4.1. Sodium Hydroxide Stagewise Precipitation
Stagewise precipitation test results with sodium hydroxide are shown in
Figure 2. The main impurities, namely aluminum and iron, underwent precipitation in the pH range from 2 to 5. The concentration of these elements decreased gradually from pH 2 to 4. Notably, at pH 5, the aluminum concentration significantly dropped from 3089 mg/L at pH 4 to 19.61 mg/L, and iron exhibited a similar trend, decreasing from 257.25 mg/L at pH 4 to 1.41 mg/L at pH 5. Additional elements, including silicon and phosphorus, also experienced significant reduction at this pH. Conversely, at pH 5, the cumulative precipitation rates were 28.91 wt.% for manganese, 40.41 wt.% for cobalt, and 44.87 wt.% for nickel. This represents a content reduction of 11.99 mg/L, 64.35 mg/L, and 23.85 mg/L, respectively, from their initial concentrations. Consequently, pH 5 emerged as a promising value for impurity removal while maintaining suitable concentrations of cobalt, manganese, and nickel despite the presence of magnesium and calcium in the solution.
Based on previously gathered information, the decision was made to collect solids in two distinct pH ranges (2 to 5 and 5 to 10). This aimed to produce a pre-concentrate of cobalt, manganese, and nickel, predominantly obtained from the pH 5 to 10 range. At pH 9, approximately 99 wt.% of nickel and cobalt was precipitated, while manganese achieved a cumulative precipitation of 74 wt.%. Around 99 wt.% of manganese precipitation was accomplished at pH 11, but it also led to the precipitation of other impurities, such as magnesium. Therefore, solid collection ceased at pH 10, where manganese precipitation reached 94 wt.% while avoiding additional impurity precipitation.
ICP-MS analysis of the collected solids from both pH ranges revealed their elemental compositions and the grades of critical minerals and impurities, as shown in
Table 3. As anticipated, the low pH range precipitated most impurities, resulting in an Al and Fe grade of 8.3 wt.% and 1.2 wt.%, respectively. Solids generated in the high pH range had content percentages by weight of 2.7 wt.%, 0.35 wt.%, and 0.67 wt.% for Mn, Co, and Ni, separately.
To validate the results obtained by the experimental procedure, Visual MINTEQ 3.1 was used to simulate the stagewise precipitation, which began at an initial pH value of zero and was completed at pH 14. The simulation allows it to predict the chemical species precipitated at different pH values based on the elemental composition and water chemistry of the feedstock solution used in laboratory testing.
Table 3 presents the predicted species with their respective saturation index values for pH 5 and pH 10, respectively. As known, saturation index (SI) is a quantity commonly used to determine whether the solution is saturated, undersaturated, or supersaturated with respect to the given mineral and is calculated according to the following equation:
where
IAP is the ion activity product, and
Ksp is the equilibrium solubility product constant [
27].
A positive SI value indicates precipitation, while negative values suggest that the mineral remains dissolved in the solution. As seen in
Table 4, the results obtained from sodium hydroxide precipitation are well correlated to the predicted results. The chemical species predicted to precipitate are mostly aluminum and iron compounds at pH 5, including Boehmite (Al
2O
3·H
2O), Diaspore (AlO (OH)
3), Gibbsite (Al(OH)
3), Hercynite (FeAl
2O
4), Mirabilite (Na
2SO
4.10H
2O), and other compounds such as Al
4(OH)
10SO
4(s) and AlOHSO
4(s). On the other hand, at pH 10, compounds of Mn, Co, and Ni are precipitated according to the simulation, including Co(OH)
2, CoO(s), Ni(OH)
2, Ni
4(OH)
6SO
4(s), and Pyrochroite (Mn(OH)
2).
4.2. Nitric Acid Dissolution
Through NaOH precipitation in the pH range of 5 to 10, enriched solids with Co, Ni, and Mn were collected at pH 10 after 10 min of reaching the desired value. Then, these solids underwent a 0.5 M nitric acid dissolution process to selectively dissolve Co and Ni while keeping Mn in the solid phase. Throughout the process, approximately 94% of the initial mass was dissolved in nitric acid, resulting in a dissolved solution and a solid residue. ICP-MS analysis results of the solid residue indicate a significantly elevated Mn purity from an initial value of 2.77 wt.% to 46.99 wt.%. On the other hand, cobalt and nickel experienced slight decreases in their weight percentages, resulting in grades of 0.6 wt.% and 0.24 wt.%, respectively. Detailed elemental compositions of the dissolved solution and solid residue are shown in
Table 4 and
Table 5, respectively.
Figure 3 shows that the nitric acid dissolved solution contained approximately 393.02 mg/L of Co and 619.87 mg/L of Ni, which holds promise for further recovery of these two elements. On the other hand, around 2053.40 mg/L of Mn and 2437.21 mg/L of Mg were also present in the solution, along with other impurities, such as Al and Zn. Therefore, the nitric acid dissolved solution was subsequently subjected to Na
2S precipitation to separate different CMs in the solution. In the meantime, a Mn concentrate with a purity of 46.99 wt.% was produced from the same experimental procedure.
4.3. Sodium Sulfide Precipitation
The solids obtained from the 3-stage precipitation exhibited promising results for the potential separation of Ni, Co, Mn, and Mg. In the pH range of 1 to 5, the precipitated solids were combined, and ICP-MS analyses of the mixed solid indicate that it contained approximately 8.35 wt.% of Ni and 5.25 wt.% of Co. However, aluminum was also present with a concentration of 4.27 wt.%. Upon increasing the pH from 5 to 10, a solid product with a Mn grade of 20.15 wt.% was generated, indicating an additional stream for Mn extraction. Finally, a Mg concentrate was obtained at pH 10–12 with a purity of 27.49% wt.%. The ICP-MS characterization of the solids generated from the 3-stage Na
2S precipitation is detailed in
Table 6.
Like sodium hydroxide precipitation, validation of the results obtained by experimental testing was also conducted using Visual MINTEQ 3.1 to predict the mineral species to be formed with the given water chemistry. Based on the experimental observations, most of the cobalt and nickel compounds were precipitated at pH 5; this fact can be validated by the simulation results shown in
Table 7. As seen from the table, cobalt and nickel compounds are precipitated at pH 5, including CoS (alpha), CoS (beta), NiS (alpha), and NiS (beta). Moreover, at a pH range of 5 to 10, manganese sulfide (MnS) is likely precipitated. The third stage precipitation, which was performed in the pH region of 10 to 12, produced a solid magnesium product with a purity of 27.49 wt.%. According to
Table 7, the solid species likely to appear at this pH region are pyrochroite (Mn(OH)
2) and spinel (MgAl
2O
4). Spinel appears at both pH 10 and 12, indicating that the mineral starts forming at a pH value close to 10.
4.4. Hydrochloric Acid Dissolution
Following Na
2S precipitation, solid redissolution was tested at varying acid concentrations to identify the optimal dissolution rate and favorable elemental composition for subsequent Co and Ni recovery. Two tests were conducted, one with a hydrochloric acid (HCl) concentration of 2M and the other with 4M. Unexpectedly, the 4M HCl experiment yielded a total mass dissolution of 85%, lower than the 97.59% achieved with 2M HCl. To validate the results, the experiment was repeated, and a similar dissolution rate was observed. Despite being unexpected, this phenomenon can be attributed to factors such as the common ion effect, which decreases the dissolution of solids in the chemical reaction due to the high production of Cl
−. Under this phenomenon, the reaction is reversed to relieve the excess of Cl
− ions, which reduce the solubility of the substance, leading to a low dissolution rate [
28,
29].
As indicated in
Table 8, the 4M HCl test produced superior redissolution of the main elements, leaving Mn in the solid residue, resulting in a purer solution in terms of Co and Ni concentrations. The corresponding Co and Ni contents were 243 mg/L and 490 mg/L, respectively. In contrast, the 2M HCl test produced 206 mg/L of Co and 338 mg/L of Ni in the solution. The most notable difference was observed in the concentration of Mn, with a content value of 102.4 mg/L generated by 4M HCl, significantly lower than the 973.4 mg/L of Mn obtained from 2M HCl. Therefore, 4M HCl was selected as the acid concentration for redissolving the solid preconcentrate for Co and Ni extraction. This process generated two products: a dissolved solution containing Co and Ni as the main elements and others with lower amounts, such as Mn and Al, and a precipitated solid with approximately 9.92 wt.% Co, 10.93 wt.% Ni, and 0.28 wt.% Mn.
4.5. Solvent Extraction with Cyanex 272
4.5.1. Effect of Feed pH
The separation of cobalt from nickel in the HCl dissolved solution was conducted through solvent extraction by adjusting experimental parameters such as O:A ratio, extraction time, extractant concentration, feed pH, and equilibrium pH. Initially, to assess the pH’s effect on cobalt extraction, the experiment varied the feed pH from 4 to 5, maintaining an O:A ratio of 1:1, extractant concentration of 0.5 M, extraction time of 20 min, and a measured equilibrium pH of 7.5.
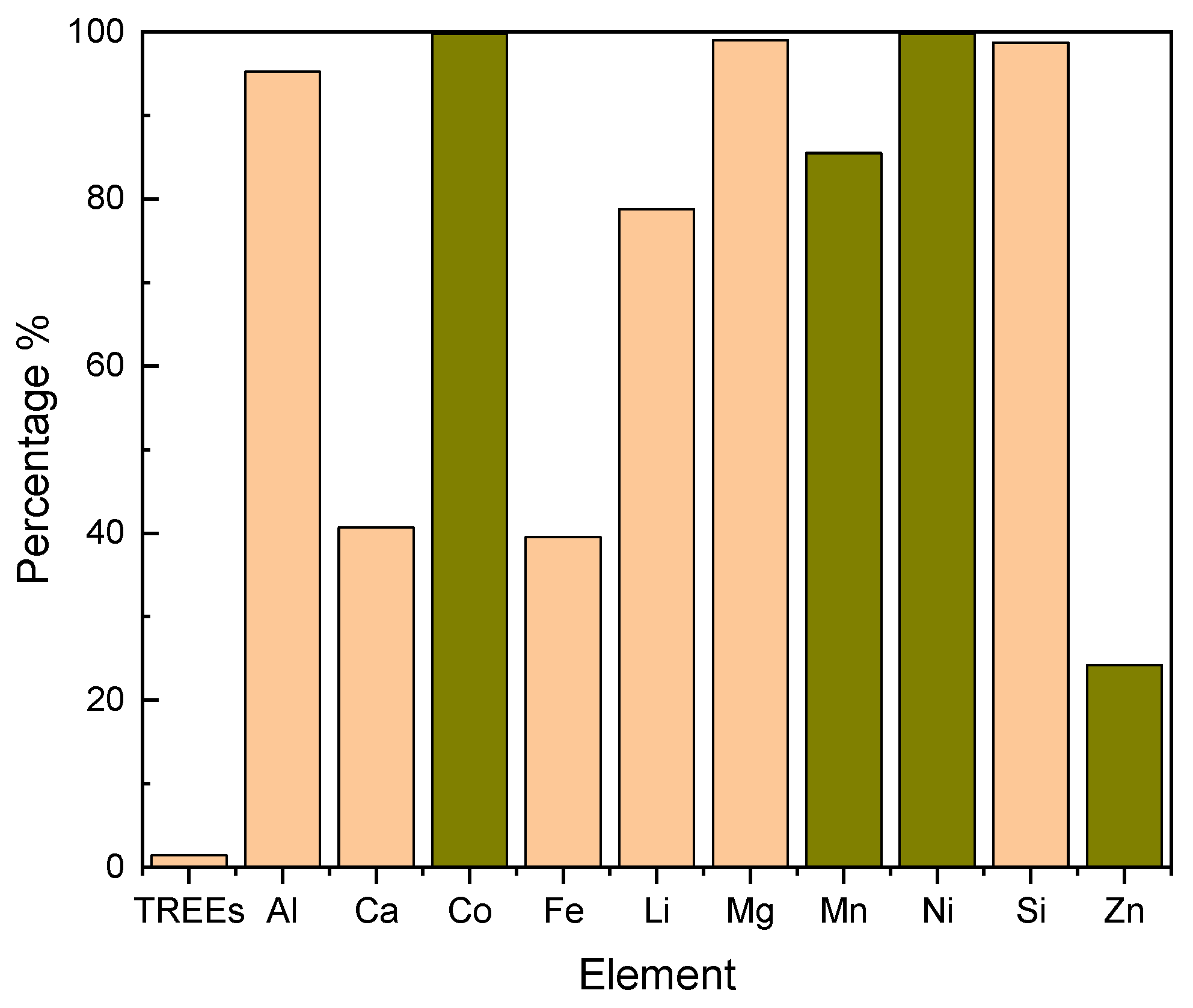
Results in
Figure 4 indicated that while the process effectively extracted elements from the feed solution, it could not separate cobalt from nickel, suggesting that feed pH had no significant influence on element separation. The recovery percentage in
Figure 4 demonstrates that approximately 99% of Co, Mn, and Ni, along with other impurities like Mg and Al, were all extracted to the organic solution, while Fe remained in the feed solution across the three-test run.
Although the tested feed pH range did not directly impact the separation of cobalt and nickel, it plays a crucial role in the extraction of elements. Swain et al. (2007) explored the distribution coefficient (DSX) of cobalt, a parameter linked to the stability of metal species, influencing the efficiency of metal extraction in a solvent extraction process. In the feed pH range of 4 to 6, cobalt extraction varied from 84% to 88%, and the study identified the optimal feed pH for cobalt extraction as 6 [
30]. To prevent the loss of important minerals, the feed pH for the experiments in this study was set at 5, based on previous results from NaOH and Na
2S precipitation.
4.5.2. Effect of Equilibrium pH
As previously mentioned, the feed pH was set at 5, with constant values for other parameters such as O:A ratio and separation time (1:1 and 20 min, respectively). After mixing the organic and aqueous solutions, the equilibrium pH was raised to approximately 7.5. It has been established that Ni and Co are co-extracted after the pH reaches 6.5 in chloride solutions but with negligible extraction before pH 3 [
31]. Therefore, an equilibrium pH of 4.7 was selected, where most cobalt can be extracted along with a low amount of nickel.
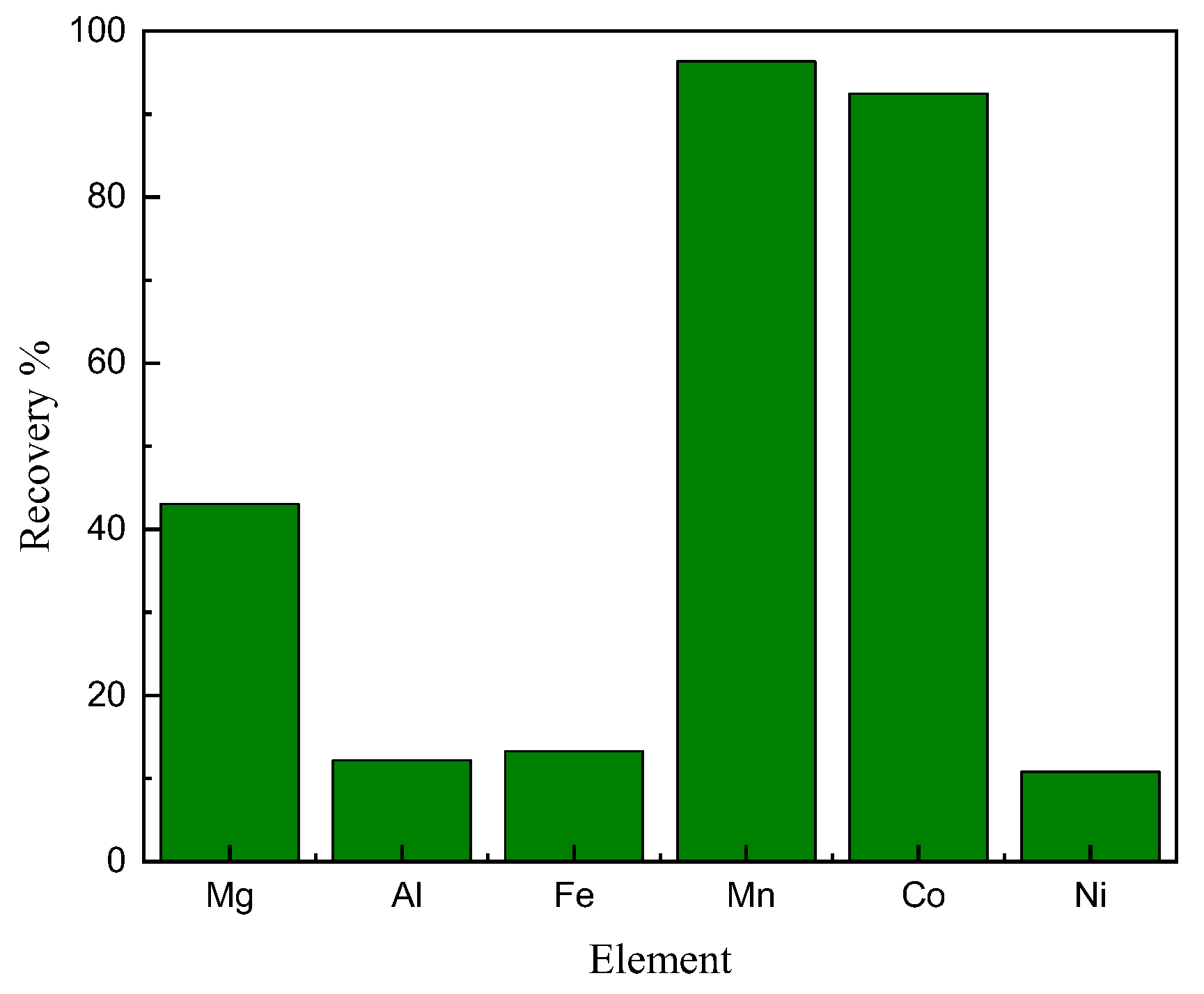
In the extraction process, 2M hydrochloric acid was gradually added to the organic–aqueous solution, lowering the equilibrium pH from 7.5 to 4.7 to achieve the desired value. Under this controlled condition, the process successfully extracted 96% of cobalt and 10% of nickel into the organic solution, as shown in
Figure 5. Additionally, manganese and magnesium were extracted at percentages of 92% and 43%, respectively. Following extraction, the organic solution loaded with cobalt, manganese, and other elements underwent stripping, leaving a raffinate solution enriched with nickel and low amounts of other impurities.
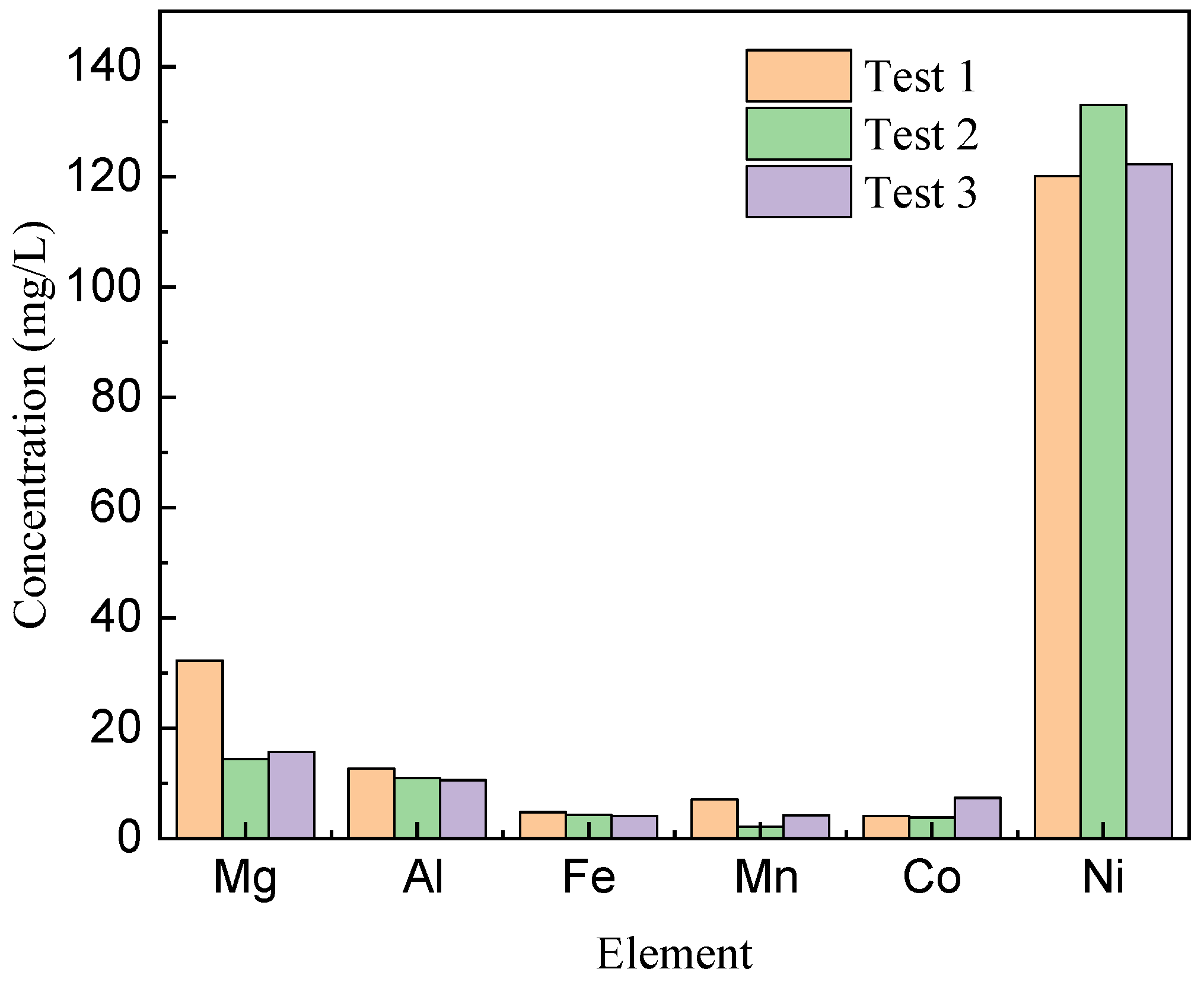
Replicate tests were conducted under the specified conditions, maintaining an equilibrium pH of 4.7. Across the three tests, the resulting raffinate solution exhibited slight variations in nickel content, ranging from 120 to 133 mg/L, cobalt concentrations from 3.8 to 7.3 mg/L, and manganese levels between 2.1 and 7.1 mg/L. Furthermore, certain impurities, including magnesium (an average concentration of 20.8 mg/L), aluminum (an average concentration of 11.4 mg/L), and iron (an average concentration of 5.0 mg/L), persisted in the raffinate solution (
Figure 6). The blue color of the organic phase during solvent extraction indicates cobalt enrichment with saponified Cyanex 272, as shown in
Figure 7.
4.5.3. Stripping of the Loaded Organic Solution
Following solvent extraction, the organic solution underwent a stripping process using 2M sulfuric acid (H
2SO
4). The procedure involved mixing the organic solution with 2M sulfuric acid at an O:A ratio of 1:1 for 5 min at 600 RPM. During this stage, the blue color of the organic solution changed to milky white, indicating the transfer of metals, particularly cobalt, into the acid solution (
Figure 6). Subsequently, the organic and aqueous solutions were separated, and the aqueous solution underwent ICP-MS analysis to determine its elemental composition.
The analysis revealed a cobalt recovery of 82% from the organic solution, corresponding to a concentration of 52 mg/L in the aqueous solution. Additionally, manganese and zinc were extracted into the sulfuric solution, with concentrations of 95.5 mg/L and 106 mg/L, respectively. While these data provide insight into the efficiency of solvent extraction in separating nickel from cobalt, it is important to note that the organic solution also contained other elements, thereby reducing the purity of the strip solution in terms of cobalt.
4.5.4. Na2S Precipitation of the Raffinate and Stripping Solution
During the solvent extraction process using Cyanex 272, two aqueous solutions with varying critical mineral contents were produced. These solutions were then treated with sodium sulfide (Na2S) to yield solid products of nickel and cobalt. The raffinate solution, predominantly containing nickel, had its initial pH raised from 5 to 10, resulting in the formation of solids. Similarly, the strip solution containing cobalt and manganese had its pH adjusted from 0 to 5, leading to the precipitation of solids in the solution, which were subsequently separated via centrifugation and filtration.
In the precipitation of the raffinate solution, 99.5% of the nickel was recovered with a solid purity of 13.94 wt.%. Other detected elements had purities below 1 wt.%, except for silicon, which accounted for 2.56 wt.%. However, the precipitation of the stripping solution failed to produce a high-purity cobalt product despite recovering 80% of the cobalt. The low cobalt purity (0.4 wt.%) could be attributed to factors such as the low cobalt concentration in the stripping solution and the presence of other elements like silicon and zinc, which accounted for 0.83 wt.% and 1.47 wt.%, respectively, in the precipitated solid.
4.7. Experimental Flowsheet
Based on the studies conducted throughout this research and the results presented above, a laboratory experimental flowsheet was developed to extract multiple CMs from a coal waste stream. As mentioned earlier, this research served as a continuation of a previously published study [
20]. A coal AMD treatment sludge was used as the feedstock, and a process was developed to produce a mixed REO product with a purity greater than 80 wt.% [
20]. This presented study utilized the raffinate from REE SX for the continuous extraction of additional CMs, including manganese, cobalt, and nickel. As seen in
Figure 13, the developed flowsheet consists of multi-stage precipitation, solid dissolution, followed by sodium sulfide precipitation, redissolution, solvent extraction, and finally, product precipitation. Operating conditions of each stage were explored and determined based on the target process selectivity, with the ultimate objective of generating purer products. Resulting from this developed hydrometallurgical extraction process, multiple products were generated, including manganese, nickel, magnesium, and aluminum concentrates, with a purity of 47 wt.%, 14 wt.%, 27 wt.%, and 8.34 wt.%, respectively. In addition, an intermediate solid product was also obtained from hydrochloric acid redissolution, which contains approximately 10 wt.% of cobalt and 11 wt.% of nickel sulfides.