Submerged Membrane Bioreactor Configurations for Biological Nutrient Removal from Urban Wastewater: Experimental Tests and Model Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pilot Plant
2.2. Wastewater Characterisation
2.3. Calibration Methodology
2.4. Simulation
3. Results and Discussion
3.1. Calibration
3.2. N-REM Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Alk | Alkalinity |
| ASM 1 | Activated Sludge Model |
| BNR | Biological nutrient removal |
| (d−1) | |
| Rate constant for lysis and decay (d−1) | |
| CAS | Conventional activated sludge |
| COD | Chemical oxygen demand |
| C-O | Carbon oxidation |
| DO | Dissolved oxygen |
| Inert soluble COD fraction | |
| Substrate half-saturation coefficient | |
| Inhibition coefficient for oxygen | |
| Hydrolysis rate constant | |
| Saturation coefficient for oxygen | |
| Substrate half-saturation coefficient | |
| HRT | Hydraulic retention time |
| MLSS | Mixed liquor suspended solids |
| MLVS | Mixed liquor volatile suspended solids |
| NH3 | Ammonia |
| NO2− | Nitrite |
| NO3− | Nitrate |
| N-REM | Nitrogen removal |
| OLR | Organic loading rate |
| RAS | Return activated sludge |
| SCOD | Soluble chemical oxygen demand |
| SMBR | Submerged membrane bioreactor |
| SMP | Soluble microbial product |
| SRT | Sludge retention time |
| Inert soluble COD | |
| Readily biodegradable COD | |
| SP | Soluble phosphorus |
| TCOD | Total chemical oxygen demand |
| TN | Total nitrogen |
| TNU | Total nitrogen unit |
| TSS | Total suspended solids |
| TP | Total phosphorus |
| VSS | Volatile suspended solids |
| WWTP | Wastewater treatment plant |
| Autotrophic organisms | |
| Heterotrophic organisms | |
| Inert particulate matter | |
| Hydrolysis of particulate substrate | |
| Yield of autotrophic biomass | |
| Yield coefficient | |
| Maximum growth on substrate | |
| Mean | |
| Standard deviation | |
| Number of readings | |
| Reduction factor for denitrification |
Appendix A
| Parameter | Units | Default Values (20 °C) | Run No. | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Heterotrophic organisms: | ||||||
| gCOD/gCOD | 0.67 | 0.38 | 0.40 | 0.52 | 0.30 | |
| day−1 | 6.00 | 6.00 | 6.00 | 6.0 | 5.00 | |
| day−1 | 0.62 | 0.40 | 0.20 | 0.20 | 0.20 | |
| gCOD/m3 | 20 | 25 | 20 | 20 | 25 | |
| gO2/m3 | 0.20 | 0.30 | 1.00 | 3.00 | 0.10 | |
| Hydrolysis of particulate substrate: | ||||||
| day−1 | 3.00 | 3.50 | 3.50 | 3.50 | 3.00 | |
| gCOD/gCOD | 0.03 | 0.03 | 0.03 | 0.03 | 0.14 | |
| - | 0.40 | 0.75 | 0.75 | 0.75 | 0.30 | |
| Autotrophic organisms: | ||||||
| gCOD/gN | 0.24 | 0.25 | 0.25 | 0.25 | 0.25 | |
| day−1 | 0.80 | 0.50 | 0.80 | 0.80 | 1.00 | |
| day−1 | 0.04 | 0.12 | 0.40 | 0.40 | 0.20 | |
| gO2/m3 | 0.40 | 0.20 | 0.17 | 0.17 | 0.15 | |
| Parameter | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z1—Anoxic | Z2—Anaerobic | Z3—Aerobic | SMBR | Effluent | ||||||
| M | S | M | S | M | S | M | S | M | S | |
| TSS (gSS/m3) | 13,370 | 12,666 | 13,240 | 12,654 | 13,710 | 12,644 | 18,990 | 18,634 | ||
| VSS (gMLVSS/m3) | 9426 | 9117 | 9348 | 9105 | 9766 | 9095 | 13,483 | 13,399 | ||
| Inert solids (gSS/m3) | 3944 | 3549 | 3893 | 3549 | 3944 | 3549 | 5507 | 5235 | ||
| CODsol (mg/L) | 55 | 36 | 51 | 31 | 46 | 30 | 32 | 29 | 32 | 29 |
| NH3 (mgN/L) | 14 | 13 | 9 | 8 | 5 | 3 | 0.6 | 0.44 | 0.6 | 0.44 |
| Nitrogen oxides (mgN/L) | 1.0 | 0.7 | 1.0 | 0.3 | 4.2 | 4.3 | 7.8 | 7.5 | 16 | 7.5 |
| DO (mg/L) | 0.13 | 0.06 | 0.13 | 0.08 | 1.0 | 1.0 | 2.0 | 2.3 | ||
| Parameter | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Z1—Anaerobic | Z2—Anoxic | SMBR | Effluent | |||||
| M | S | M | S | M | S | M | S | |
| TSS (gSS/m3) | 4740 | 4845 | 6660 | 6038 | 7230 | 7193 | ||
| VSS (gMLVSS/m3) | 3570 | 3730 | 4870 | 4645 | 5350 | 5532 | ||
| Inert solids (gSS/m3) | 1170 | 1115 | 1790 | 1394 | 1880 | 1661 | ||
| CODsol (mg/L) | 58 | 25 | 59 | 23 | 57 | 22 | 26 | 22 |
| NH3 (mgN/L) | 22 | 17 | 10 | 7.1 | 2.3 | 2.4 | 1.5 | 2.4 |
| Nitrogen oxides (mgN/L) | 2.4 | 3.1 | 6.5 | 8.3 | 13 | 13 | 17 | 13 |
| DO (mg/L) | 0.16 | 0.18 | 0.22 | 0.21 | 4.8 | 4.5 | ||
| Parameter | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Z1—Anaerobic | Z2—Anoxic | SMBR | Effluent | |||||
| M | S | M | S | M | S | M | S | |
| TSS (gSS/m3) | 9480 | 9063 | 11,920 | 11,764 | 13,770 | 14,164 | ||
| VSS (gMLVSS/m3) | 6470 | 6247 | 8060 | 8105 | 9340 | 9758 | ||
| Inert solids (gSS/m3) | 3010 | 2816 | 3860 | 3659 | 4430 | 4407 | ||
| CODsol (mg/L) | 25 | 27 | 28 | 24 | 28 | 24 | 20 | 24 |
| NH3 (mgN/L) | 16 | 16 | 8.5 | 7.4 | 2.13 | 3.3 | 1.8 | 3.3 |
| Nitrogen oxides (mgN/L) | 0.9 | 0.9 | 2.9 | 3.9 | 7.7 | 7.4 | 9.5 | 7.4 |
| DO (mg/L) | 0.05 | 0.14 | 0.08 | 0.12 | 3.9 | 3.5 | ||
| Parameter | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z1—Anaerobic | Z2—Aerobic | Z3-Anoxic | SMBR | Effluent | ||||||
| M | S | M | S | M | S | M | S | M | S | |
| TSS (gSS/m3) | 6780 | 6441 | 9370 | 9974 | 9610 | 9969 | 11,980 | 13,223 | ||
| VSS (gMLVSS/m3) | 4620 | 4374 | 6230 | 6761 | 6390 | 6756 | 7970 | 8958 | ||
| Inert solids (gSS/m3) | 2200 | 2067 | 3200 | 3213 | 3300 | 3213 | 4100 | 4265 | ||
| CODsol (mg/L) | 45 | 38 | 28 | 24 | 39 | 24 | 39 | 24 | 23 | 24 |
| NH3 (mgN/L) | 10 | 11 | 4.0 | 4.4 | 3.0 | 3.5 | 2.0 | 1.1 | 0.5 | 1.1 |
| Nitrogen oxides (mgN/L) | 0.5 | 0.1 | 3.0 | 4.6 | 4.0 | 3.9 | 7.0 | 6.5 | 8.0 | 6.5 |
| DO (mg/L) | 0.06 | 0.02 | 1.3 | 1.2 | 0.05 | 0.05 | 5.1 | 4.9 | ||


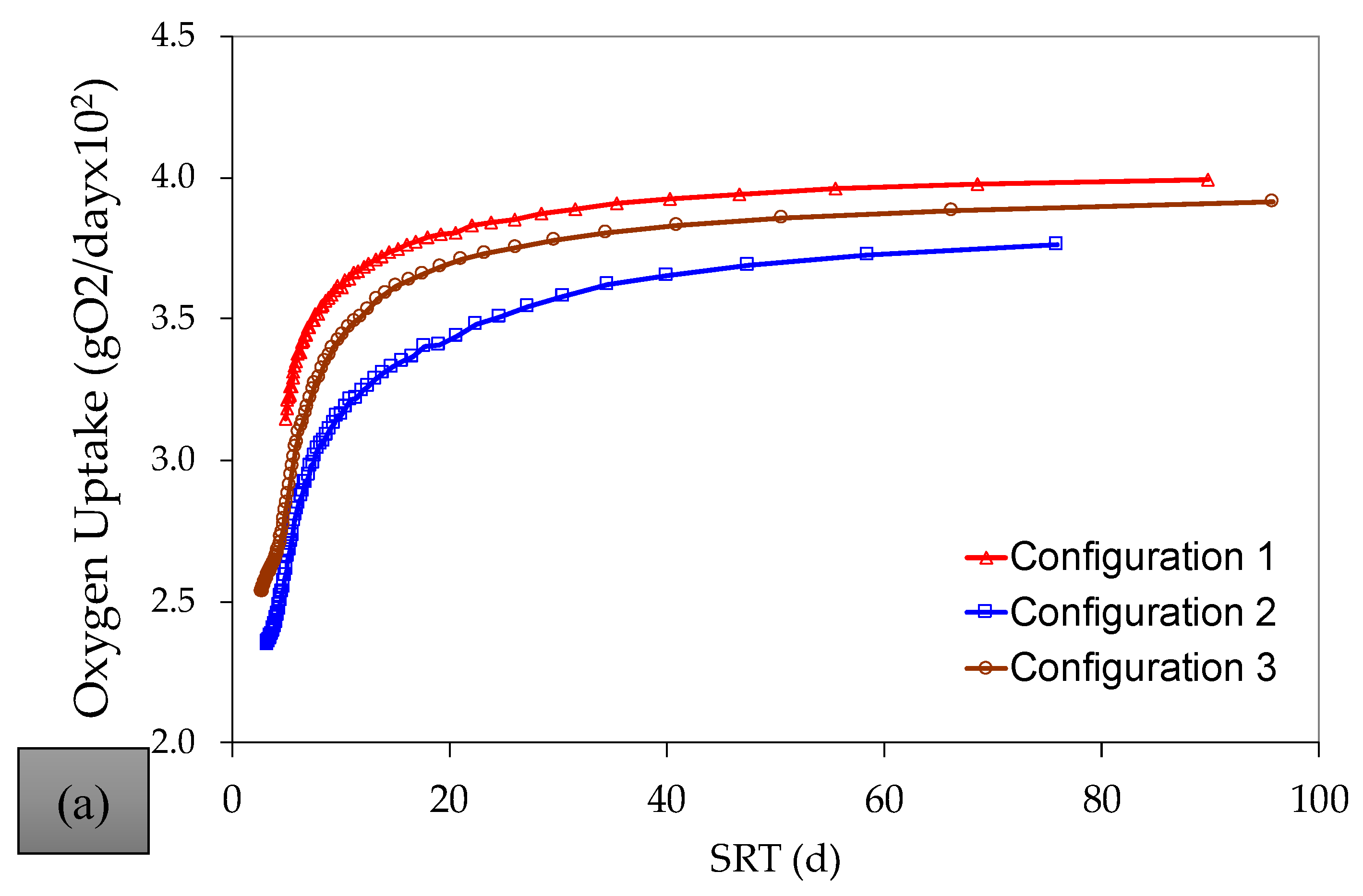
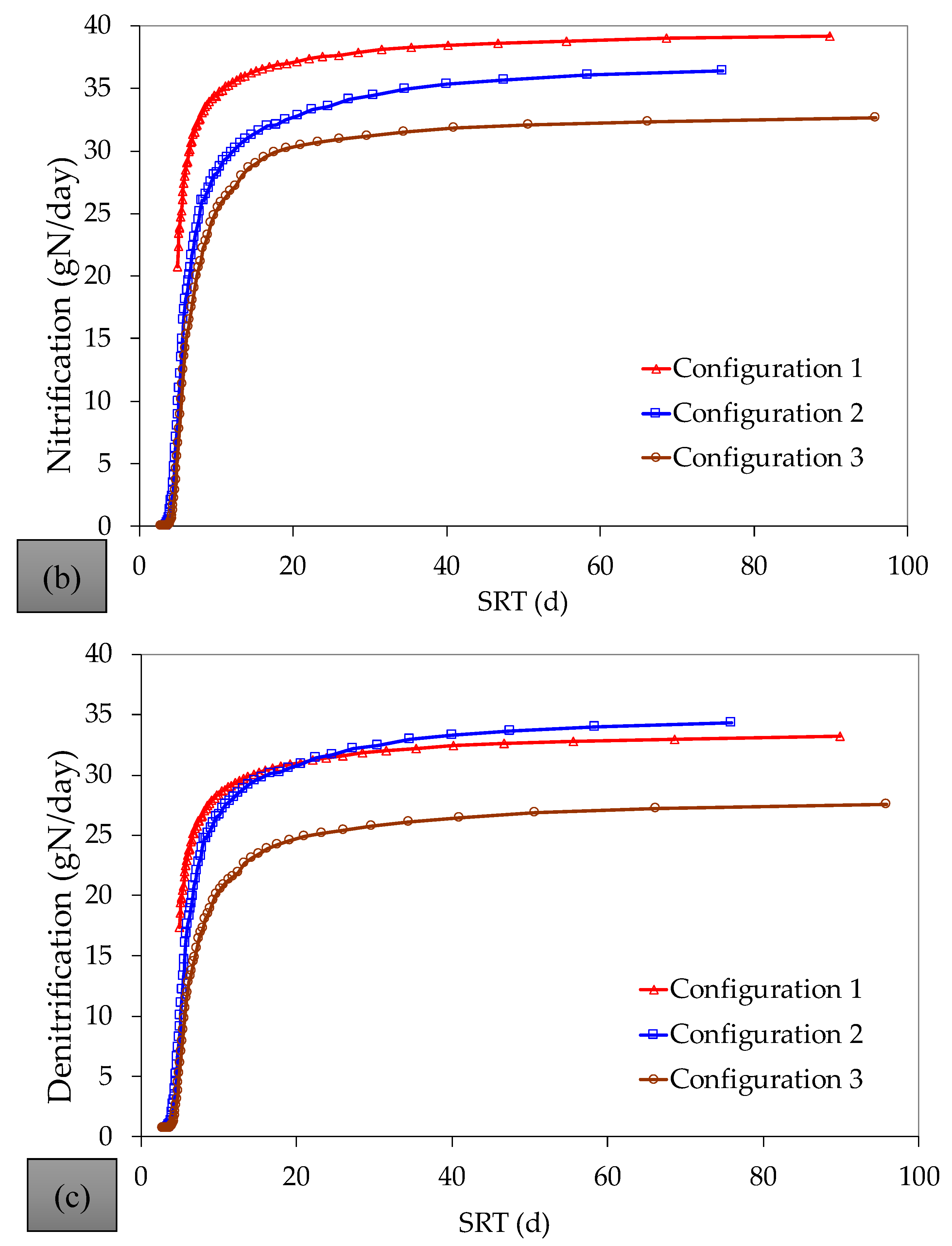
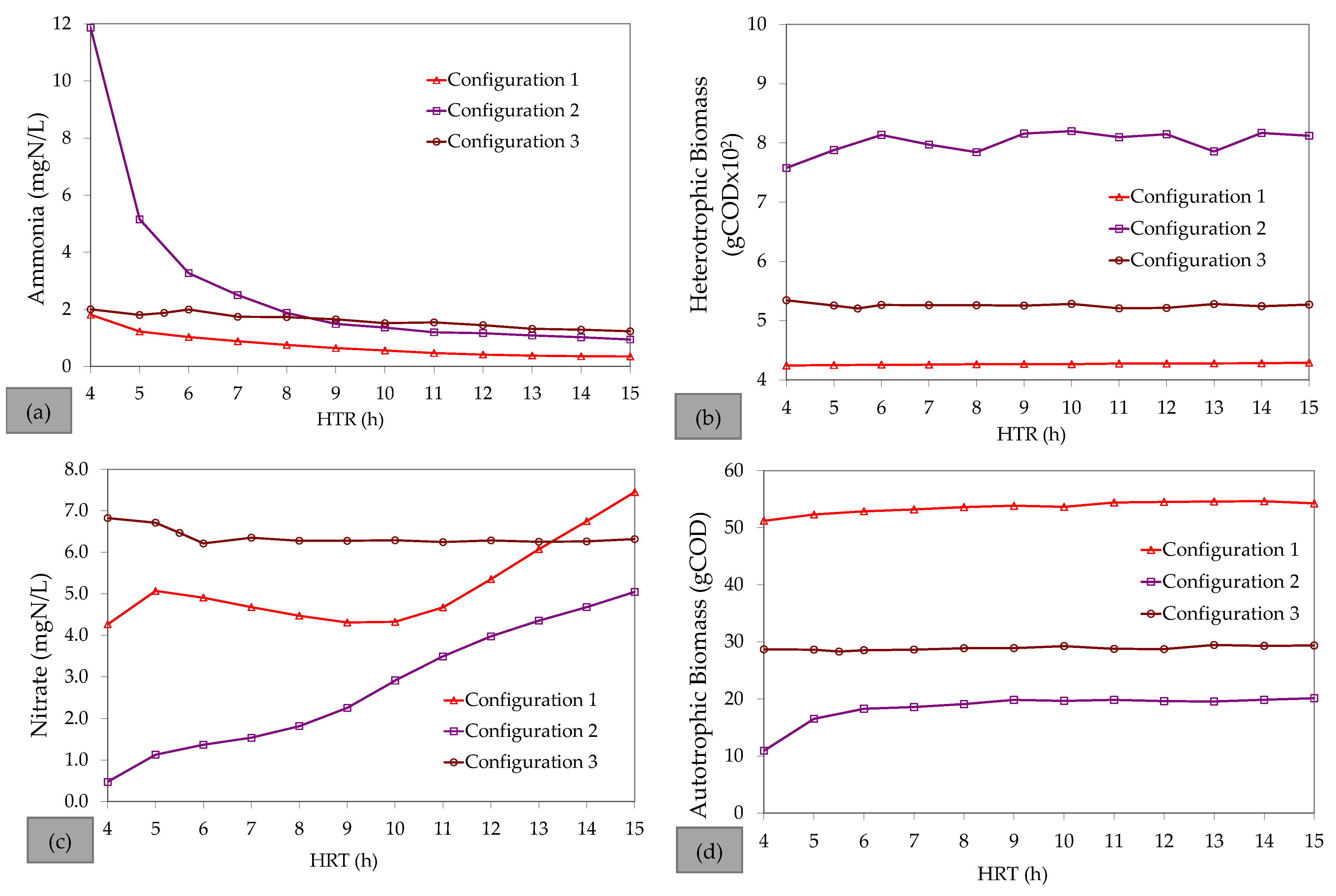
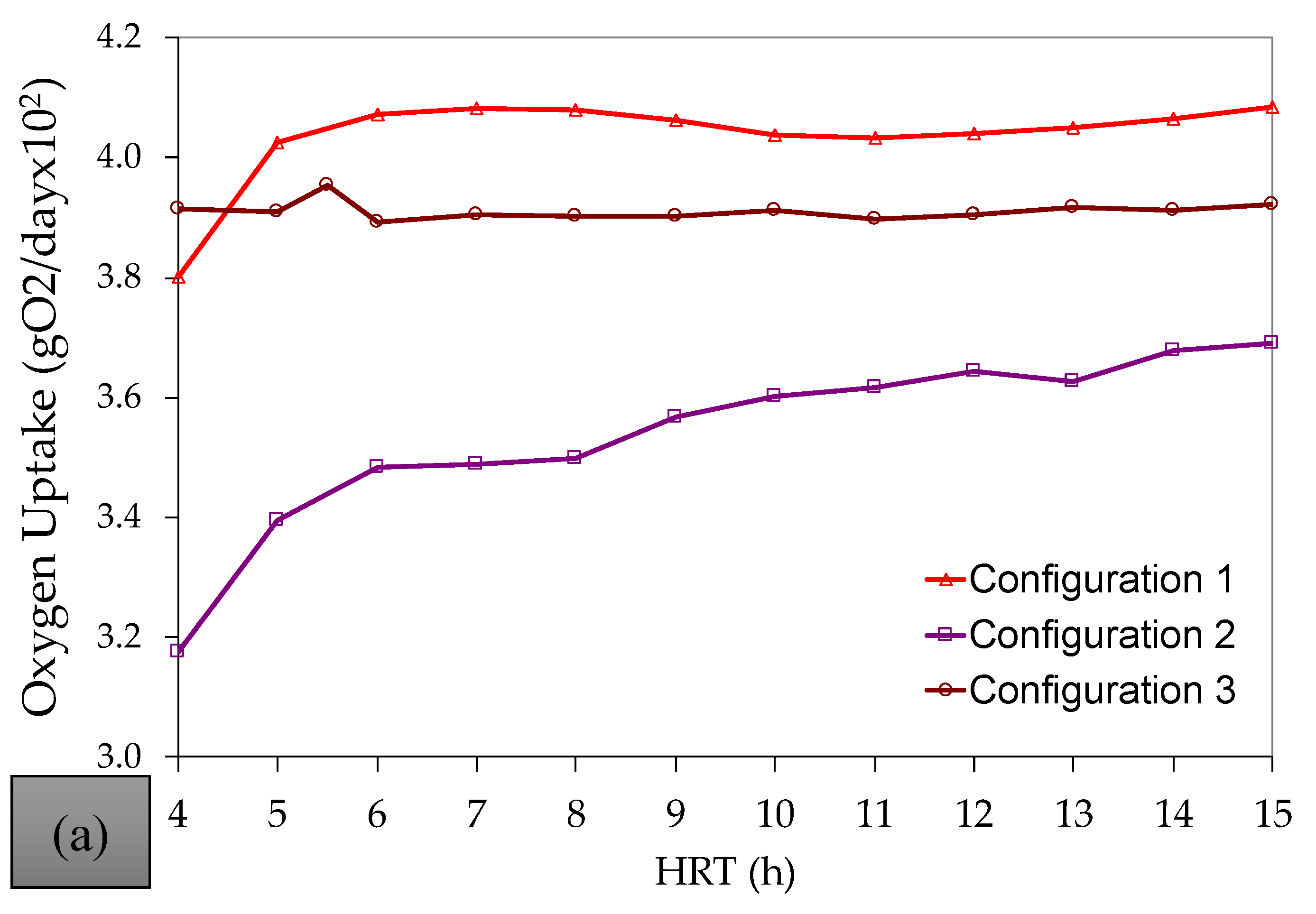
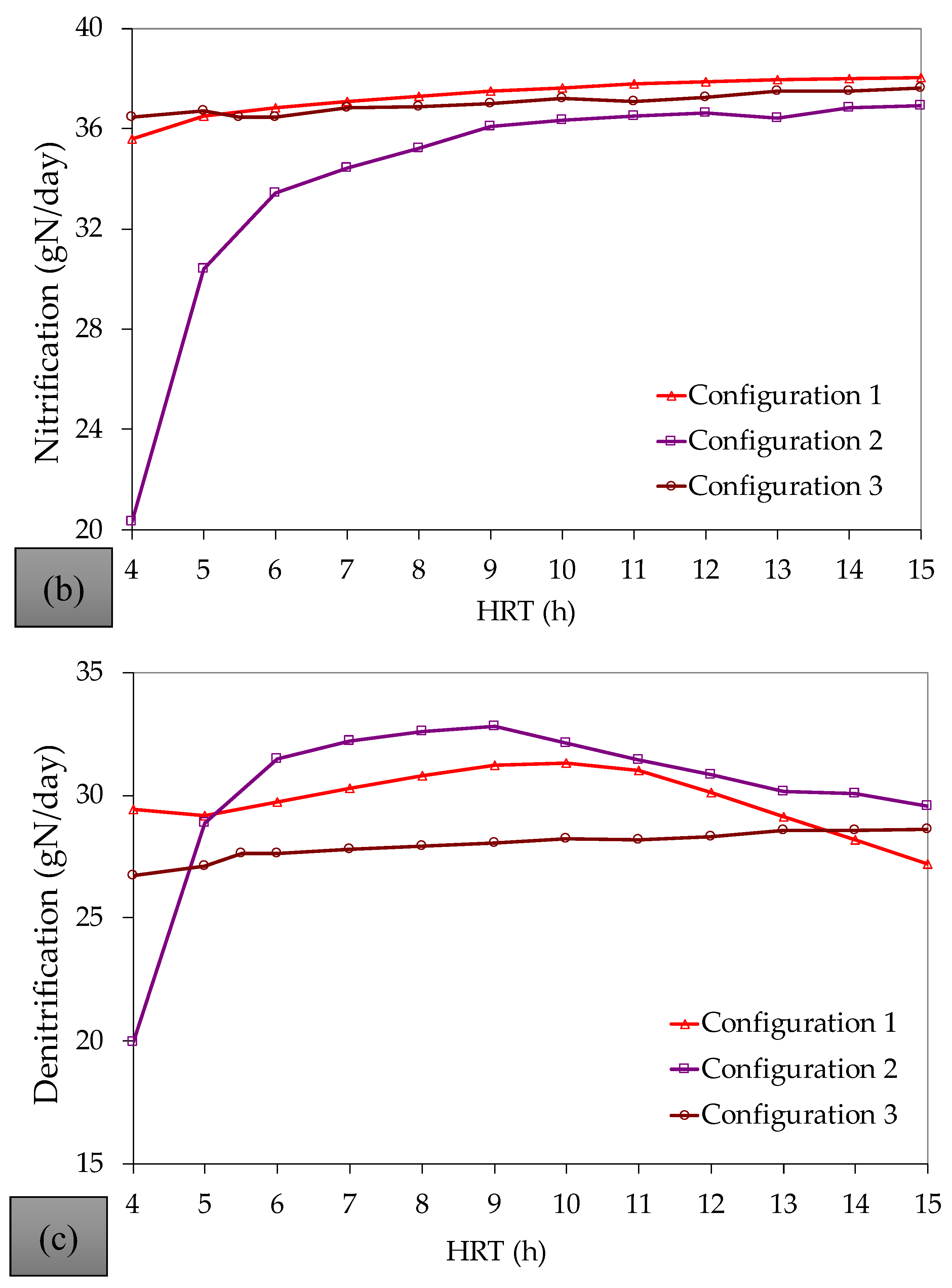
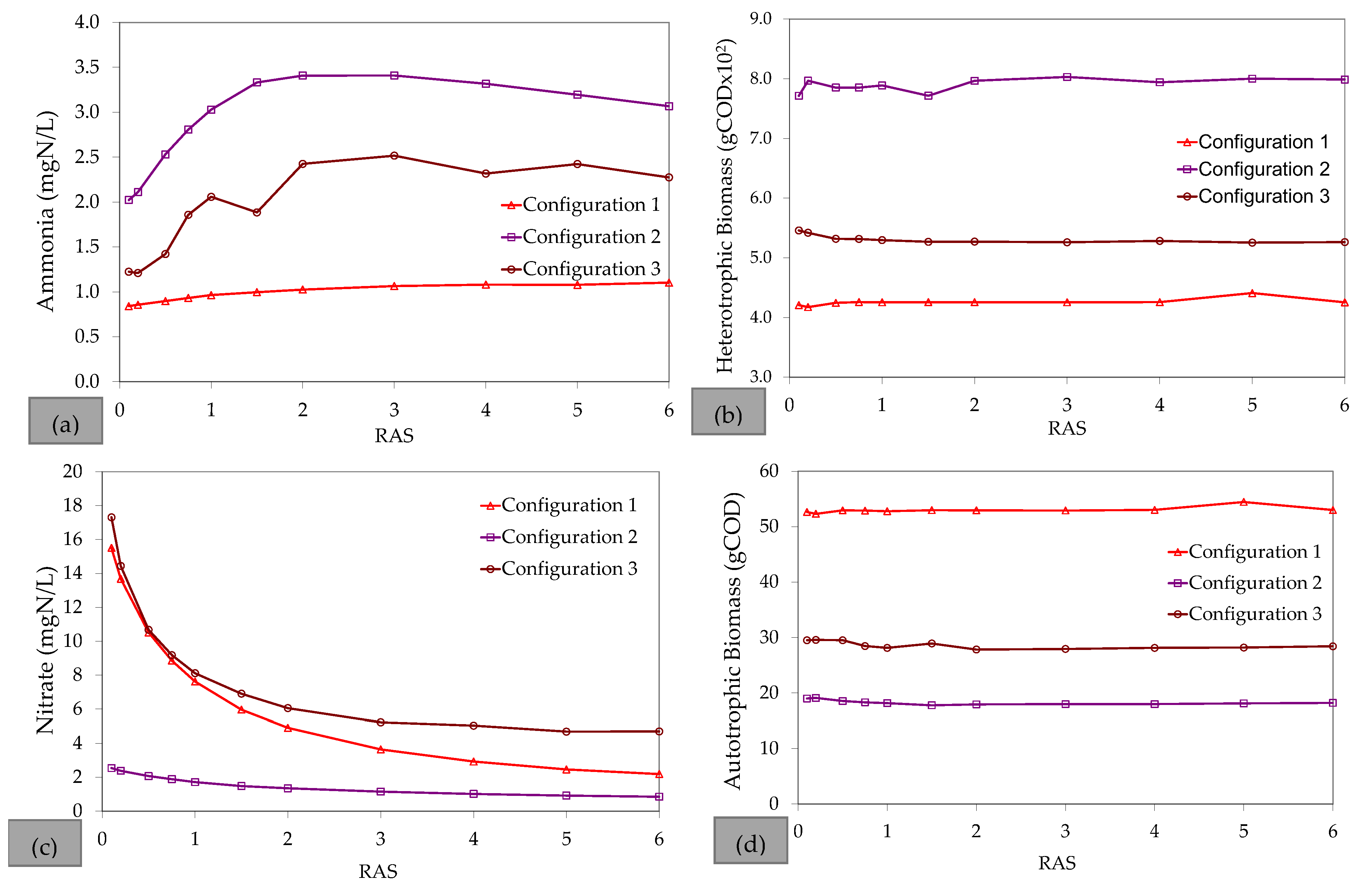
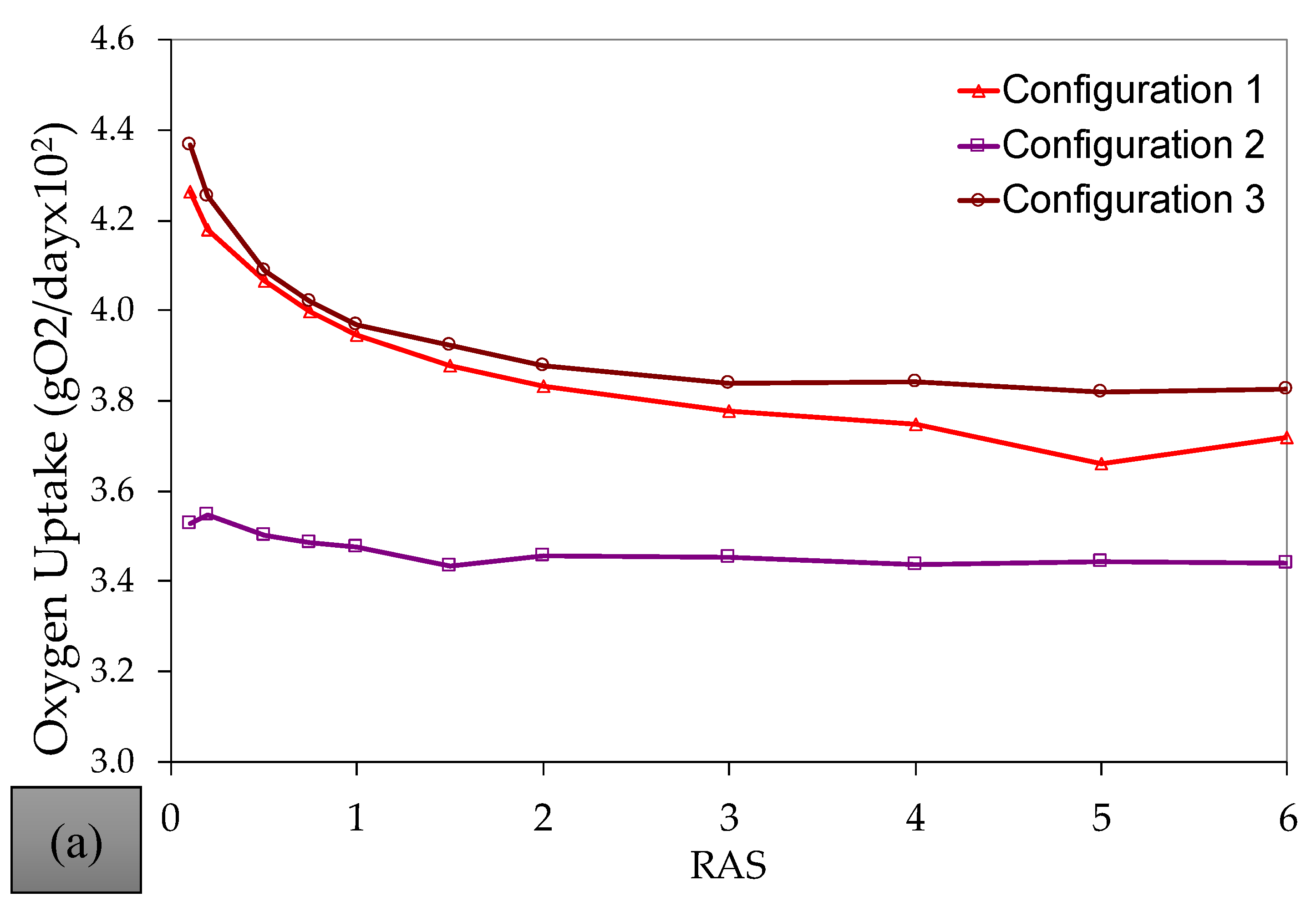
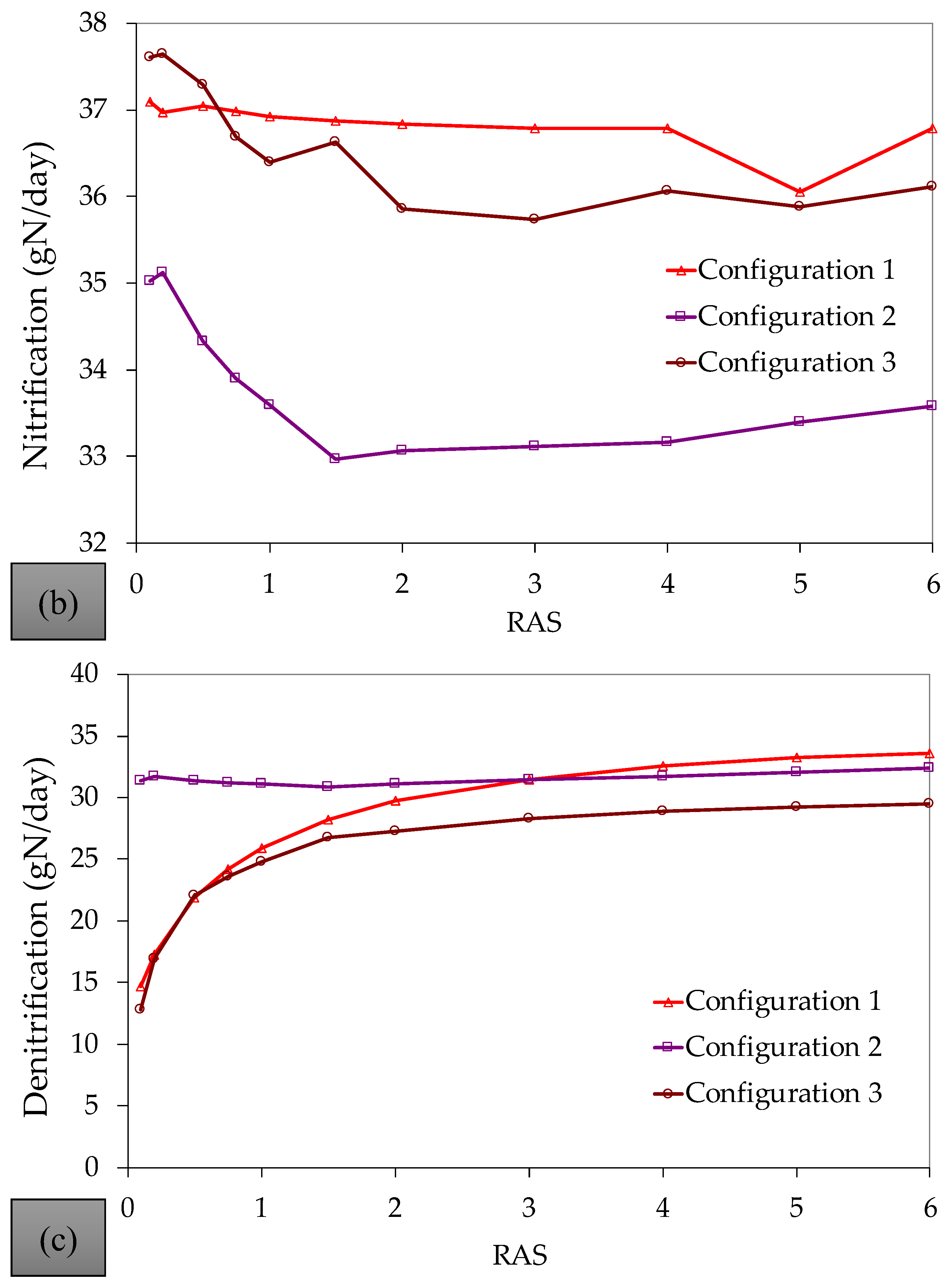

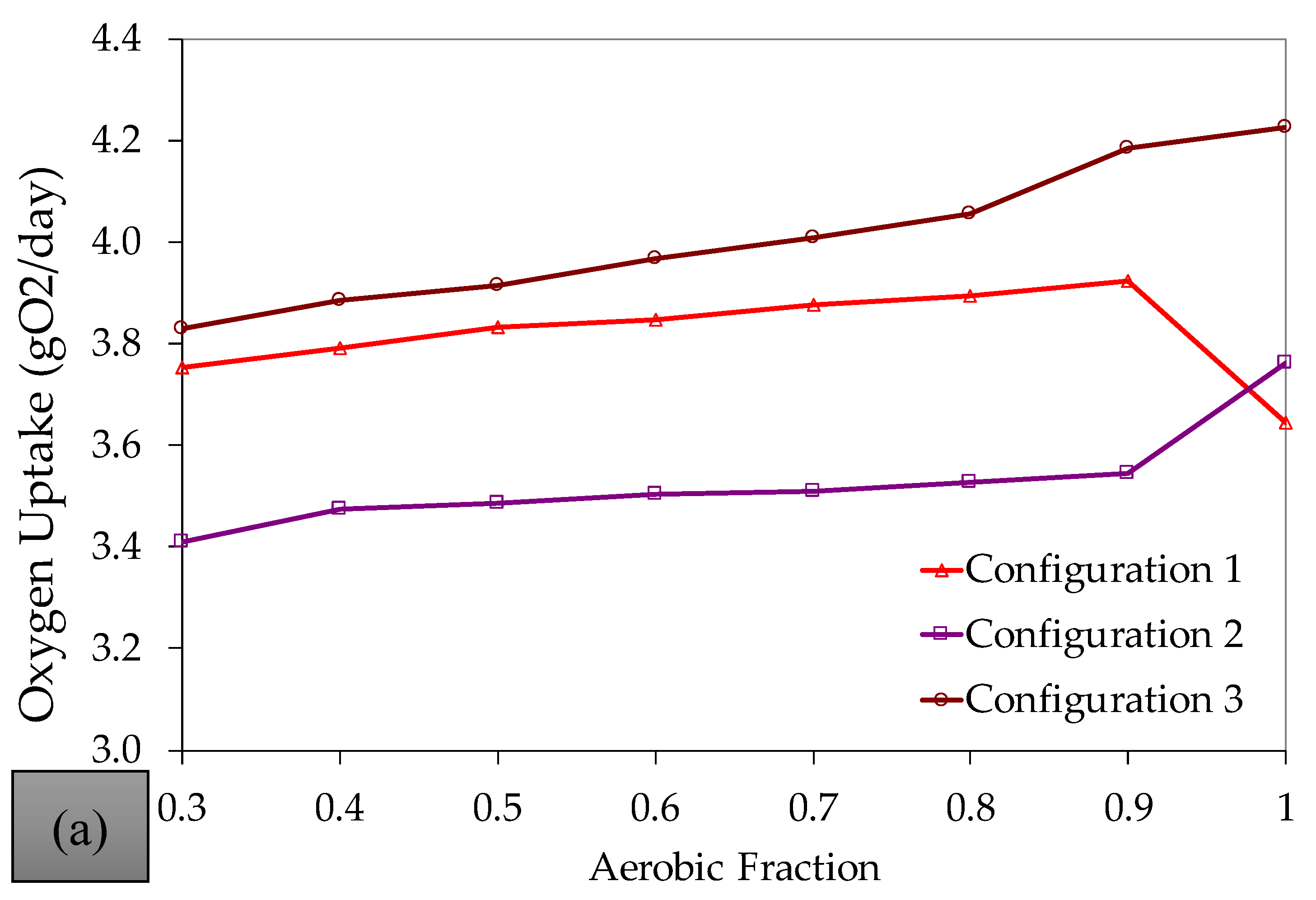

References
- Arumugham, T.; Khudzari, J.; Abdullah, N.; Yuzir, A.; Iwamoto, K.; Homma, K. Research Trends and Future Directions on Nitrification and Denitrification Processes in Biological Nitrogen Removal. J. Environ. Chem. Eng. 2024, 12, 111897. [Google Scholar] [CrossRef]
- Hamedi, H.; Mohammadzadeh, O.; Rasouli, S.; Zendehboudi, S. A Critical Review of Biomass Kinetics and Membrane Filtration Models for Membrane Bioreactor Systems. J. Environ. Chem. Eng. 2021, 9, 106406. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane Bioreactor for Wastewater Treatment: A Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Mannina, G.; Ni, B.J.; Makinia, J.; Harmand, J.; Alliet, M.; Brepols, C.; Ruano, M.V.; Robles, A.; Heran, M.; Gulhan, H.; et al. Biological Processes Modelling for MBR Systems: A Review of the State-of-the-Art Focusing on SMP and EPS. Water Res. 2023, 242, 120275. [Google Scholar] [CrossRef]
- Mannina, G.; Alliet, M.; Brepols, C.; Comas, J.; Harmand, J.; Heran, M.; Kalboussi, N.; Makinia, J.; Robles, Á.; Rebouças, T.F.; et al. Integrated Membrane Bioreactors Modelling: A Review on New Comprehensive Modelling Framework. Bioresour. Technol. 2021, 329, 124828. [Google Scholar] [CrossRef]
- Al-Sayed, A.; Hellal, M.S.; Al-Shemy, M.T.; Hassan, G.K. Performance Evaluation of Submerged Membrane Bioreactor for Municipal Wastewater Treatment: Experimental Study and Model Validation with GPS-X Software Simulator. Water Environ. J. 2023, 37, 480–492. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Khoiruddin, K.; Ting, Y.P.; Boopathy, R.; Wenten, I.G. Advances in Membrane Bioreactor: High Performance and Antifouling Configurations. Curr. Pollut. Rep. 2022, 8, 98–112. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, K. Evaluation of Autotrophic Process Influencing Extracellular Polymeric Substances in Aerobic Membrane Bieactor with Expanded ASM Model. Sci. Total Environ. 2024, 928, 172207. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Y.-H.; Chen, Z.; Luo, L.-W.; Wang, Y.-H.; Tong, X.; Bai, Y.; Wang, H.-B.; Xu, Y.-Q.; Zhang, Z.-W.; et al. Enhanced extracellular polymeric substances production and aggravated membrane fouling potential caused by different disinfection treatment. J. of Membrane Sci. 2022, 642, 120007. [Google Scholar] [CrossRef]
- Yilmaz, T.; Demir, E.K.; Asik, G.; Başaran, S.T.; Çokgör, E.U.; Sözen, S.; Sahinkaya, E. Effect of Sludge Retention Time on the Performance and Sludge Filtration Characteristics of an Aerobic Membrane Bioreactor Treating Textile Wastewater. J. Water Process Eng. 2023, 51, 103390. [Google Scholar] [CrossRef]
- Al-Sayed, A.; El-gohary, F.A. Wastewater Remediation by Aerobic Membrane Bioreactor: Effect of Sludge Retention Time on Treatment Performance and Filtration Process. Egypt. J. Chem. 2024, 67, 383–392. [Google Scholar] [CrossRef]
- Ma, Q.; Han, F.; Lyu, F.; Yang, X. Municipal Sewage Treatment Technology: A2/O-VMBR Integrated Technology for Municipal Treatment and Improved Pollutant Removal. Water 2023, 15, 1574. [Google Scholar] [CrossRef]
- Tiar, S.M.; Bessedik, M.; Abdelbaki, C.; ElSayed, N.B.; Badraoui, A.; Slimani, A.; Kumar, N. Steady-State and Dynamic Simulation for Wastewater Treatment Plant Management: Case Study of Maghnia City, North-West Algeria. Water 2024, 16, 269. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Huang, P.-W.; Whang, L.-M. Optimization of Biological Nitrogen Removal in Full-Scale Municipal WWTPs Using Activated Sludge Model Simulation. Chemosphere 2024, 362, 142939. [Google Scholar] [CrossRef]
- James, S.N.; Vijayanandan, A. Recent Advances in Simultaneous Nitrification and Denitrification for Nitrogen and Micropollutant Removal: A Review. Biodegradation 2023, 34, 103–123. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mazumder, D. Mathematical Modelling of Simultaneous Nitrification and Denitrification in Biological Reactor Systems—A Review. Int. J. Environ. Sci. Technol. 2023, 20, 8105–8126. [Google Scholar] [CrossRef]
- Bai, X.; Hazi, F.; Takacs, I.; Wadhawan, T.; Parker, W.J. A Comprehensive Floc Model for Simulating Simultaneous Nitrification, Denitrification, and Phosphorus Removal. Sci. Total Environ. 2024, 927, 172023. [Google Scholar] [CrossRef]
- Bai, X.; McKnight, M.; Neufeld, J.; Parker, W. Simultaneous nitrification, denitrification, and phosphorus removal from municipal wastewater at low temperature. Bioresource Techn. 2023, 368, 128261. [Google Scholar] [CrossRef]
- Witzig, R.; Manz, W.; Rosenberger, S.; Krüger, U.; Kraume, M.; Szewzyk, U. Microbiological Aspects of a Bioreactor with Submerged Membranes for Aerobic Treatment of Municipal Wastewater. Water Res. 2002, 36, 394–402. [Google Scholar] [CrossRef]
- Van Huynh, V.; Nguyen, M.B.; Saenchan, S.; Truc-Ly, L.-H.; Ueyama, T.; Shirayanagi, S.; Itayama, T. Investigation of Performance in MBR Operated with Low DO for Low C/N Ratio Wastewater. Water Air Soil. Pollut. 2024, 235, 540. [Google Scholar] [CrossRef]
- Liss, S.N.; Liao, B.Q.; Droppo, I.G.; Allen, D.G.; Leppard, G.G. Effect of Solids Retention Time on Floc Structure. Water Sci. Technol. 2002, 46, 431–438. [Google Scholar] [CrossRef]
- Manser, R.; Gujer, W.; Siegrist, H. Consequences of Mass Transfer Effects on the Kinetics of Nitrifiers. Water Res. 2005, 39, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Cicek, N.; Macomber, J.; Davel, J.; Suidan, M.T.; Audic, J.; Genestet, P. Effect of Solids Retention Time on the Performance and Biological Characteristics of a Membrane Bioreactor. Water Sci. Technol. 2001, 43, 43–50. [Google Scholar] [CrossRef]
- Bertanza, G.; Canato, M.; Laera, G.; Vaccari, M.; Svanström, M.; Heimersson, S. A Comparison between Two Full-Scale MBR and CAS Municipal Wastewater Treatment Plants: Techno-Economic-Environmental Assessment. Environ. Sci. Pollut. Res. 2017, 24, 17383–17393. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Effects of Environmental Conditions on the Performance of Nitrogen Removal Activated Sludge Systems. Ph.D Dissertation, The University of Manitoba, Winnipeg, MB, Canada, 2004. [Google Scholar]
- Petropoulos, P.; Gilbride, K.A. Nitrification in Activated Sludge Batch Reactors Is Linked to Protozoan Grazing of the Bacterial Population. Can. J. Microbiol. 2005, 51, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Al-Malack, M.H. Determination of Biokinetic Coefficients of an Immersed Membrane Bioreactor. J. Memb. Sci. 2006, 271, 47–58. [Google Scholar] [CrossRef]
- IWA. Task Group on Mathematical Modelling for Design and Operation of Biological Wastewater Treatment (2000). Activated Sludge Models ASM1, ASM2, ASM2D and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Ramphao, M.; Wentzel, M.C.; Merritt, R.; Ekama, G.A.; Young, T.; Buckley, C.A. Impact of Membrane Solid–Liquid Separation on Design of Biological Nutrient Removal Activated Sludge Systems. Biotechnol. Bioeng. 2005, 89, 630–646. [Google Scholar] [CrossRef]
- Lesjean, B.; Gnirss, R.; Adam, C. Process Configurations Adapted to Membrane Bioreactors for Enhanced Biological Phosphorous and Nitrogen Removal. Desalination 2002, 149, 217–224. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Water and Wastewater Determination; APHA: Washington, DC, USA, 1995. [Google Scholar]
- Mouthón-Bello, J.; Zhou, H. Performance of a Submerged Membrane Bioreactor System for Biological Nutrient Removal. Water Environ. Res. 2006, 78, 538–545. [Google Scholar] [CrossRef]
- Dold, P.; Jones, R.; Takacs, I. Practical Guide for WWTP Model Calibration and Associated Data Gathering Requirements. In Proceedings of the WEFTEC® 2003 Conference, Los Angeles, CA, USA, 12–16 October 2003. [Google Scholar]
- Vanrolleghem, P.; Insel, G.; Petersen, B.; Sin, G.; De Pauw, D.; Nopens, I.; Dovermann, H.; Weijers, S.; Gernay, K. A Comprehensive Model Calibration Procedure for Activated Sludge Models. In Proceedings of the WEFTEC® 2003 Conference, Los Angeles, CA, USA, 12–16 October 2003. [Google Scholar]
- Grady Jr, C.L.; Daigger, G.T.; Love, N.G.; Filipe, C.D. Biological Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kraume, M.; Bracklow, U.; Vocks, M.; Drews, A. Nutrients Removal in MBRs for Municipal Wastewater Treatment. Water Sci. Technol. 2005, 51, 391–402. [Google Scholar] [CrossRef]
- Innocenti, L.; Bolzonella, D.; Pavan, P.; Cecchi, F. Effect of Sludge Age on the Performance of a Membrane Bioreactor: Influence on Nutrient and Metals Removal. Desalination 2002, 146, 467–474. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.v.R.; Van Loosdrecht, M.C.M. Activated Sludge Model No.2d, ASM2D. Water Sci. Technol. 1999, 39, 165–182. [Google Scholar] [CrossRef]
- Grady, L.; Daigger, G.; Lim, H. Biological Wastewater Treatment; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Van Loosdrecht, M.C.M.; Henze, M. Maintenance, Endogeneous Respiration, Lysis, Decay and Predation. Water Sci. Technol. 1999, 39, 107–117. [Google Scholar] [CrossRef]
- Gasmi, A.; Heran, M.; Elboughdiri, N.; Kolsi, L.; Ghernaout, D.; Hannachi, A.; Grasmick, A. Steady State Modeling of Autotrophic Membrane Bioreactor—A New Approach to Quantify Biomass. Arab. Gulf J. Sci. Res. 2023, 42, 920–941. [Google Scholar] [CrossRef]
- Choubert, J.-M.; Racault, Y.; Grasmick, A.; Beck, C.; Heduit, A. Nitrogen Removal from Urban Wastewater by Activated Sludge Process Operated over the Conventional Carbon Loading Rate Limit at Low Temperature. Water SA 2005, 31, 503–510. [Google Scholar] [CrossRef]
- Larrea, L.; Irizar, I.; Hildago, M.E. Improving the Predictions of ASM2d through Modelling in Practice. Water Sci. Technol. 2002, 45, 199–208. [Google Scholar] [CrossRef]
- Qrenawi, L.I.; Rabah, F.K.J. Studying the Effect of HRT, SRT, and MLSS on Membrane Bioreactor Performance for Wastewater Treatment. Egypt. J. Chem. 2024, 67, 423–437. [Google Scholar] [CrossRef]
- Moussa, M.S.; Hooijmans, C.M.; Lubberding, H.J.; Gijzen, H.J.; van Loosdrecht, M.C.M. Modelling Nitrification, Heterotrophic Growth and Predation in Activated Sludge. Water Res. 2005, 39, 5080–5098. [Google Scholar] [CrossRef]
- Jang, N.; Ren, X.; Cho, J.; Kim, I.S. Steady-State Modeling of Bio-Fouling Potentials with Respect to the Biological Kinetics in the Submerged Membrane Bioreactor (SMBR). J. Memb. Sci. 2006, 284, 352–360. [Google Scholar] [CrossRef]
- Tenore, A.; Vieira, J.; Frunzo, L.; Luongo, V.; Fabbricino, M. Calibration and Validation of an Activated Sludge Model for Membrane Bioreactor Wastewater Treatment Plants. Environ. Technol. 2020, 41, 1923–1936. [Google Scholar] [CrossRef]
- Ng, H.Y.; Hermanowicz, S.W. Membrane Bioreactor Operation at Short Solids Retention Times: Performance and Biomass Characteristics. Water Res. 2005, 39, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Cosenza, A.; Rebouças, T.F. A Plant-Wide Modelling Comparison between Membrane Bioreactors and Conventional Activated Sludge. Bioresour. Technol. 2020, 297, 122401. [Google Scholar] [CrossRef]
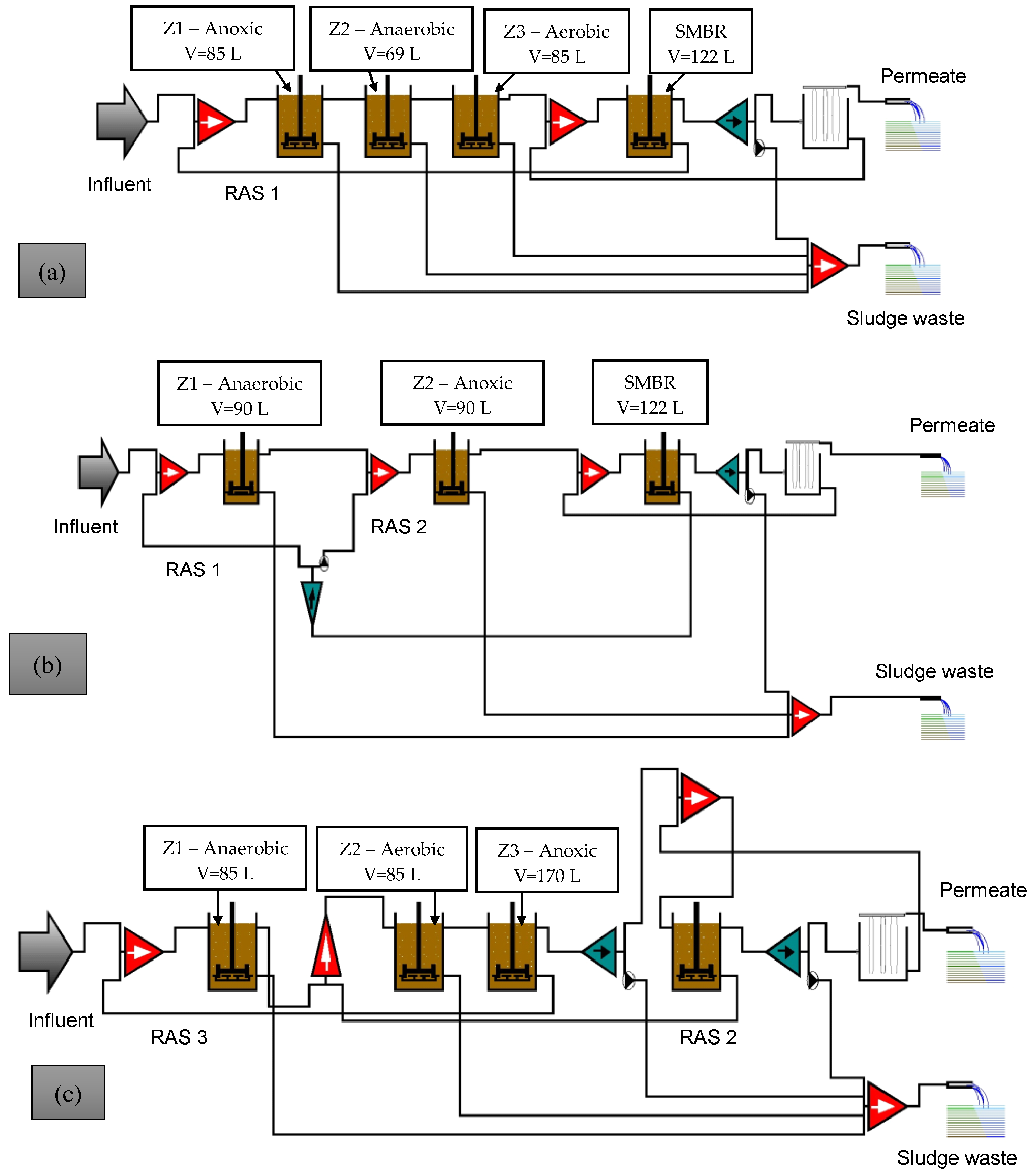
| Parameter | Units | Run No. | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Configuration No. | 1 | 2 | 2 | 3 | |
| SRT | d | 53 ± 9 | 17 ± 2 | 36 ± 9 | 31 ± 3 |
| HRT | h | 7.9 ± 0.5 | 6.2 ± 0.3 | 6.0 ± 0.2 | 6.9 ± 0.3 |
| Duration of pilot plant operation | d | 392 | 83 | 64 | 72 |
| Influent flow | L/h | 57 ± 7 | 48 ± 6 | 48 ± 7 | 67 ± 3 |
| Total reactor volume | L | 461 | 302 | 292 | 462 |
| Volume fractions (%) | |||||
| Anaerobic | 37 | 30 | 29 | 18 | |
| Anoxic | 18 | 30 | 29 | 37 | |
| Aerobic (including SMBR) | 45 | 40 | 42 | 45 | |
| Recycling ratio | |||||
| RAS 1 | 3.1 ± 0.7 | 1.0 ± 0.3 | 1.1 ± 0.3 | - | |
| RAS 2 | - | 3.2 ± 0.9 | 3.1 ± 0.8 | 3.0 ± 0.3 | |
| RAS 3 | - | - | - | 1.1 ± 0.4 | |
| Parameter | Unit | Run No. | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Configuration No. | 1 | 2 | 2 | 3 | |
| Temperature | °C | 18.7 ± 4.1 [106] | 18.6 ± 2.8 [72] | 22 ± 1.8 [56] | 20.6 ± 3 [71] |
| pH | 7.5 ± 0.3 [75] | 7.3 ± 0.2 [60] | 7.3 ± 0.2 [54] | 7.5 ± 0.2 [16] | |
| Alkalinity | mgCaCO3/L | 530 ± 58 [22] | 531 ± 62 [27] | 474 ± 65 [20] | 455 ± 47 [25] |
| TSS | g/L | 0.21 ± 0.07 [21] | 0.22 ± 0.28 [29] | 0.28 ± 0.36 [21] | 0.15 ± 0.04 [24] |
| VSS | g/L | 0.17 ± 0.06 [21] | 0.12 ± 0.15 [25] | 0.17 ± 0.25 [20] | 0.10 ± 0.04 [24] |
| COD | mg/L | 371 ± 97 [22] | 262 ± 73 [29] | 236 ± 56 [20] | 320 ± 86 [23] |
| TN | mgN/L | 62.3 ± 17.8 [27] | 60.6 ± 14.1 [29] | 44.1 ± 13.2 [20] | 39.2 ± 7.3 [24] |
| NH3 | mgN/L | 42.7 ± 11.6 [26] | 59.5 ± 15.2 [29] | 40.7 ± 14.5 [20] | 30 ± 7.9 [25] |
| NO2− | mgN/L | 1.0 ± 0.9 [26] | 0.48 ± 0.37 [29] | 0.14 ± 0.28 [20] | 0.26 ± 0.22 [25] |
| NO3− | mgN/L | 1.8 ± 2.0 [26] | 0.79 ± 0.96 [29] | 0.27 ± 1.08 [20] | 1.50 ± 2.11 [25] |
| TP | mgP/L | 6.10 ± 1.9 [19] | 5.6 ± 2.7 [29] | 5.2 ± 1.8 [20] | 6.4 ± 2.6 [26] |
| Soluble nitrogen fraction | % | 88 ± 0.6 [10] | 92 ± 13 [27] | 88 ± 29 [16] | 82 ± 21 [22] |
| Soluble phosphorous fraction | % | 17 ± 16 [7] | 21 ± 11 [29] | 6 ± 4 [20] | 9 ± 10 [25] |
| Parameter | Run No. | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| COD fractions (%) | ||||
| 42 | 38 | 35.9 | 39 |
| 9 | 9 | 10.3 | 9 |
| 20 | 19 | 12.8 | 17 |
| 33 | 29 | 25.4 | 30 |
| 38 | 43 | 51.5 | 45 |
| Soluble nitrogen fraction (%) | 88 | 92 | 88 | 82 |
| Soluble phosphorous fraction (%) | 17 | 21 | 6 | 9 |
| Parameter | Unit | Value | Fraction | Value |
|---|---|---|---|---|
| Chemical oxygen demand | mgCOD/L | 340 | VSS/TSS | 0.78 |
| TKN | mgN/L | 34 | Total soluble COD fraction | 0.39 |
| NH3 | mgN-NH4/L | 28 | Inert fraction of soluble COD | 0.17 |
| Nitrogen oxides | mgN/L | 0.5 | Fraction of slow COD | 0.62 |
| Alk | mgCaCO3/L | 490 | VFA fraction of soluble COD | 0.00 |
| TP | mgP/L | 6.0 | Orthophosphate fraction | 0.80 |
| SP | mgP/L | 4.0 | NH₃ fraction | 1.00 |
| XCOD/VSS | 1.76 | |||
| BOD5/BODu | 0.66 |
| Variable Operating Parameter | SRT (d) | HRT (h) | RAS (L/h) | Aerobic Fraction |
|---|---|---|---|---|
| SRT | 5–100 | 6 | 120 | Table 1 |
| HRT | 20 | 4–15 | 120 | Table 1 |
| RAS | 20 | 6 | 6–360 | Table 1 |
| Aerobic volume fraction | 20 | 6 | 120 | 0.3–1 |
| Parameter | Run | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Flows (L/h) | ||||
| Sludge waste | 0.25 (0.27) | 0.55 (0.51) | 0.27 (0.27) | 0.50 (0.47) |
| RAS 1 | 120 (176) | 250 (201) | 230 (202) | - |
| RAS 2 | - | 152 (154) | 147 (149) | 204 (201) |
| RAS 3 | - | - | - | 120 (74) |
| Influent fractions | ||||
| XCOD/VSS | 1.88 | 1.88 | 1.74 | 1.76 |
| Soluble COD | 0.29 (0.42 ± 0.18) | 0.29 (0.38 ± 0.11) | 0.33 (0.36 ± 0.12) | 0.39 (0.39 ± 0.14) |
| Inert COD | 0.25 (0.18) | 0.25 (0.18) | 0.31 (0.22) | 0.17 (0.19) |
| VSS/TSS | 0.85 (0.81) | 0.85 (0.71) | 0.73 (0.74) | 0.78 (0.73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouthón-Bello, J.A.; Coronado-Hernández, O.E.; Fuertes-Miquel, V.S. Submerged Membrane Bioreactor Configurations for Biological Nutrient Removal from Urban Wastewater: Experimental Tests and Model Simulation. Environments 2024, 11, 260. https://doi.org/10.3390/environments11110260
Mouthón-Bello JA, Coronado-Hernández OE, Fuertes-Miquel VS. Submerged Membrane Bioreactor Configurations for Biological Nutrient Removal from Urban Wastewater: Experimental Tests and Model Simulation. Environments. 2024; 11(11):260. https://doi.org/10.3390/environments11110260
Chicago/Turabian StyleMouthón-Bello, Javier A., Oscar E. Coronado-Hernández, and Vicente S. Fuertes-Miquel. 2024. "Submerged Membrane Bioreactor Configurations for Biological Nutrient Removal from Urban Wastewater: Experimental Tests and Model Simulation" Environments 11, no. 11: 260. https://doi.org/10.3390/environments11110260
APA StyleMouthón-Bello, J. A., Coronado-Hernández, O. E., & Fuertes-Miquel, V. S. (2024). Submerged Membrane Bioreactor Configurations for Biological Nutrient Removal from Urban Wastewater: Experimental Tests and Model Simulation. Environments, 11(11), 260. https://doi.org/10.3390/environments11110260








