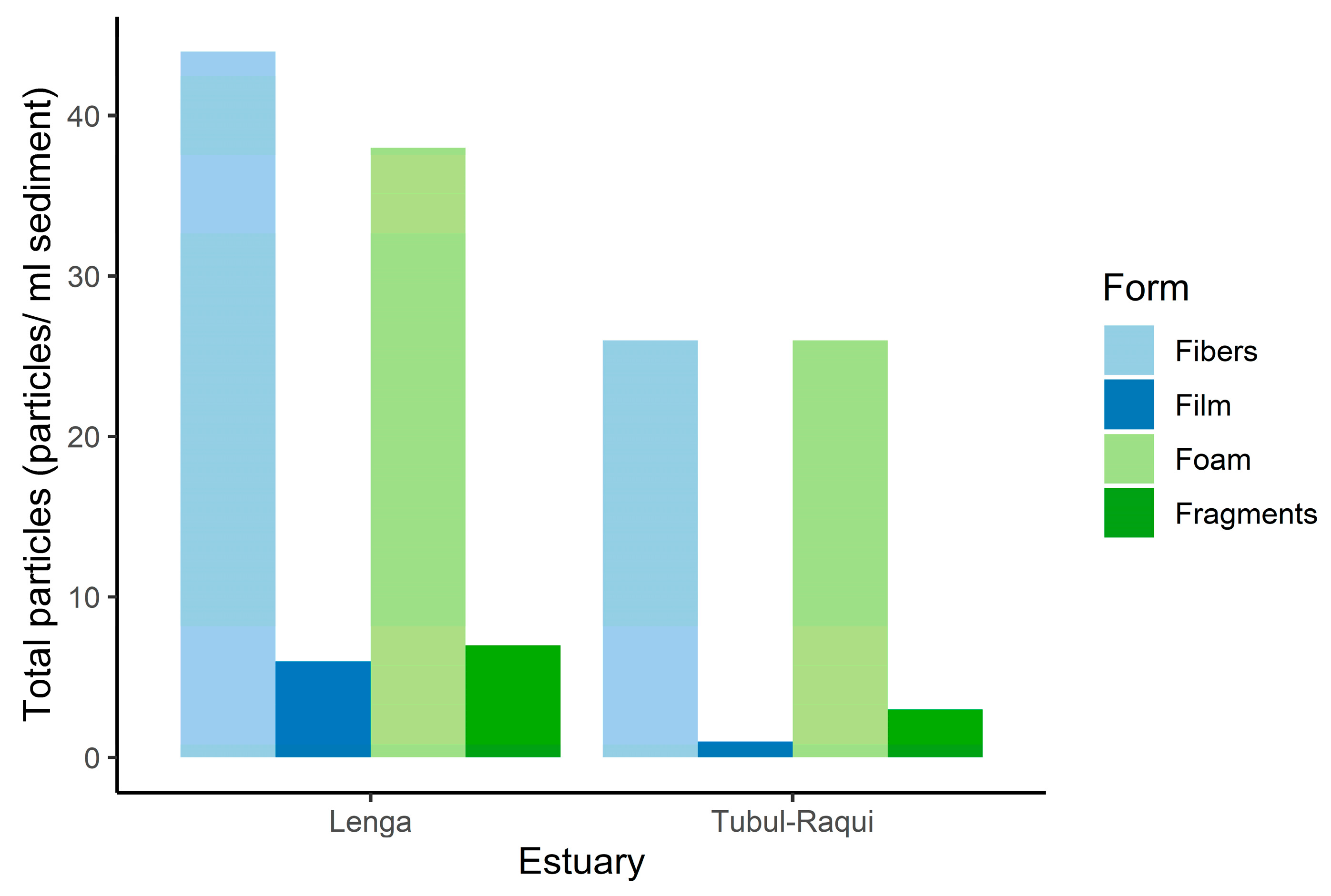
3.1. Microplastic Abundance and Concentration
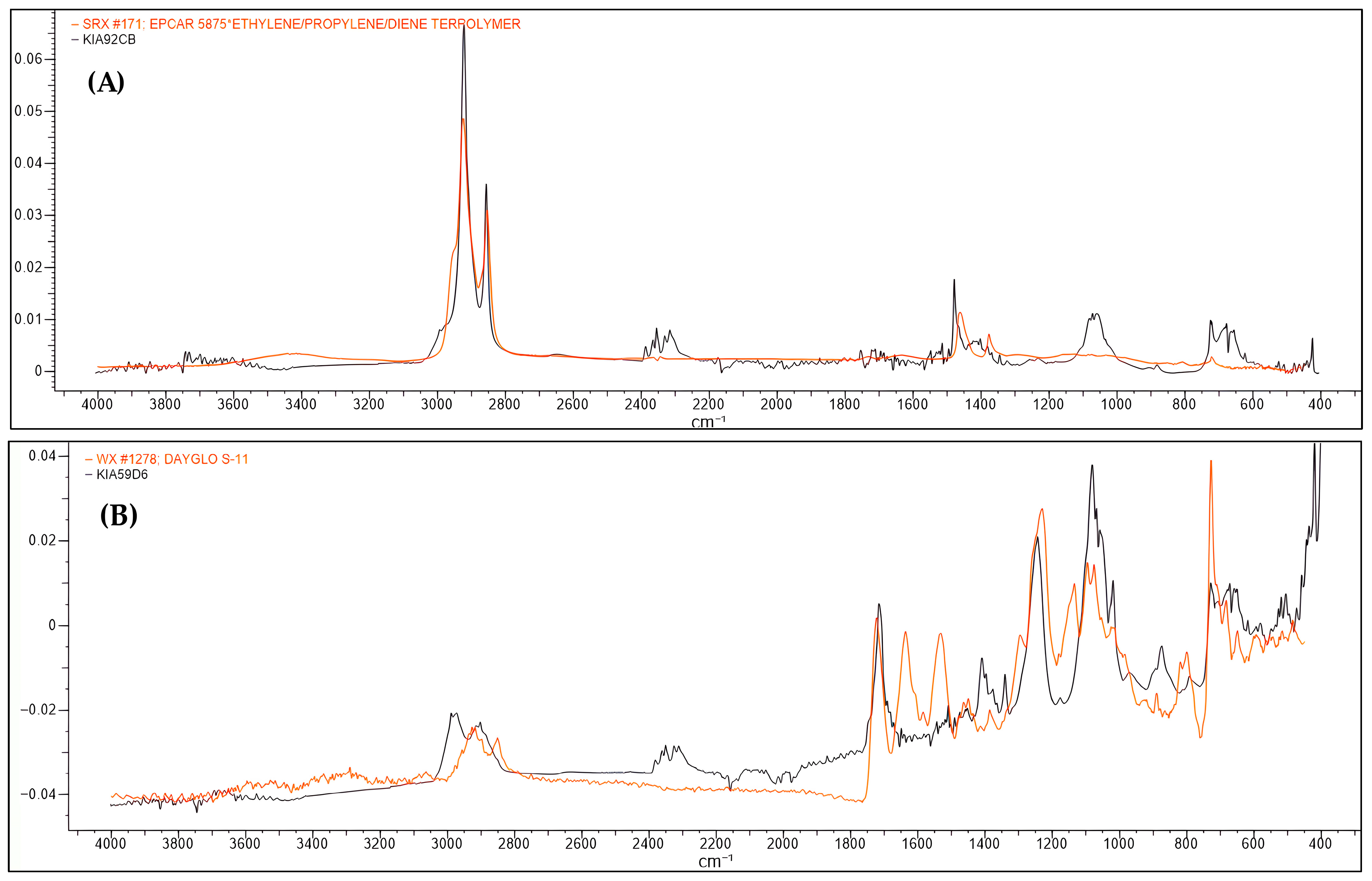
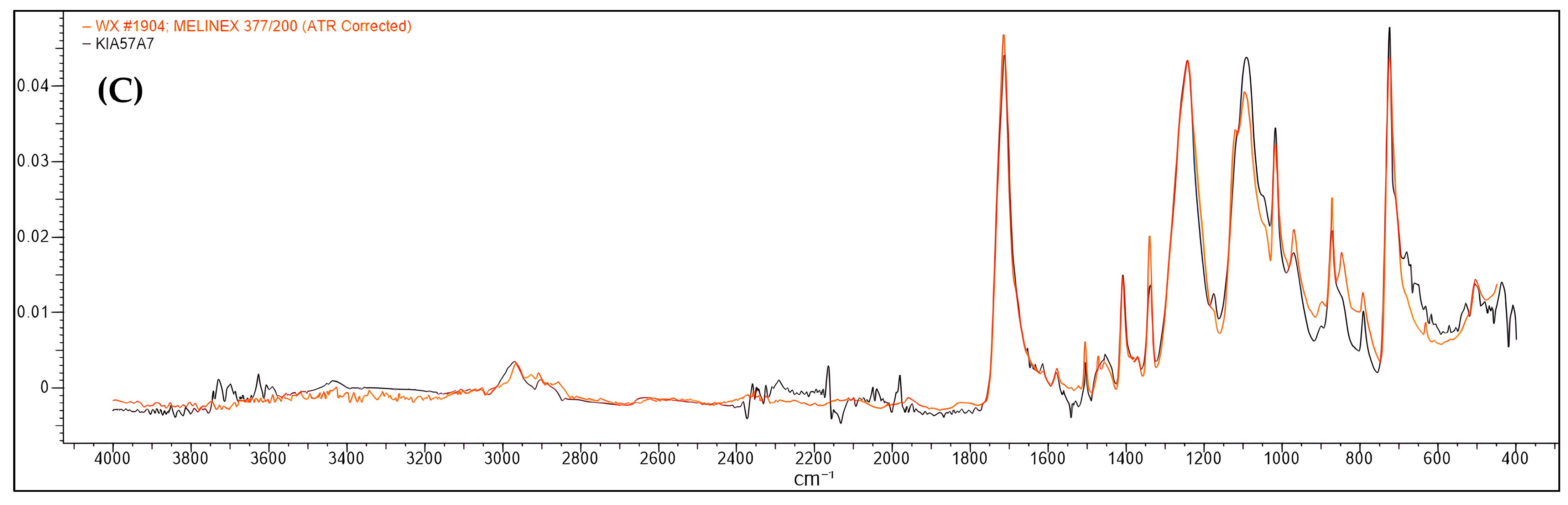
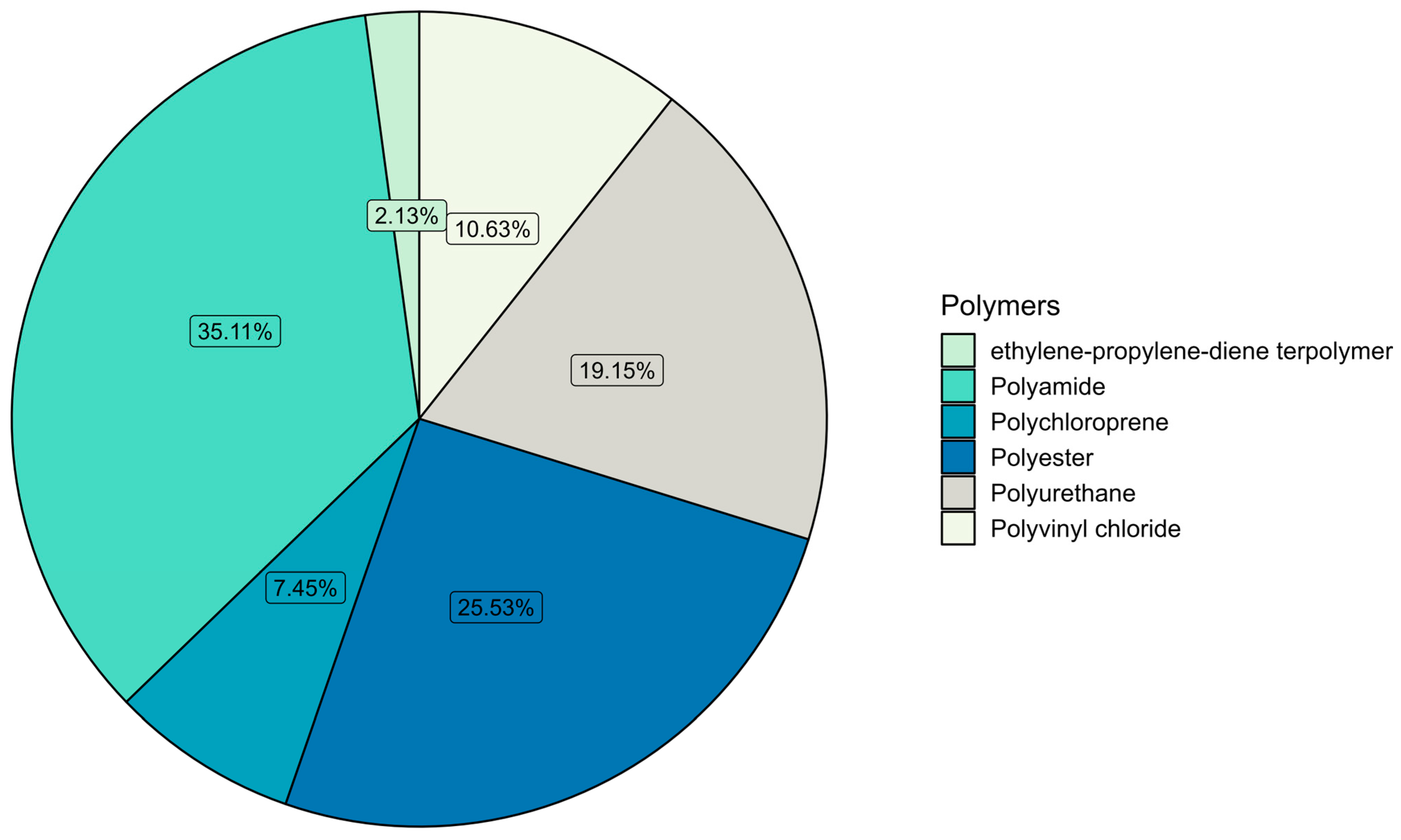
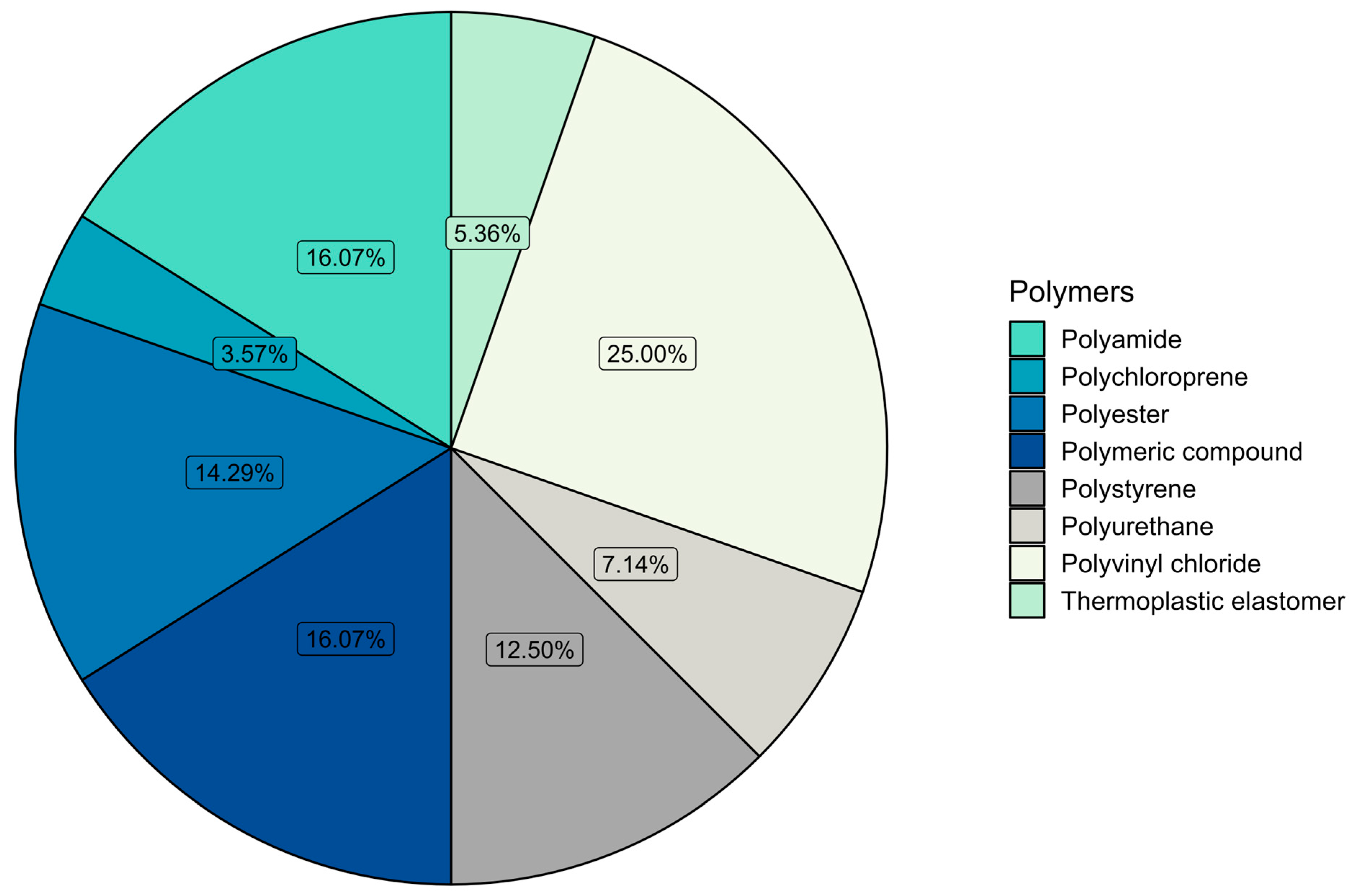
3.2. Microplastic Polymer Types
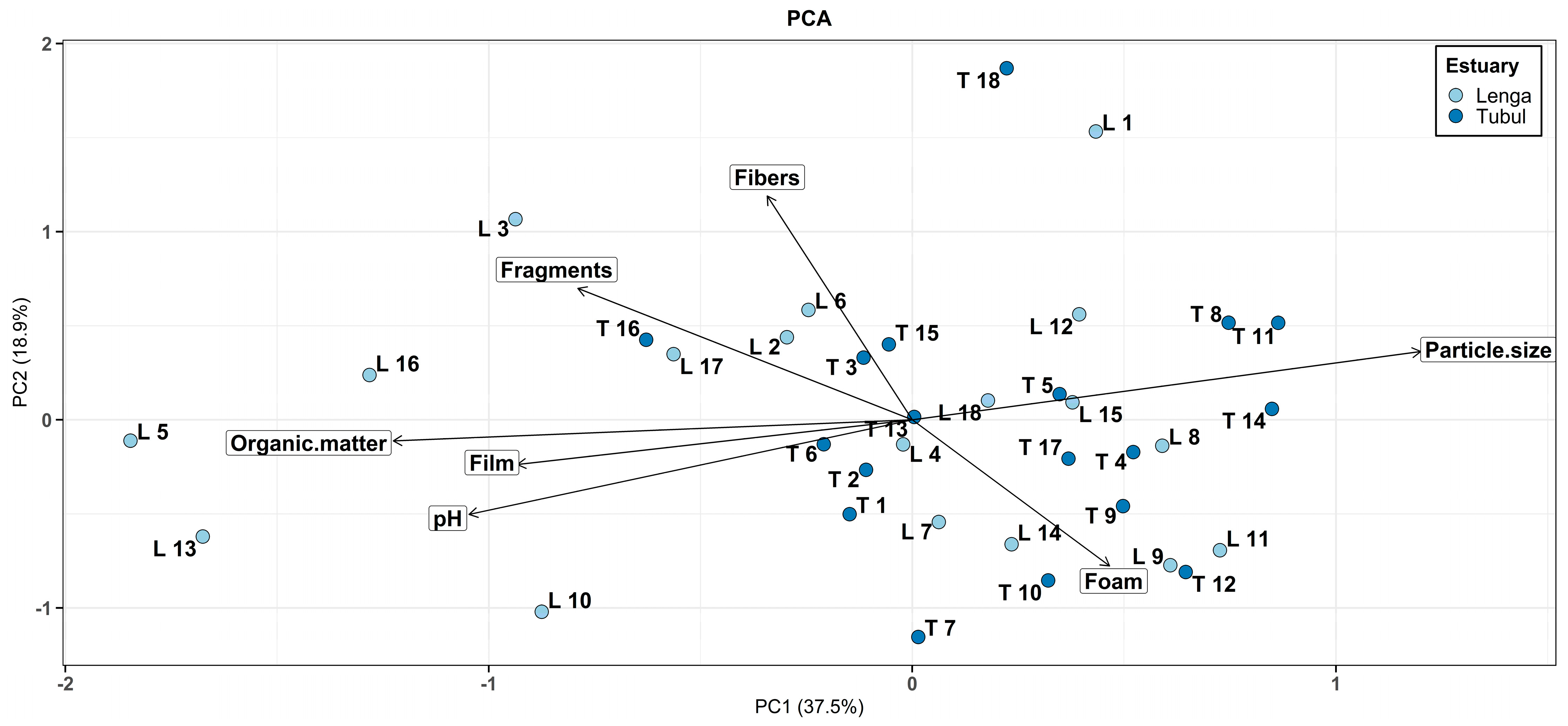
3.3. Principal Component Analysis (PCA)
4. Discussion
| Estuary | Microplastics Concentration (Particles/kg Sediment d.w.) | References |
|---|---|---|
| Wanquan River, China | 1065.0 ± 696.0 | [5] |
| Jagir, Indonesia | 92.0–590.0 | [16] |
| Kollidam River, India | 1580.0 ± 563.0 | [36] |
| Red River, Vietnam | 2188.0 ± 1499.0 | [37] |
| Loire, France | 4440.0 (summer)–1443.0 (winter) | [39] |
| Liaohe, China | 120.0 ± 46.0 | [40] |
| Uppanar and Gadilam, India | 36.3 ± 3.39–51.6 ± 2.05 | [41] |
| Kayamkulam, India | 433.3 | [46] |
| Sebou, Morocco | 10.0–300.0 | [47] |
| Tecolutla, Mexico | 121.0 | [29] |
| Bahia Blanca, Argentina | 1693.0 ± 2315.0 | [38] |
| Chubut River, Argentina | 175.4 ± 63.5 | [43] |
| Salado, Ecuador | 720–7090 | [44] |
| Guanabara Bay, Brazil | 528.0 ± 30.0 | [45] |
| Lenga, Chile | 106.9 ± 67.2 | Present study |
| Tubul-Raqui, Chile | 49.3 ± 30.9 | Present study |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Polymer compound |
| EPDM | Ethylene propylene diene polymer |
| EPR | Extended producer responsibility |
| GESAMP | Group of Experts on the Scientific Aspects of Marine Environmental Protection |
| MPs/MP | Microplastics/microplastic |
| PA | Polyamide |
| Pb | Lead |
| PCA | Principal component analysis |
| PC1 | First principal component |
| PC2 | Second principal component |
| PU | Polyurethane |
| PVC | Polyvinylchloride |
| Zn | Zinc |
Appendix A
| Estuary | Sampling Point | Particle Size (μm) | Organic Matter (%) | pH |
|---|---|---|---|---|
| Lenga | 1 | 149.0 | 1.84 | 7.02 |
| 2 | 131.1 | 12.60 | 6.78 | |
| 3 | 86.48 | 7.88 | 7.95 | |
| 4 | 145.7 | 2.35 | 8.25 | |
| 5 | 28.84 | 11.61 | 8.44 | |
| 6 | 102.9 | 5.26 | 7.96 | |
| 7 | 142.1 | 2.16 | 8.52 | |
| 8 | 116.3 | 1.77 | 7.04 | |
| 9 | 122.3 | 2.06 | 7.44 | |
| 10 | 38.16 | 11.50 | 8.42 | |
| 11 | 168.6 | 1.64 | 7.60 | |
| 12 | 155.5 | 1.91 | 7.48 | |
| 13 | 43.65 | 7.40 | 8.85 | |
| 14 | 154.7 | 2.33 | 7.76 | |
| 15 | 164.1 | 1.88 | 7.84 | |
| 16 | 45.86 | 9.69 | 8.74 | |
| 17 | 57.13 | 4.29 | 7.97 | |
| 18 | 138.4 | 1.84 | 8.01 | |
| Mean ± SD | 110.60 ± 48.11 | 5.00 ± 4.00 | 7.89 ± 0.59 | |
| Tubul-Raqui | 1 | 59.84 | 4.56 | 7.71 |
| 2 | 85.29 | 4.89 | 7.85 | |
| 3 | 66.01 | 4.35 | 7.43 | |
| 4 | 169.8 | 2.74 | 7.54 | |
| 5 | 132.1 | 2.39 | 7.46 | |
| 6 | 66.18 | 5.64 | 7.66 | |
| 7 | 81.4 | 5.48 | 7.87 | |
| 8 | 218.1 | 1.58 | 7.36 | |
| 9 | 147.0 | 2.31 | 7.53 | |
| 10 | 95.34 | 2.88 | 7.55 | |
| 11 | 153.3 | 1.91 | 6.45 | |
| 12 | 178.1 | 3.22 | 7.62 | |
| 13 | 84.51 | 5.03 | 7.38 | |
| 14 | 223.2 | 1.56 | 7.33 | |
| 15 | 121.6 | 3.01 | 7.61 | |
| 16 | 89.06 | 5.61 | 7.54 | |
| 17 | 127.9 | 2.65 | 7.39 | |
| 18 | 197.0 | 1.91 | 7.40 | |
| Mean ± SD | 127.54 ± 53.16 | 3.43 ± 1.46 | 7.48 ± 0.30 |
References
- Giraldez Alvarez, L.D.; Braz de Jesus, F.; Lacerda Costa, A.P.; Ferraz Bastos, L.E.; Moura De Souza, D.A.; Gonçalves da Silva, D. Efectos de los microplásticos en el medio ambiente: Un macroproblema emergente. Rev. Cienc. Tecnol. 2020, 33, 100–107. [Google Scholar] [CrossRef]
- Paredes-Osses, E.; Pozo, K.; Opazo-Capurro, A.; Bahamonde, P.; Cabrera-Pardo, J.R. Microplastics Pollution in Chile: Current Situation and Future Prospects. Front. Environ. Sci. 2021, 9, 796989. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; GESAMP Reports and Studies, No. 99; GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; 130p. [Google Scholar] [CrossRef]
- Wang, T.; Tang, W.; Wu, D.; Yu, X.; Wang, G.; Cai, X.; Shao, S.; Wang, S.; Mo, L.; Liu, Y.; et al. Abundance and characteristics of microplastics in the Wanquan River estuary, Hainan Island. Mar. Pollut. Bull. 2023, 189, 114810. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.; Martinez-Perez, S.; Dafouz, R.; Hurley, R.; Vighi, M.; Rico, A. Effects of Polyester Fibers and Car Tire Particles on Freshwater Invertebrates. Environ. Toxicol. Chem. 2022, 41, 1555–1567. [Google Scholar] [CrossRef]
- Razi, A.; Hassimi, O.; Hasan, A.; Hafizuddin, M.; Nur, M.; Ismail, I. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Ahrendt, C.; Galbán-Malagón, C.; Gómez, V.; Torres, M.; Mattar, C.; DeCoite, M.; Guida, Y.; Příbylová, P.; Pozo, K. Marine debris and associated organic pollutants in surface waters of Chiloé in the Northern Chilean Patagonia (42°–44°S). Mar. Pollut. Bull. 2023, 187, 114558. [Google Scholar] [CrossRef]
- Castillo, C.; Fernández, C.; Gutiérrez, M.H.; Aranda, M.; Urbina, M.A.; Yáñez, J.; Álvarez, Á.; Pantoja-Gutiérrez, S. Water column circulation drives microplastic distribution in the Martínez-Baker channels; A large fjord ecosystem in Chilean Patagonia. Mar. Pollut. Bull. 2020, 160, 111591. [Google Scholar] [CrossRef]
- Jorquera, A.; Castillo, C.; Murillo, V.; Araya, J.; Pinochet, J.; Narváez, D.; Pantoja-Gutiérrez, S.; Urbina, M.A. Physical and anthropogenic drivers shaping the spatial distribution of microplastics in the marine sediments of Chilean fjords. Sci. Total Environ. 2022, 814, 152506. [Google Scholar] [CrossRef]
- Pozo, K.; Gomez, V.; Torres, M.; Vera, L.; Nuñez, D.; Oyarzún, P.; Mendoza, G.; Clarke, B.; Fossi, M.C.; Baini, M.; et al. Presence and characterization of microplastics in fish of commercial importance from the Biobío region in central Chile. Mar. Pollut. Bull. 2019, 140, 315–319. [Google Scholar] [CrossRef]
- Perez-Venegas, D.J.; Seguel, M.; Pavés, H.; Pulgar, J.; Urbina, M.; Ahrendt, C.; Galbán-Malagón, C. First detection of plastic microfibers in a wild population of South American fur seals (Arctocephalus australis) in the Chilean Northern Patagonia. Mar. Pollut. Bull. 2018, 136, 50–54. [Google Scholar] [CrossRef]
- Nacaratte, F.; Cuevas, P.; Becerra-Herrera, M.; Manzano, C.A. Early screening of suspected microplastics in bottled water in the Santiago Metropolitan Region of Chile. Environ. Pollut. 2023, 334, 1222118. [Google Scholar] [CrossRef] [PubMed]
- Summers, E.; Du, J.; Park, K.; Wharton, M.; Kaiser, K. Importance of the water-sediment bed interactions in simulating microplastic particles in an estuarine system. Front. Mar. Sci. 2024, 11, 1414459. [Google Scholar] [CrossRef]
- Ridall, A.; Ingels, J. Seasonal and spatial variations in microplastics abundances in St. Andrew Bay, Florida. Sci. Total Environ. 2022, 852, 158422. [Google Scholar] [CrossRef]
- Firdaus, M.; Trihadiningrum, Y.; Lestari, P. Microplastic pollution in the sediment of Jagir Estuary, Surabaya City, Indonesia. Mar. Pollut. Bull. 2020, 150, 110790. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Sáez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Fulfer, V.M.; Walsh, J.P. Extensive estuarine sedimentary storage of plastics from city to sea: Narragansett Bay, Rhode Island, USA. Sci. Rep. 2023, 13, 10195. [Google Scholar] [CrossRef]
- Alava, J.J.; Kazmiruk, T.N.; Douglas, T.; Schuerholz, G.; Heath, B.; Flemming, S.A.; Bendell, L.; Drever, M.C. Occurrence and size distribution of microplastics in mudflat sediments of the Cowichan-Koksilah Estuary, Canada: A baseline for plastic particle contamination in an anthropogenic-influenced estuary. Mar. Pollut. Bull. 2021, 173, 113033. [Google Scholar] [CrossRef]
- Díaz-Jaramillo, M.; da Rocha, A.M.; Chiang, G.; Buchwalter, D.; Monserrat, J.M.; Barra, R. Biochemical and behavioral responses in the estuarine polychaete Perinereis gualpensis (Nereididae) after in situ exposure to polluted sediments. Ecotoxicol. Environ. Saf. 2013, 89, 182–188. [Google Scholar] [CrossRef]
- Pozo, K.; Urrutia, R.; Mariottini, M.; Rudolph, A.; Banguera, J.; Pozo, K.; Parra, O.; Focardi, S. Levels of Persistent Organic Pollutants (POPs) in sediments from Lenga estuary, central Chile. Mar. Pollut. Bull. 2014, 79, 338–341. [Google Scholar] [CrossRef]
- Daniel, I.; DeGrandpre, M.; Farías, L. Greenhouse gas emissions from the Tubul-Raqui estuary (central Chile 36°S). Estuar. Coast. Shelf Sci. 2013, 134, 31–44. [Google Scholar] [CrossRef]
- Díaz-Jaramillo, M.; Martins da Rocha, A.; Gomes, V.; Bianchini, A.; Monserrat, J.M.; Sáez, K.; Barra, R. Multibiomarker approach at different organization levels in the estuarine Perinereis gualpensis (Polychaeta; Nereididae) under chronic and acute pollution conditions. Sci. Total Environ. 2011, 410–411, 126–135. [Google Scholar] [CrossRef]
- Novoa, V.; Rojas, O.; Ahumada-Rudolph, R.; Katia, S.; Fierro, P. Coastal Wetlands: Ecosystems Affected by Urbanization? Water 2020, 12, 698. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olson, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zheng, H.; Wang, J.; Chen, C. Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary, China. Environ. Sci. Eur. 2020, 32, 15. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 104, 101–110. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Bergeron, J.; Wilkinson, N.; Hanlon, E.A. Soil pH and Electrical Conductivity: A County Extension Soil Laboratory Manual. Edis 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.J.; Ramírez-Romero, P.; Rodríguez-González, F.; Ramos-Sánchez, V.H.; Márquez Montes, R.A.; Romero-Paredes Rubio, H.; Sujitha, S.B.; Jonathan, M.P. Seasonal evidence of microplastics in environmental matrices of a tourist-dominated urban estuary in the Gulf of Mexico, Mexico. Chemosphere 2021, 277, 130261. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Molano, D.; Molina, A.; Duque, G. Distribución espacial y aumento a través del tiempo de microplásticos en sedimentos de la Bahía de Buenaventura, Pacífico colombiano. Boletín Investig. Mar. Costeras 2021, 50, 27–42. Available online: http://boletin.invemar.org.co/ojs/index.php/boletin/article/view/1021 (accessed on 23 September 2025).
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Vassilenko, E.; Watkins, M.; Chastain, S.; Mertens, J.; Posacka, A.M.; Patankar, S.; Ross, P.S. Domestic laundry and microfiber pollution: Exploring fiber shedding from consumer apparel textiles. PLoS ONE 2021, 16, 250346. [Google Scholar] [CrossRef]
- Nagalakshmi, R.; Joseph, A.; Balaji, V.A.; Saichand, V.; Begum, M.; Sambandam, M.; Kaviarasan, T.; Mishra, P. Microplastic Contamination in Kollidam River Estuary, East Coast of India: A Comparative Study Across Inner, Outer and Mangrove Estuarine Regions. Water Air Soil Pollut. 2024, 235, 475. [Google Scholar] [CrossRef]
- Le, D.; Hoang, H.; Duong, T.T.; Phuong, N.; Le, P.; Nguyen, T.D.; Binh, P.; Le, T.; Le, T.; Thi Huong, V.; et al. Microplastics in the Surface Sediment of the main Red River Estuary. Vietnam J. Earth Sci. 2022, 45, 19–32. [Google Scholar] [CrossRef]
- Arias, A.H.; Alvarez, G.; Pozo, K.; Pribylova, P.; Klanova, J.; Rodríguez Pirani, L.S.; Picone, A.L.; Alvarez, M.; Tombesi, N. Beached microplastics at the Bahia Blanca Estuary (Argentina): Plastic pellets as potential vectors of environmental pollution by POPs. Mar. Pollut. Bull. 2023, 187, 114520. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Aminot, Y.; Bely, N.; Pollono, C.; Idjaton, B.I.T.; Bizzozero, L.; Pierre-Duplessix, O.; Phuong, N.N.; Gasperi, J. Organophosphate ester additives and microplastics in benthic compartments from the Loire estuary (French Atlantic coast). Mar. Pollut. Bull. 2024, 201, 116256. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, R.; Sun, M.; Gao, Y.; An, L. Microplastics in sediments from an interconnected river-estuary region. Sci. Total Environ. 2020, 729, 139025. [Google Scholar] [CrossRef]
- Nithin, A.; Sundaramanickam, A.; Saha, M.; Hassanshahian, M.; Thangaraj, M.; Rathore, C. Risk assessments of microplastics accumulated in estuarine sediments at Cuddalore, Tamil Nadu, southeast coast of India. Environ. Monit. Assess. 2023, 195, 890. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.; Espinosa-Díaz, L.F.; Canals, M. Seasonal variation in plastic litter pollution in mangroves from two remote tropical estuaries of the Colombian Pacific. Mar. Pollut. Bull. 2023, 193, 115210. [Google Scholar] [CrossRef] [PubMed]
- Giarratano, E.; Di Mauro, R.; Silva, L.I.; Tomba, J.P.; Hernández-Moresino, R.D. The Chubut River estuary as a source of microplastics and other anthropogenic particles into the Southwestern Atlantic Ocean. Mar. Pollut. Bull. 2022, 185, 114267. [Google Scholar] [CrossRef]
- Arteaga, I.; Pinos-Vélez, V.; Capparelli, M.; Moulatlet, G.M.; Cipriani-Avila, I.; Cabrera, M.; Rebolledo, E.; Arnés-Urgellés, C.; Cazar, M.E. Microplastic occurrence and distribution in the Gulf of Guayaquil, Ecuador. Mar. Pollut. Bull. 2024, 209, 117288. [Google Scholar] [CrossRef]
- Alves, V.E.N.; Figueiredo, G.M. Microplastics in the sediments of a highly eutrophic tropical estuary. Mar. Pollut. Bull. 2019, 146, 326–335. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivapriya, V.; Rajkumar, A.; Akramkhan, N.; Prakasheswar, P.; Krishnakumar, S.; Hussain, S.M. Characterization and distribution of microplastics in estuarine surface sediments, Kayamkulam estuary, southwest coast of India. Mar. Pollut. Bull. 2021, 168, 112389. [Google Scholar] [CrossRef]
- Haddout, S.; Gimiliani, G.T.; Priya, K.L.; Hoguane, A.M.; Casila, J.C.C.; Ljubenkov, I. Microplastics in Surface Waters and Sediments in the Sebou Estuary and Atlantic Coast, Morocco. Anal. Lett. 2022, 55, 256–268. [Google Scholar] [CrossRef]
- Taha, Z.D.; Md Amin, R.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in seawater and zooplankton: A case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef]
- Jiwarungrueangkul, T.; Phaksopa, J.; Sompongchaiyakul, P.; Tipmanee, D. Seasonal microplastic variations in estuarine sediments from urban canal on the west coast of Thailand: A case study in Phuket province. Mar. Pollut. Bull. 2021, 168, 112452. [Google Scholar] [CrossRef]
- Islam, S.; Apitius, L.; Jakob, F.; Schwaneberg, U. Targeting microplastic particles in the void of diluted suspensions. Environ. Int. 2019, 123, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.; Westhead, E.K.; Jayaratne, R.; Newport, D. Microplastic abundance in the Thames River during the New Year period. Mar. Pollut. Bull. 2022, 177, 113534. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Misidentification of PVC microplastics in marine environmental samples. Trends Anal. Chem. 2022, 153, 116649. [Google Scholar] [CrossRef]
- Luo, Y.; Al Amin, M.; Gibson, C.T.; Chuah, C.; Tang, Y.; Naidu, R.; Fang, C. Raman imaging of microplastics and nanoplastics generated by cutting PVC pipe. Environ. Pollut. 2022, 298, 118857. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xu, B.; Liu, F.; Li, W.; Sy, N.; Zhou, X.; Yan, B. Effects of chemical and natural ageing on the release of potentially toxic metal additives in commercial PVC microplastics. Chemosphere 2021, 283, 131274. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef]
- Miller, R.R.; Newhook, R.; Poole, A. Styrene production, use, and human exposure. Crit. Rev. Toxicol. 1994, 24 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef]
- Muñoz Prieto, E.d.J. Síntesis y copolimerización de poliimidas a partir de aminas aromáticas mediante irradiación por microondas. Cienc. Desarro. 2016, 7, 161. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. New soybean oil-styrene-divinylbenzene thermosetting copolymers. II. Dynamic mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2721–2738. [Google Scholar] [CrossRef]
- Tang, H.; Gong, F.; Liu, C.; Ren, Q.; Yang, Y.; Jiang, B.; Liu, C.; Chen, J. Studies on the Preparation of Branched Polymers from Styrene and Divinylbenzene. J. Appl. Polym. Sci. 2007, 116, 2658–2667. [Google Scholar] [CrossRef]
- Muñoz, C.A.; Pardo, L.M.; Henríquez, L.A.; Palma, A.T. Variaciones temporales en la composición y abundancia de cuatro especies de Cancer (Decapoda: Brachyura: Cancridae) capturadas con trampas en bahía San Vicente, Concepción (Chile central). Investig. Mar. 2006, 34, 9–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019, 254, 112953. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Arias, A.H.; Ronda, A.C.; Piccolo, M.C. Continental microplastics: Presence, features, and environmental transport pathways. Sci. Total Environ. 2021, 799, 149447. [Google Scholar] [CrossRef]
- Malli, A.; Corella-Puertas, E.; Hajjar, C.; Boulay, A. Transport mechanisms and fate of microplastics in estuarine compartments: A review. Mar. Pollut. Bull. 2022, 177, 113553. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Stuardo, J.; Valdovinos, C. Estuarios y lagunas costeras: Ecosistemas importantes del Chile central. Amb. Des. 1989, 5, 107–115. [Google Scholar]
- Khalil, K.; Raimonet, M.; Laverman, A.M.; Yan, C.; Andrieux-Loyer, F.; Viollier, E.; Deflandre, B.; Ragueneau, O.; Rabouille, C. Spatial and Temporal Variability of Sediment Organic Matter Recycling in Two Temperate Eutrophicated Estuaries. Aquat. Geochem. 2013, 19, 517–542. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Mustafa, A.; Zedan, A.; Amer, S.; Albeialy, N.; Alkharsawey, D.; Aeash, N.; Abuomar, A.; Hamd, R.; Elbltagy, H.; et al. The Effect of Microplastic Pollution on Soil, Plants, and Soil Microbes and Its Remediation. Egypt. J. Soil Sci. 2022, 62, 331–340. [Google Scholar] [CrossRef]
- Choong, W.S.; Hadibarata, T. Abundance and Distribution of Microplastics in the Water and Riverbank Sediment in Malaysia—A Review. Biointerface Res. Appl. Chem. 2021, 11, 11700–11712. [Google Scholar] [CrossRef]
| Estuary | n | Particle Size (μm) | Classification | Organic Matter (%) | pH | Moisture (%) |
|---|---|---|---|---|---|---|
| Lenga | 18 | 110.60 ± 48.11 | Very fine sand | 5.00 ± 4.00 | 7.89 ± 0.59 | 66.77 ± 41.63 |
| Tubul-Raqui | 18 | 127.54 ± 53.16 | Fine sand | 3.43 ± 1.46 | 7.48 ± 0.30 | 38.59 ± 11.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-González, B.; Perfetti-Bolaño, A.; Araneda, A.; Lardies, M.A.; Leppes, N.; Barra, R.O. Sediment Microplastic Pollution in Contrasting Estuarine Environments of the Biobío Region South-Central Chile. Environments 2025, 12, 340. https://doi.org/10.3390/environments12100340
Cáceres-González B, Perfetti-Bolaño A, Araneda A, Lardies MA, Leppes N, Barra RO. Sediment Microplastic Pollution in Contrasting Estuarine Environments of the Biobío Region South-Central Chile. Environments. 2025; 12(10):340. https://doi.org/10.3390/environments12100340
Chicago/Turabian StyleCáceres-González, Belén, Alessandra Perfetti-Bolaño, Alberto Araneda, Marco A. Lardies, Nicolás Leppes, and Ricardo O. Barra. 2025. "Sediment Microplastic Pollution in Contrasting Estuarine Environments of the Biobío Region South-Central Chile" Environments 12, no. 10: 340. https://doi.org/10.3390/environments12100340
APA StyleCáceres-González, B., Perfetti-Bolaño, A., Araneda, A., Lardies, M. A., Leppes, N., & Barra, R. O. (2025). Sediment Microplastic Pollution in Contrasting Estuarine Environments of the Biobío Region South-Central Chile. Environments, 12(10), 340. https://doi.org/10.3390/environments12100340







