Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Clay Minerals
2.2. Reagents
2.3. Sorption Studies
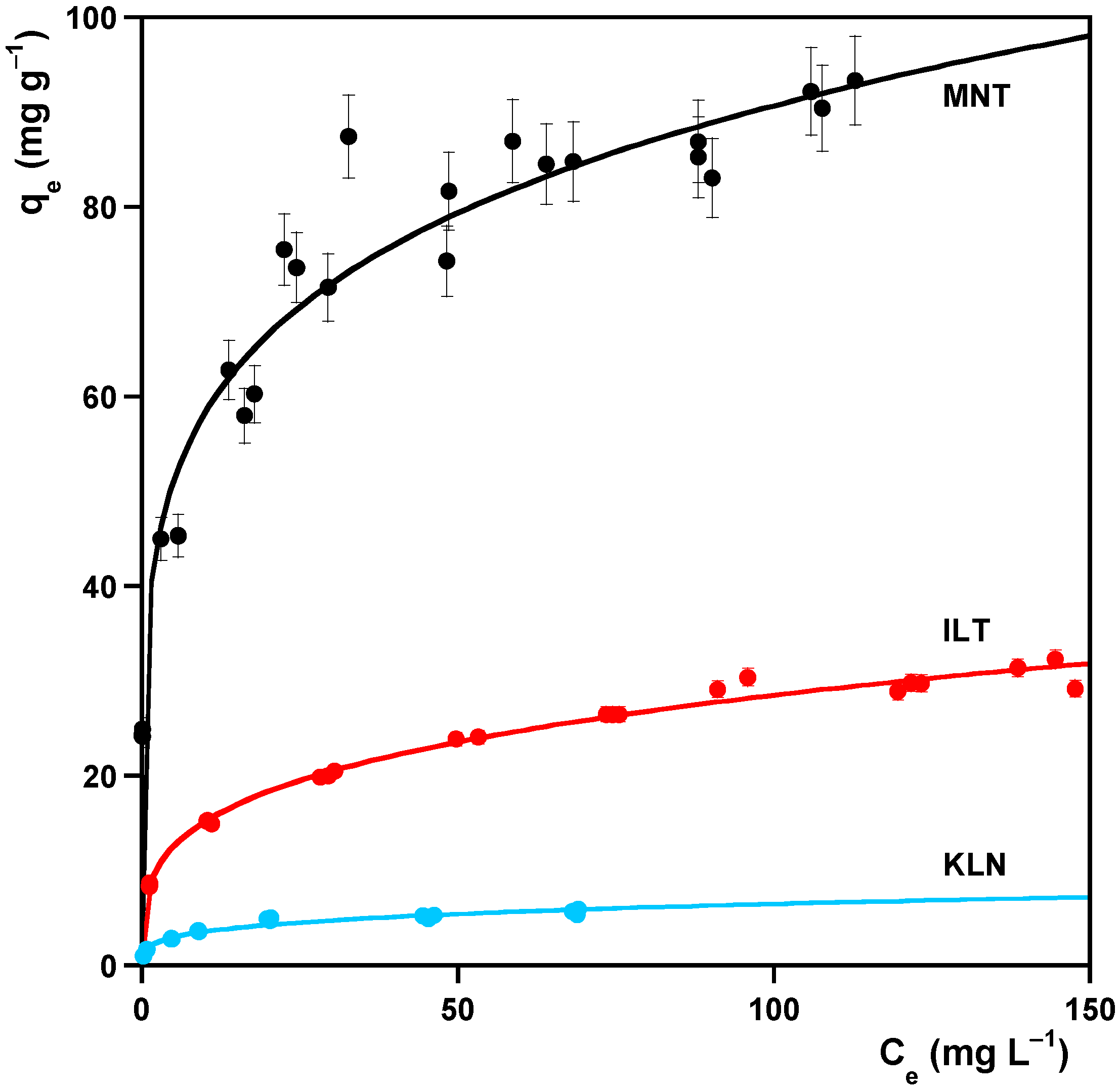
2.3.1. Sorption Isotherms
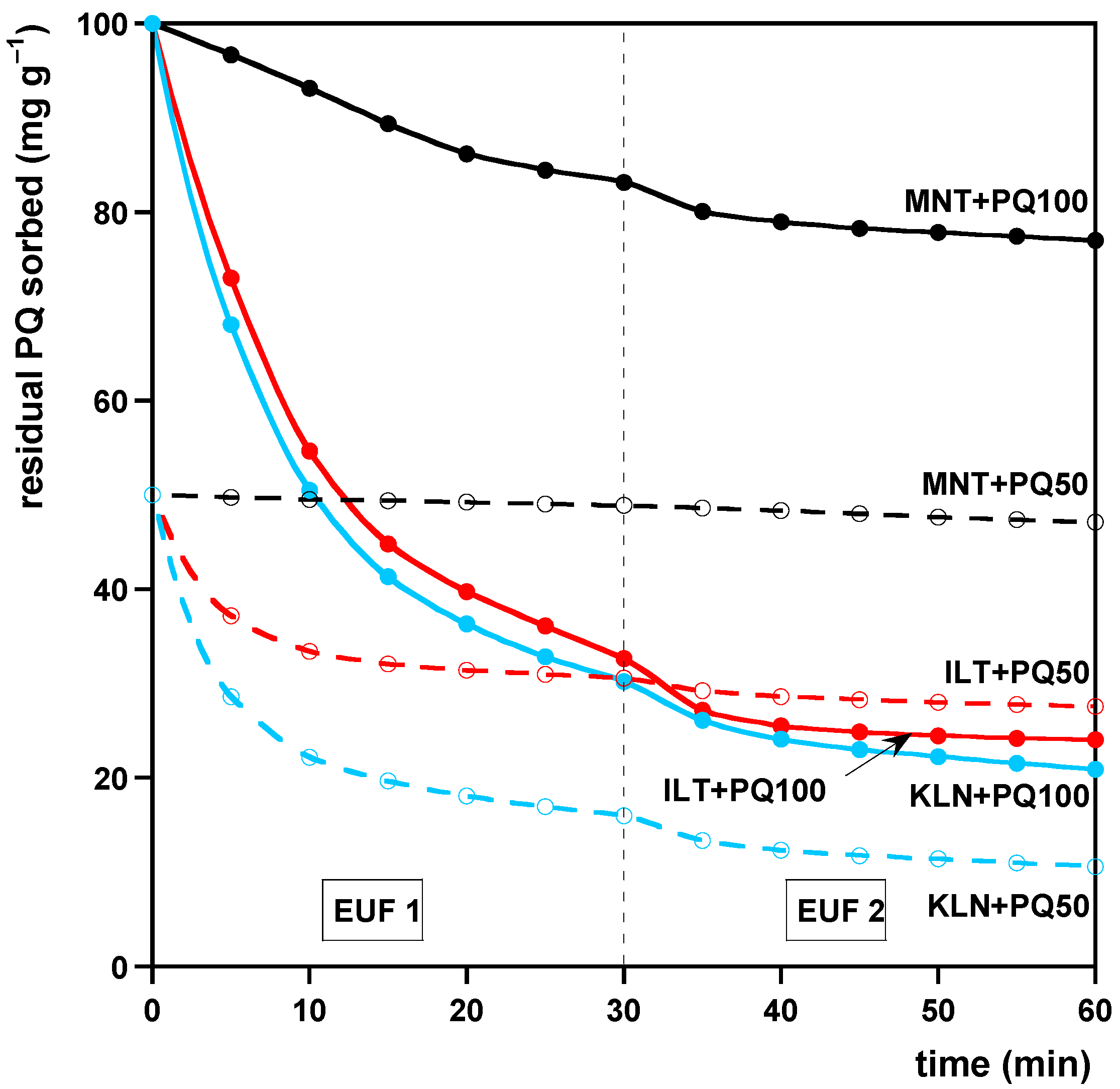
2.4. Desorption Studies
2.4.1. Addition of PQ to the Clays
- (i)
- Clays containing an amount of PQ corresponding to their CEC value. We added 10 mL of aqueous solutions containing the suitable amount of PQ to 200 mg of KLN, ILT, or MNT into stoppered 100 mL polypropylene centrifuge tubes. The suspensions were kept under agitation as described for the sorption studies and then gently evaporated to dryness in an oven at 40 °C for 3 days, allowing the residual PQ in the liquid phase to deposit on the clays. The samples prepared in this way were denoted as KLN + PQCEC, ILT + PQCEC, and MNT + PQCEC, respectively.
- (ii)
- Clays containing an amount of PQ in the ratios of 10:1 and 20:1 (w:w), roughly corresponding to the average PQ amount sorbable by KLT or MNT and the highest PQ amount sorbable by MNT in the sorption experiments (see Section 2.3.1), respectively. We added 10 mL of aqueous solutions containing 10 or 20 mg of PQ to 200 mg of each clay. The samples were then prepared as described above in (i) and hereafter denoted as KLN + PQ100, ILT + PQ100, and MNT + PQ100 or KLN + PQ50, ILT + PQ50, and MNT + PQ50, respectively.
- (iii)
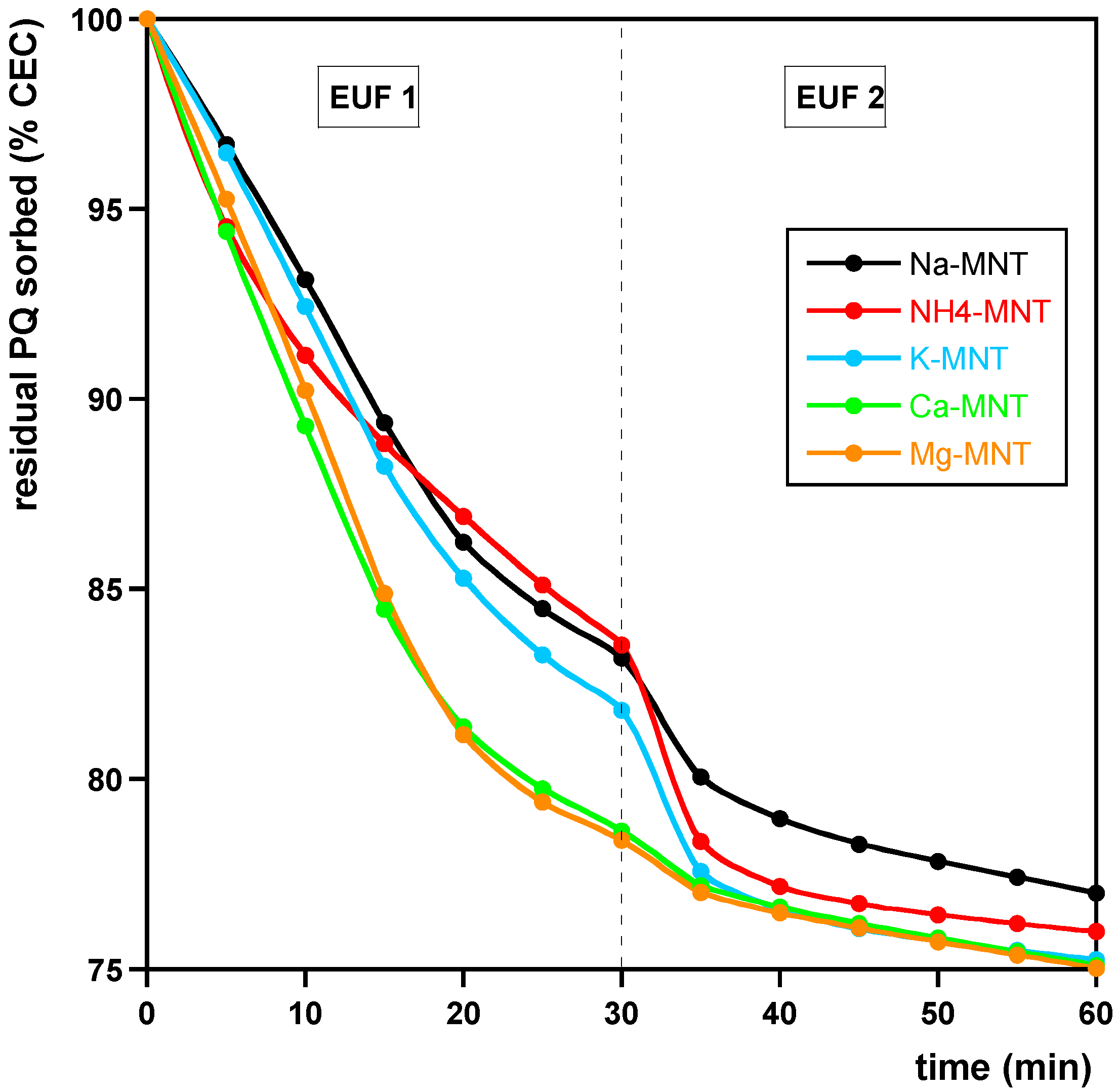
- Samples containing amounts of PQ corresponding to the CEC of MNT, in which MNT was saturated with K+, NH4+, Ca2+, or Mg2+ ions according to the procedure described for Na saturation (see Section 2.1). The samples were prepared as in (i) and henceforth are referred to as Na- K-, NH4-, Ca-, and Mg-MNT, respectively.
2.4.2. Desorption of PQ by EUF (EUF-PQ)
2.5. Determination of PQ in Solution
2.6. Data modelling
- = amount of sorbate taken up per unit mass of sorbent at a given equilibrium concentration in solution;
- = maximum amount of sorbate that may be bound;
- = equilibrium Langmuir constant;
- = Freundlich constant;
- = heterogeneity index of the Freundlich model;
- = Jovanović constant.
- = residual sorbed amount of PQ per mass of clay at time t;
- t = extraction time;
- and = kinetic constants related to the EUF release rate of PQ;
- = initial content of desorbable PQ;
- = maximum amount of PQ per mass of clay that can be released.
3. Results
3.1. Sorption
3.2. Desorption
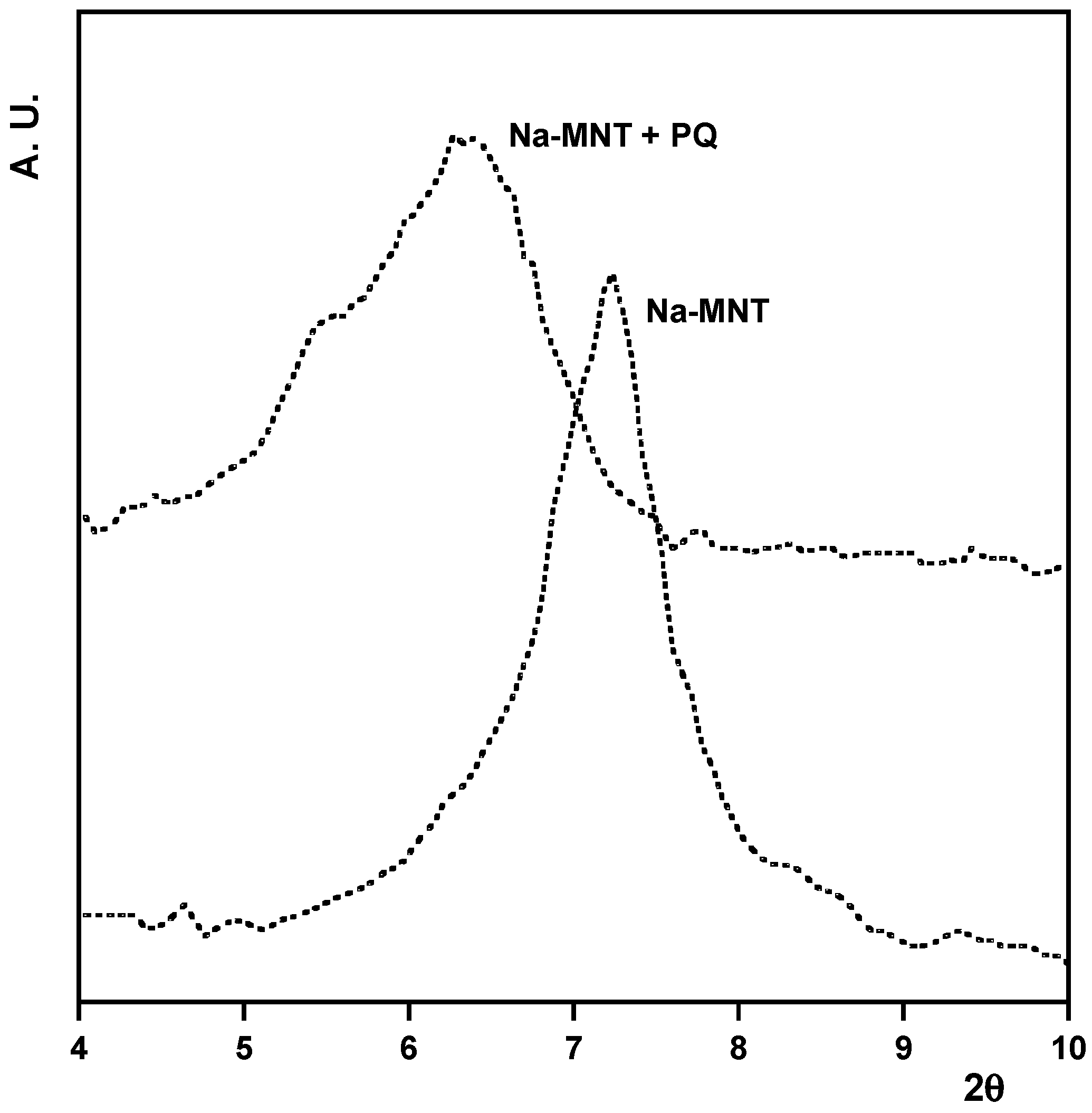
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PQ | Paraquat |
| KLN | Kaolinite |
| ILT | Illite |
| MNT | Montmorillonite |
| EUF | Electro–Ultrafiltration |
| EUF 1 | First extraction step at low energy |
| EUF 2 | Second extraction step at high energy |
| CEC | Cation-exchange capacity (cmol kg−1) |
| KLN + PQCEC | KLN containing an amount of PQ corresponding to its CEC value |
| ILT + PQCEC | ILT containing an amount of PQ corresponding to its CEC value |
| MNT + PQCEC | MNT containing an amount of PQ corresponding to its CEC value |
| KLN + PQ100 | KLN containing an amount of PQ at a ratio of 10:1 (w:w) |
| ILT + PQ100 | ILT containing an amount of PQ at a ratio of 10:1 (w:w) |
| MNT + PQ100 | MNT containing an amount of PQ at a ratio of 10:1 (w:w) |
| KLN + PQ50 | KLN containing an amount of PQ at a ratio of 20:1 (w:w) |
| ILT + PQ50 | ILT containing an amount of PQ at a ratio of 20:1 (w:w) |
| MNT + PQ50 | MNT containing an amount of PQ at a ratio of 20:1 (w:w) |
| Na-MNT | MNT saturated with Na+ containing amounts of PQ corresponding to its CEC value |
| K-MNT | MNT saturated with K+ containing amounts of PQ corresponding to its CEC value |
| NH4-MNT | MNT saturated with NH4+ containing amounts of PQ corresponding to its CEC value |
| Ca-MNT | MNT saturated with Ca2+ containing amounts of PQ corresponding to its CEC value |
| Mg-MNT | MNT saturated with Mg2+ containing amounts of PQ corresponding to its CEC value |
| Sorbed amount per mass of clay at equilibrium (mg g−1) | |
| Maximum sorbable amount per mass of clay (mg g−1) | |
| Equilibrium Langmuir constant (L mg−1) | |
| Freundlich constant (LN mg1−N g−1) | |
| Heterogeneity index of the Freundlich model (adimensional) | |
| Jovanović constant (L mg−1) | |
| PFO | Pseudo-first-order kinetic model |
| PSO | Pseudo-second-order kinetic model |
| Residual sorbed amount per mass of clay at time t | |
| t | Extraction time |
| Kinetic rate constant related to the PFO kinetic model | |
| Kinetic constants related to the EUF release rate of PQ | |
| Initial content of releasable PQ | |
| Maximum amount of releasable PQ per mass of clay |
References
- Syngenta Crop Protection. Available online: https://www.syngenta.com/en/protecting-crops/products-list/paraquat (accessed on 12 January 2024).
- Wesseling, C.; Van Wendel De Joode, B.; Ruepert, C.; León, C.; Monge, P.; Hermosillo, H.; Partanen, L.J. Paraquat in Developing Countries. Int. J. Occup. Environ. Health 2001, 7, 275–286. [Google Scholar] [CrossRef]
- Donaher, S.E.; Van den Hurk, P. Ecotoxicology of the herbicide paraquat: Effects on wildlife and knowledge gaps. Ecotoxicology 2023, 32, 1187–1199. [Google Scholar] [CrossRef]
- Ardiwinata, A.N.; Harsanti, E.S.; Kurnia, A.; Sulaeman, E. The distribution of paraquat and carbosulfan residues in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012033. [Google Scholar] [CrossRef]
- Bromilow, R.H. Paraquat and sustainable agriculture. Pest Manag. Sci. 2004, 60, 340–349. [Google Scholar] [CrossRef]
- Wang, H.; Pei, Z.; Chen, G.; Xing, B. Mutual influence of copper and paraquat on their adsorption in soil. Pedosphere 2023, 33, 857–864. [Google Scholar] [CrossRef]
- Pateiro-Moure, M.; Arias-Estévez, M.; Simal-Gándara, J. Competitive and non-competitive adsorption/desorption of paraquat, diquat and difenzoquat in vineyard-devoted soils. J. Hazard. Mater. 2010, 178, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Canzano, S.; Iovino, P.; Leone, V.; Capasso, S. Modelling the biphasic sorption of simazine, imidacloprid, and boscalid in water/soil systems. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Yurdakoç, K. Paraquat adsorption onto clays and organoclays from aqueous solution. J. Colloid Interface Sci. 2005, 287, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Spark, K.M.; Swift, R.S. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R.; Dyson, J.S.; Lane, M.C.G. Deactivation of the biological activity of paraquat in the soil environment: A review of long-term environmental fate. J. Agric. Food Chem. 2002, 50, 3623–3631. [Google Scholar] [CrossRef]
- Weber, J.B.; Meek, R.C.; Weed, S.B. The Effect of Cation-Exchange Capacity on the Retention of Diquat2+ and Paraquat2+ by Three-Layer Type Clay Minerals: II. Plant Availability of Paraquat. Soil Sci. Soc. Am. J. 1969, 33, 382–385. [Google Scholar] [CrossRef]
- Summers, L.A. The Bipyridinium Herbicides; Academic Press: London, UK; New York, NY, USA, 1980; p. 449. [Google Scholar]
- Moyer, J.R.; Lindwall, C.W. Persistence and availability of paraquat in a Lethbridge clay loam soil. Can. J. Soil Sci. 1985, 65, 523–529. [Google Scholar] [CrossRef]
- Tucker, B.V.; Pack, D.E.; Ospenson, J.N.; Omid, A.; Thomas, W.D. Paraquat Soil Bonding and Plant Response. Weed Sci. 1969, 17, 448–451. [Google Scholar] [CrossRef]
- Boyd, N.S. Pepper and Tomato Root Uptake of Paraquat and Flumioxazin. Weed Technol. 2014, 28, 626–632. [Google Scholar] [CrossRef]
- Ipor, I.B.; Price, C.E. Uptake, translocation and activity of paraquat on Mikania micrantha H.B.K. grown in different light conditions. Int. J. Pest Manag. 1994, 40, 40–45. [Google Scholar] [CrossRef]
- Hart, J.J.; Di Tomaso, J.M.; Linscott, D.L.; Kochian, L.V. Characterization of the transport and cellular compartmentation of paraquat in roots of intact maize seedlings. Pestic. Biochem. Physiol. 1992, 43, 212–222. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Hart, J.J.; Kochian, L.V. Compartmentation Analysis of Paraquat Fluxes in Maize Roots as a Means of Estimating the Rate of Vacuolar Accumulation and Translocation to Shoots. Plant Physiol. 1993, 102, 467–472. [Google Scholar] [CrossRef]
- Cheah, U.B.; Kirkwood, R.C.; Lum, K.Y. Adsorption, desorption and mobility of four commonly used pesticides in Malaysian agricultural soils. Pestic. Sci. 1997, 50, 53–63. [Google Scholar] [CrossRef]
- Kumar, J.; Nisar, K.; Shakil, N.A.; Walia, S.; Parsad, R. Controlled release formulations of metribuzin: Release kinetics in water and soil. J. Environ. Sci. Health Part B 2010, 45, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Vanore, P.; Iovino, P.; Leone, V.; Capasso, S. Adsorption of simazine and boscalid onto acid-activated natural clinoptilolite. Environ. Eng. Manag. J. 2015, 14, 1705–1712. [Google Scholar] [CrossRef]
- Németh, K. The availability of nutrients in the soil as determined by electro-ultrafiltration (EUF). Adv. Agron. 1980, 31, 155–188. [Google Scholar] [CrossRef]
- Sager, M. Modelling the Plant Uptake of Metals from Release Rates Obtained by the EUF Method. Plants 2021, 11, 85. [Google Scholar] [CrossRef]
- Chen, G.; Song, W.; Qi, B.; Lu, J.; Wan, Y. Recycling cellulase from enzymatic hydrolyzate of acid treated wheat straw by electroultrafiltration. Bioresour. Technol. 2013, 144, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yi, X.; Gao, Y. Effect of Electric Field on Membrane Fouling and Membrane Performance in Reverse Osmosis Treatment of Brackish Water. Appl. Sci. 2024, 14, 575. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Li, M.; Zuo, K.; Liang, S.; Xiao, K.; Liang, P.; Wang, X.; Huang, X. Electrically Tuning Ultrafiltration Behavior for Efficient Water Purification. Environ. Sci. Technol. 2020, 54, 11536–11545. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, A.; Felleca, D.; Bufo, S.A.; Pizzigallo, M.D.R.; Testini, C. Comparison between electro-ultrafiltration and extraction methods for the determination of k fractions in some soils of southern Italy. Commun. Soil Sci. Plant Anal. 1988, 19, 239–258. [Google Scholar] [CrossRef]
- Jelecevic, A.; Horn, D.; Eigner, H.; Sager, M.; Liebhard, P.; Moder, K.; Vollprecht, D. Kinetics of lead release from soils at historic mining and smelting sites, determined by a modified electro-ultrafiltration. Plant Soil Environ. 2019, 65, 298–306. [Google Scholar] [CrossRef]
- Varga, I.; Jović, J.; Rastija, M.; Markulj Kulundžić, A.; Zebec, V.; Lončarić, Z.; Iljkić, D.; Antunović, M. Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen 2022, 3, 170–185. [Google Scholar] [CrossRef]
- Postolache, S.; Sebastião, P.; Viegas, V.; Postolache, O.; Cercas, F. IoT-Based Systems for Soil Nutrients Assessment in Horticulture. Sensors 2022, 23, 403. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Jovanović, D.S. Physical adsorption of gases—I: Isotherms for monolayer and multilayer adsorption. Kolloid-Z. Z. Polym. 1969, 235, 1203–1213. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; Debord, J.; Harel, M.; Salvestrini, S.; Bollinger, J.C. The Jovanović adsorption isotherm in water contaminant research: Unmasking spurious versions and spotlighting the real thing. Chem. Eng. Sci. 2023, 281, 119127. [Google Scholar] [CrossRef]
- Salvestrini, S. A modification of the Langmuir rate equation for diffusion-controlled adsorption kinetics. React. Kinet. Mech. Catal. 2019, 128, 571–586. [Google Scholar] [CrossRef]
- Debord, J.; Chu, K.H.; Harel, M.; Salvestrini, S.; Bollinger, J.C. Yesterday, Today, and Tomorrow. Evolution of a Sleeping Beauty: The Freundlich Isotherm. Langmuir 2023, 39, 3062–3071. [Google Scholar] [CrossRef] [PubMed]
- Ilari, R.; Etcheverry, M.; Waiman, C.V.; Zanini, G.P. A simple cation exchange model to assess the competitive adsorption between the herbicide paraquat and the biocide benzalkonium chloride on montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125797. [Google Scholar] [CrossRef]
- Wang, M.; Orr, A.A.; He, S.; Dalaijamts, C.; Chiu, W.A.; Tamamis, P.; Phillips, T.D. Montmorillonites Can Tightly Bind Glyphosate and Paraquat Reducing Toxin Exposures and Toxicity. ACS Omega 2019, 4, 17702–17713. [Google Scholar] [CrossRef] [PubMed]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of interlayer cations on montmorillonite swelling: Comparison between molecular dynamic simulations and experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]

| Stage | Temperature (°C) | Maximum Electric Field Strength (V) | Current Intensity (mA) | Extraction Time (min) |
|---|---|---|---|---|
| EUF 1 | 20 | 200 | 15 | 0–30 |
| EUF 2 | 80 | 400 | 150 | 30–60 |
| Clay | Model | (mg g−1) | (L mg−1) | (LN mg1−N g−1) | (L mg−1) | R2 | |
|---|---|---|---|---|---|---|---|
| MNT | Langmuir | 95 ± 2 | 0.13 ± 0.02 | 0.9939 | |||
| MNT | Freundlich | 0.18 ± 0.02 | 40 ± 3 | 0.9925 | |||
| MNT | Jovanović | 87 ± 1 | 0.0081 ± 0.009 | 0.9915 | |||
| ILT | Langmuir | 33 ± 1 | 0.070 ± 0.010 | 0.9991 | |||
| ILT | Freundlich | 0.27 ± 0.01 | 8.1 ± 0.4 | 0.9998 | |||
| ILT | Jovanović | 28.9 ± 0.8 | 0.050 ± 0.007 | 0.9982 | |||
| KLN | Langmuir | 5.7 ± 0.2 | 0.24 ± 0.03 | 0.9998 | |||
| KLN | Freundlich | 0.25 ± 0.02 | 2.1 ± 0.1 | 0.9999 | |||
| KLN | Jovanović | 5.2 ± 0.2 | 0.16 ± 0.02 | 0.9997 |
| Model | (%) | (min−1) | (min−1) | |
|---|---|---|---|---|
| KLN | ||||
| PFO (EUF-PQCEC) | 65 ± 1 | 0.029 ± 0.001 | 0.9931 | |
| PSO (EUF-PQCEC) | 91 ± 2 | 0.00027 ± 0.00002 | 0.9967 | |
| PFO (EUF-PQ50) | 79.6 ± 0.2 | 0.072 ± 0.001 | 0.9906 | |
| PSO (EUF-PQ50) | 87.6 ± 0.6 | 0.0068 ± 0.0004 | 0.9901 | |
| PFO (EUF-PQ100) | 79.9 ± 0.1 | 0.074 ± 0.001 | 0.9947 | |
| PSO (EUF-PQ100) | 87.4 ± 0.7 | 0.0018 ± 0.0001 | 0.9826 | |
| ILT | ||||
| PFO (EUF-PQCEC) | 42.2 ± 0.2 | 0.056 ± 0.001 | 0.9958 | |
| PSO (EUF-PQCEC) | 50 ± 1 | 0.0016 ± 0.0001 | 0.9891 | |
| PFO (EUF-PQ50) | 45.5 ± 0.1 | 0.069 ± 0.001 | 0.9955 | |
| PSO (EUF-PQ50) | 50.6 ± 0.2 | 0.0104 ± 0.0004 | 0.9940 | |
| PFO (EUF-PQ100) | 76.4 ± 0.1 | 0.15 ± 0.09 | 0.9782 | |
| PSO (EUF-PQ100) | 81 ± 1 | 0.0034 ± 0.0006 | 0.9072 | |
| MNT | ||||
| PFO (EUF-PQCEC) | 24.2 ± 0.1 | 0.051 ± 0.001 | 0.9950 | |
| PSO (EUF-PQCEC) | 29 ± 1 | 0.0022 ± 0.0002 | 0.9868 | |
| PFO (EUF-PQ50) | 105.6 ± 0.2 | 0.099 ± 0.005 | 0.9995 | |
| PSO (EUF-PQ50) | 110.4 ± 0.4 | 0.014 ± 0.001 | 0.9999 | |
| PFO (EUF-PQ100) | 24.1 ± 0.1 | 0.051 ± 0.001 | 0.9950 | |
| PSO (EUF-PQ100) | 29 ± 1 | 0.0022 ± 0.0002 | 0.9868 | |
| Model | (%) | (min−1) | (min−1) | |
|---|---|---|---|---|
| Na-MNT | ||||
| PFO (EUF-PQCEC) | 24.1 ± 0.1 | 0.051 ± 0.001 | 0.9950 | |
| PSO (EUF-PQCEC) | 29 ± 1 | 0.0022 ± 0.0002 | 0.9868 | |
| K-MNT | ||||
| PFO (EUF-PQCEC) | 25.3 ± 0.1 | 0.063 ± 0.002 | 0.9856 | |
| PSO (EUF-PQCEC) | 29 ± 1 | 0.0036 ± 0.0005 | 0.9577 | |
| NH4-MNT | ||||
| PFO (EUF-PQCEC) | 24.7 ± 0.2 | 0.062 ± 0.003 | 0.9616 | |
| PSO (EUF-PQCEC) | 28 ± 1 | 0.0036 ± 0.0007 | 0.9228 | |
| Ca-MNT | ||||
| PFO (EUF-PQCEC) | 25.2 ± 0.1 | 0.065 ± 0.002 | 0.9724 | |
| PSO (EUF-PQCEC) | 28.4 ± 0.1 | 0.0040 ± 0.0002 | 0.9964 | |
| Mg-MNT | ||||
| PFO (EUF-PQCEC) | 25.3 ± 0.1 | 0.067 ± 0.002 | 0.9709 | |
| PSO (EUF-PQCEC) | 28.3 ± 0.1 | 0.0044 ± 0.0002 | 0.9962 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvestrini, S.; Grilli, E.; Coppola, E. Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques. Environments 2024, 11, 53. https://doi.org/10.3390/environments11030053
Salvestrini S, Grilli E, Coppola E. Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques. Environments. 2024; 11(3):53. https://doi.org/10.3390/environments11030053
Chicago/Turabian StyleSalvestrini, Stefano, Eleonora Grilli, and Elio Coppola. 2024. "Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques" Environments 11, no. 3: 53. https://doi.org/10.3390/environments11030053
APA StyleSalvestrini, S., Grilli, E., & Coppola, E. (2024). Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques. Environments, 11(3), 53. https://doi.org/10.3390/environments11030053






