Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water
Abstract
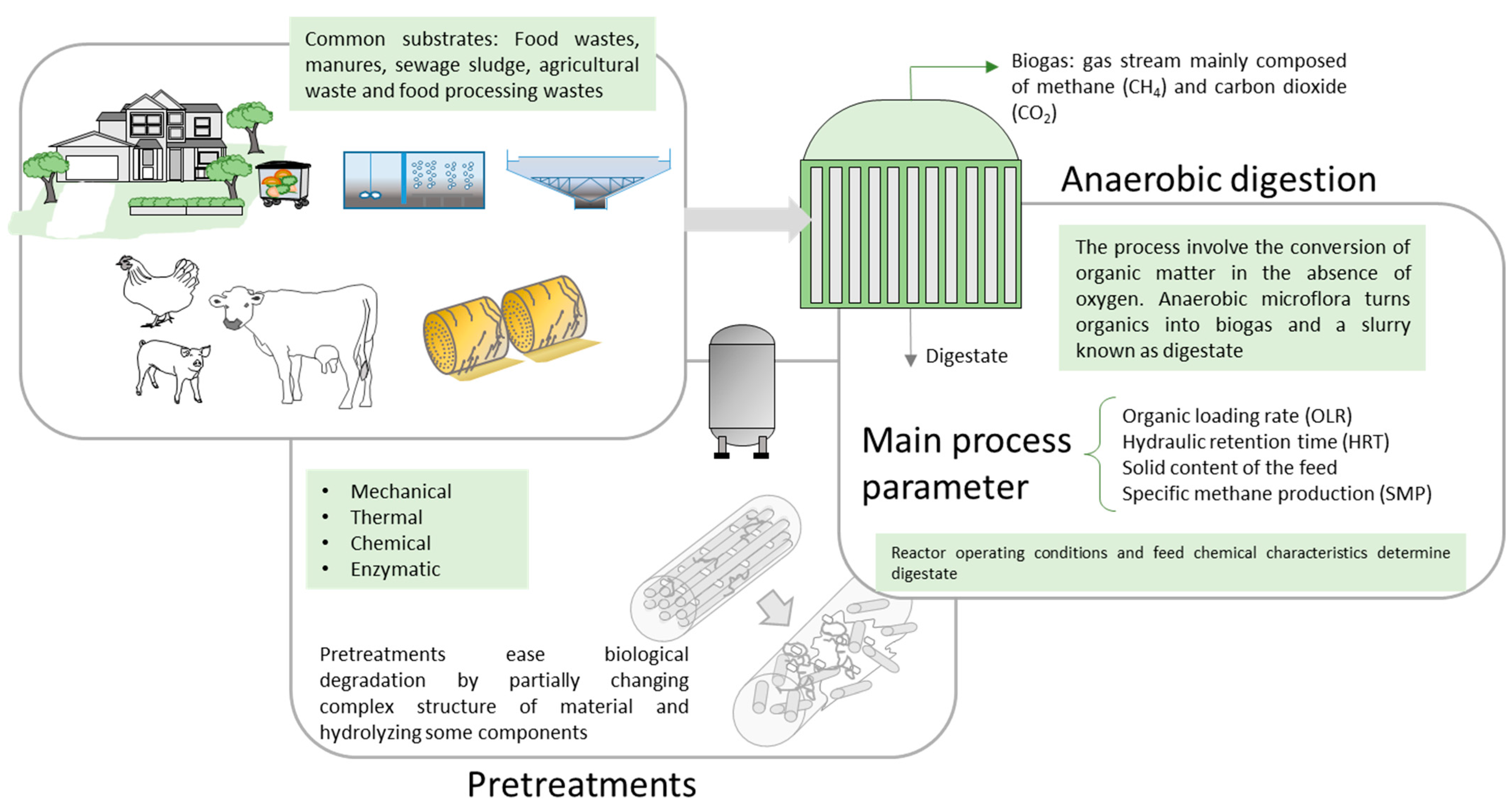
1. Introduction
2. Materials and Methods
3. Characteristics of Wastes and Digestates
3.1. Analytical Techniques for Characterizing Digestates
3.2. Thermal Analysis
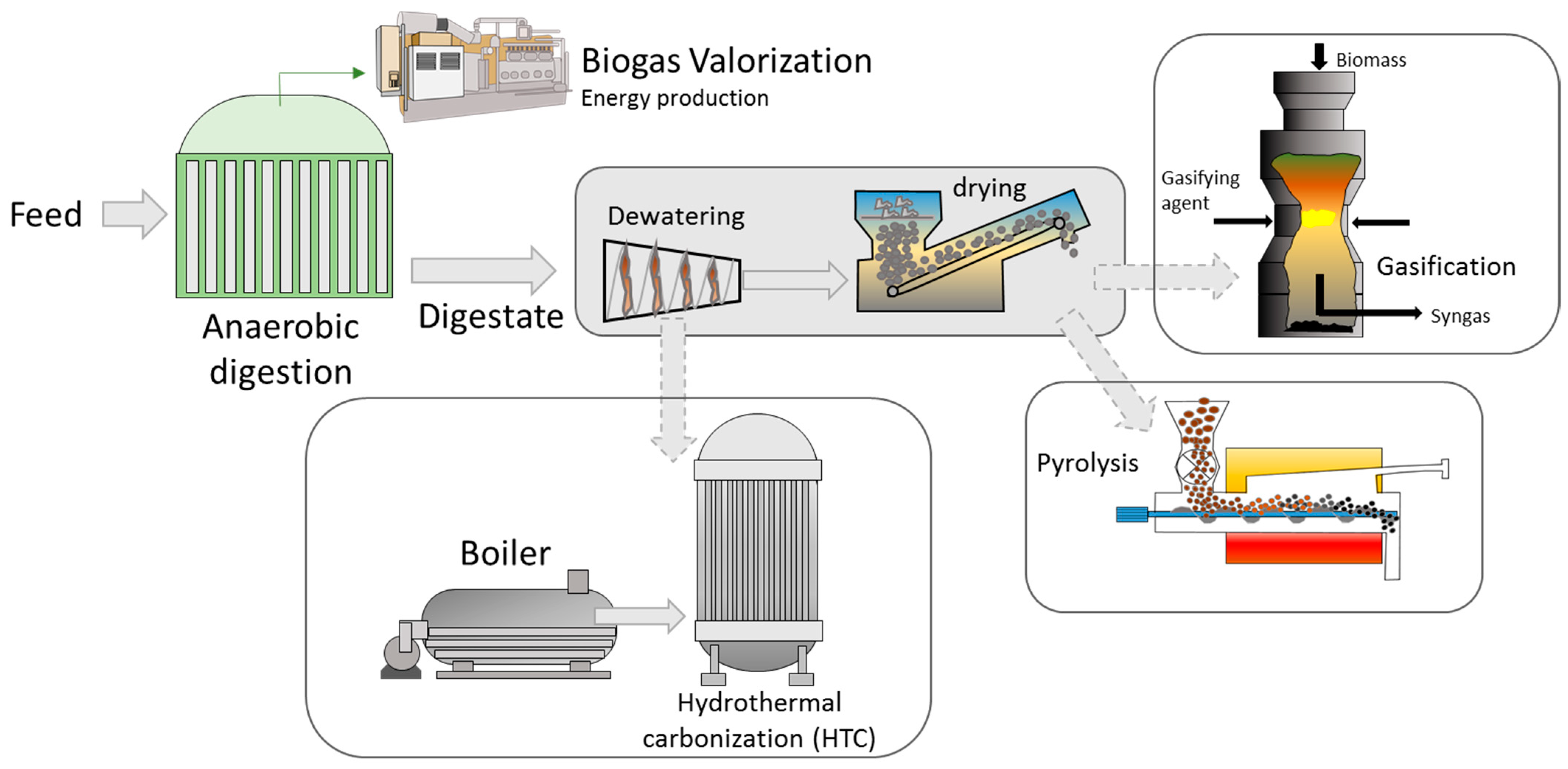
4. Thermal Valorization of Digestates
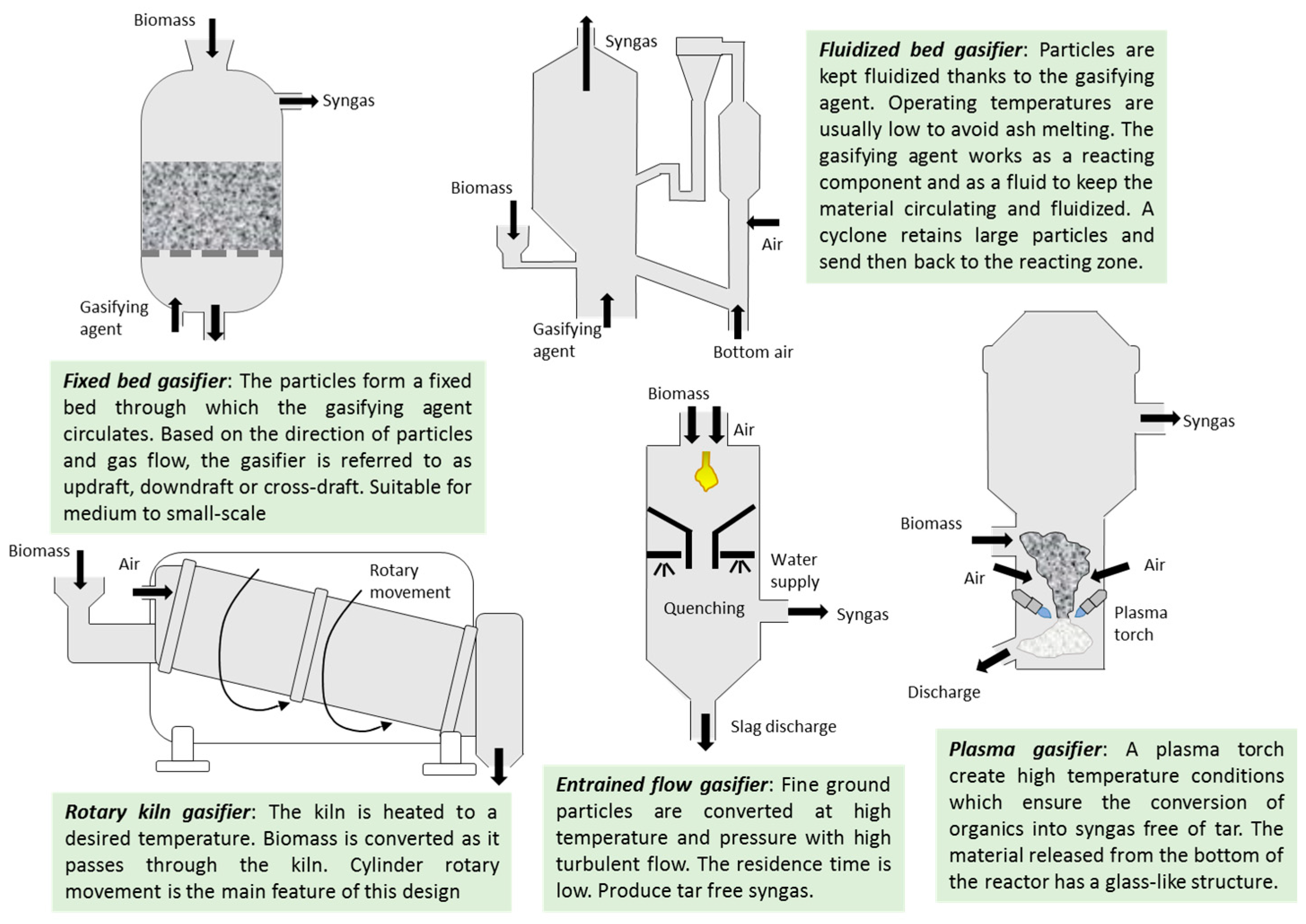
4.1. Gasification of Digestates
Supercritical Water Gasification
4.2. Pyrolysis of Digestates
4.3. HTC for Treating Digestates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Effect of Temperature and Organic Load on the Performance of Anaerobic Bioreactors Treating Grasses. Environments 2020, 7, 82. [Google Scholar] [CrossRef]
- Koley, A.; Mukhopadhyay, P.; Gupta, N.; Singh, A.; Ghosh, A.; Show, B.K.; Thakur, R.G.; Chaudhury, S.; Hazra, A.K.; Balachandran, S. Biogas production potential of aquatic weeds as the next-generation feedstock for bioenergy production: A review. Environ. Sci. Pollut. Res. 2023, 30, 111802–111832. [Google Scholar] [CrossRef]
- Yadav, D.; Barbora, L.; Bora, D.; Mitra, S.; Rangan, L.; Mahanta, P. An assessment of duckweed as a potential lignocellulosic feedstock for biogas production. Int. Biodeterior. Biodegrad. 2017, 119, 253–259. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; García-González, M.C.; González-Fernández, C.; Cuetos, M.J.; Morán, A.; Gómez, X. Anaerobic co-digestion of livestock wastes with vegetable processing wastes: A statistical analysis. Bioresour. Technol. 2010, 101, 9479–9485. [Google Scholar] [CrossRef]
- Lu, X.; Jin, W.; Xue, S.; Wang, X. Effects of waste sources on performance of anaerobic co-digestion of complex organic wastes: Taking food waste as an example. Sci. Rep. 2017, 7, 15702. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Morán, A. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol. Bioprocess Eng. 2011, 16, 1044–1052. [Google Scholar] [CrossRef]
- Ferrentino, R.; Langone, M.; Fiori, L.; Andreottola, G. Full-Scale Sewage Sludge Reduction Technologies: A Review with a Focus on Energy Consumption. Water 2023, 15, 615. [Google Scholar] [CrossRef]
- García-Cascallana, J.; Barrios, X.G.; Martinez, E.J. Thermal Hydrolysis of Sewage Sludge: A Case Study of a WWTP in Burgos, Spain. Appl. Sci. 2021, 11, 964. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- González-Arias, J.; Gil, M.V.; Fernández, R.Á.; Martínez, E.J.; Fernández, C.; Papaharalabos, G.; Gómez, X. Integrating anaerobic digestion and pyrolysis for treating digestates derived from sewage sludge and fat wastes. Environ. Sci. Pollut. Res. 2020, 27, 32603–32614. [Google Scholar] [CrossRef]
- Petrovič, A.; Vohl, S.; Cenčič Predikaka, T.; Bedoić, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability 2021, 13, 9642. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Islamova, S.I.; Gerasimov, A.V. Pyrolysis kinetics of new bioenergy feedstock from anaerobic digestate of agro-waste by thermogravimetric analysis. J. Environ. Chem. Eng. 2022, 10, 107850. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Bashkirov, V.N.; Bulygina, K.S. Thermochemical processing of digestate from biogas plant for recycling dairy manure and biomass. Biomass Convers. Biorefin. 2021, 13, 685–695. [Google Scholar] [CrossRef]
- González, R.; González, J.; Rosas, J.G.; Smith, R.; Gómez, X. Biochar and Energy Production: Valorizing Swine Manure Through Coupling Co-Digestion and Pyrolysis. C 2020, 6, 43. [Google Scholar] [CrossRef]
- Freda, C.; Nanna, F.; Villone, A.; Barisano, D.; Brandani, S.; Cornacchia, G. Air gasification of digestate and its co-gasification with residual biomass in a pilot scale rotary kiln. Int. J. Energy Environ. Eng. 2019, 10, 335–346. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates—Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- Basinas, P.; Rusín, J.; Chamrádová, K.; Kaldis, S.P. Pyrolysis of the anaerobic digestion solid by-product: Characterization of digestate decomposition and screening of the biochar use as soil amendment and as additive in anaerobic digestion. Energy Convers. Manag. 2023, 277, 116658. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Catenacci, A.; Boniardi, G.; Mainardis, M.; Gievers, F.; Farru, G.; Asunis, F.; Malpei, F.; Goi, D.; Cappai, G.; Canziani, R. Processes, applications and legislative framework for carbonized anaerobic digestate: Opportunities and bottlenecks. A critical review. Energy Convers. Manag. 2022, 263, 115691. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Pohořelý, M.; Meers, E.; Skoblia, S.; Moško, J.; Jeremiáš, M. Potential of coupling anaerobic digestion with thermochemical technologies for waste valorization. Fuel 2021, 294, 120533. [Google Scholar] [CrossRef]
- Selvaraj, P.S.; Periasamy, K.; Suganya, K.; Ramadass, K.; Muthusamy, S.; Ramesh, P.; Bush, R.; Vincent, S.G.T.; Palanisami, T. Novel resources recovery from anaerobic digestates: Current trends and future perspectives. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1915–1999. [Google Scholar] [CrossRef]
- Odirile, P.T.; Marumoloa, P.M.; Manali, A.; Gikas, P. Anaerobic Digestion for Biogas Production from Municipal Sewage Sludge: A Comparative Study between Fine Mesh Sieved Primary Sludge and Sedimented Primary Sludge. Water 2021, 13, 3532. [Google Scholar] [CrossRef]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Astals, S.; Esteban-Gutiérrez, M.; Fernández-Arévalo, T.; Aymerich, E.; García-Heras, J.; Mata-Alvarez, J. Anaerobic digestion of seven different sewage sludges: A biodegradability and modelling study. Water Res. 2013, 47, 6033–6043. [Google Scholar] [CrossRef]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef]
- Grosser, A. Determination of methane potential of mixtures composed of sewage sludge, organic fraction of municipal waste and grease trap sludge using biochemical methane potential assays. A comparison of BMP tests and semi-continuous trial results. Energy 2018, 143, 488–499. [Google Scholar] [CrossRef]
- Messaoudi, H.; Koukouch, A.; Bakhattar, I.; Asbik, M.; Bonnamy, S.; Bennouna, E.G.; Boushaki, T.; Sarh, B.; Rouboa, A. Physicochemical Characterization, Thermal Behavior, and Pyrolysis Kinetics of Sewage Sludge. Energies 2023, 17, 582. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, Y.; Kim, I. Characterization of Sewage Sludge and Food Waste-Based Biochar for Co-Firing in a Coal-Fired Power Plant: A Case Study in Korea. Sustainability 2020, 12, 9411. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Languer, M.P.; Batistella, L.; Di Domenico, M.; da Silva Filho, V.F.; Muniz Moreira, R.F.P.; José, H.J. Assessing the bioenergy potential of high-ash anaerobic sewage sludge using pyrolysis kinetics and thermodynamics to design a sustainable integrated biorefinery. Biomass Convers. Bioref. 2022, 12, 693–704. [Google Scholar] [CrossRef]
- Januševičius, T.; Mažeikienė, A.; Danila, V.; Paliulis, D. The characteristics of sewage sludge pellet biochar prepared using two different pyrolysis methods. Biomass Convers. Bioref. 2022, 14, 891–900. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Frandsen, F.J.; Henriksen, U.B. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 1: Process performance and gas product characterization. Waste Manag. 2017, 66, 123–133. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M. Thermal characteristics of the combustion process of biomass and sewage sludge. J. Therm. Anal. Calorim. 2013, 114, 519–529. [Google Scholar] [CrossRef]
- Metyouy, K.; González, R.; Gómez, X.; González-Arias, J.; Martínez, E.J.; Chafik, T.; Sánchez, M.E.; Cara-Jiménez, J. Hydrothermal carbonization vs. Anaerobic digestion to valorize fruit and vegetable waste: A comparative technical and energy assessment. J. Environ. Chem. Eng. 2023, 11, 109925. [Google Scholar] [CrossRef]
- Khan, M.A.; Hameed, B.H.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.H. Comparative Investigation of the Physicochemical Properties of Chars Produced by Hydrothermal Carbonization, Pyrolysis, and Microwave-Induced Pyrolysis of Food Waste. Polymers 2022, 14, 821. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Li, J.; Yan, B.; Chen, G. Pyrolysis of food waste and food waste solid digestate: A comparative investigation. Bioresour. Technol. 2022, 354, 127191. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Feng, Y.; Bu, T.; Zhang, Q.; Han, M.; Tang, Z.; Yuan, G.; Chen, D.; Hu, Y. Pyrolysis characteristics of anaerobic digestate from kitchen waste and availability of Phosphorus in pyrochar. J. Anal. Appl. Pyrolysis 2022, 168, 105729. [Google Scholar] [CrossRef]
- Wang, N.; Huang, D.; Zhang, C.; Shao, M.; Chen, Q.; Liu, J.; Deng, Z.; Xu, Q. Long-term characterization and resource potential evaluation of the digestate from food waste anaerobic digestion plants. Sci. Total Environ. 2021, 794, 148785. [Google Scholar] [CrossRef]
- Baek, G.; Kim, D.; Kim, J.; Kim, H.; Lee, C. Treatment of Cattle Manure by Anaerobic Co-Digestion with Food Waste and Pig Manure: Methane Yield and Synergistic Effect. Int. J. Environ. Res. Public Health 2020, 17, 4737. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Otero, M.; Lobato, A.; Cuetos, M.; Sánchez, M.; Gómez, X. Digestion of cattle manure: Thermogravimetric kinetic analysis for the evaluation of organic matter conversion. Bioresour. Technol. 2011, 102, 3404–3410. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Ábrego, J.; Gea, G.; Marías, F. Pyrolysis of dairy cattle manure: Evolution of char characteristics. J. Anal. Appl. Pyrolysis 2020, 145, 104724. [Google Scholar] [CrossRef]
- Akyürek, Z. Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy. Sustainability 2019, 11, 2280. [Google Scholar] [CrossRef]
- Thanapal, S.S.; Annamalai, K.; Sweeten, J.M.; Gordillo, G. Fixed bed gasification of dairy biomass with enriched air mixture. Appl. Energy 2012, 97, 525–531. [Google Scholar] [CrossRef]
- Gómez, X.; Cuetos, M.; García, A.; Morán, A. An evaluation of stability by thermogravimetric analysis of digestate obtained from different biowastes. J. Hazard. Mater. 2007, 149, 97–105. [Google Scholar] [CrossRef]
- Sánchez, M.; Martínez, O.; Gómez, X.; Morán, A. Pyrolysis of mixtures of sewage sludge and manure: A comparison of the results obtained in the laboratory (semi-pilot) and in a pilot plant. Waste Manag. 2007, 27, 1328–1334. [Google Scholar] [CrossRef]
- Santos, A.D.; Silva, J.R.; Castro, L.M.; Quinta-Ferreira, R.M. A biochemical methane potential of pig slurry. Energy Rep. 2022, 8, 153–158. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Z.; Fang, J.; Li, H. Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure. Sustainability 2023, 15, 9215. [Google Scholar] [CrossRef]
- Vamvuka, D.; Raftogianni, A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Appl. Sci. 2021, 11, 12011. [Google Scholar] [CrossRef]
- Lentz, Z.; Kolar, P.; Classen, J.J. Valorization of Swine Manure into Hydrochars. Processes 2019, 7, 560. [Google Scholar] [CrossRef]
- Janković, B. On-line pyrolysis kinetics of swine manure solid samples collected from rearing farm. J. Therm. Anal. Calorim. 2016, 123, 2103–2120. [Google Scholar] [CrossRef]
- Keskin, T.; Arslan, K.; Karaalp, D.; Azbar, N. The Determination of the Trace Element Effects on Basal Medium by Using the Statistical Optimization Approach for Biogas Production from Chicken Manure. Waste Biomass Valori. 2019, 10, 2497–2506. [Google Scholar] [CrossRef]
- Fierro, J.; Martínez, J.E.; Rosas, J.G.; Blanco, D.; Gómez, X. Anaerobic codigestion of poultry manure and sewage sludge under solid-phase configuration. Environ. Prog. Sustain. 2014, 33, 866–872. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Leahy, J.J.; Kwapinska, M.; Jeguirim, M.; Hamdi, H.; Kwapinski, W. Pyrolysis Process as a Sustainable Management Option of Poultry Manure: Characterization of the Derived Biochars and Assessment of their Nutrient Release Capacities. Water 2019, 11, 2271. [Google Scholar] [CrossRef]
- Tańczuk, M.; Junga, R.; Werle, S.; Chabiński, M.; Ziółkowski, Ł. Experimental analysis of the fixed bed gasification process of the mixtures of the chicken manure with biomass. Renew. Energy 2019, 136, 1055–1063. [Google Scholar] [CrossRef]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of Phosphorus and other Nutrients during Pyrolysis of Chicken Manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- González, R.; Blanco, D.; Cascallana, J.G.; Carrillo-Peña, D.; Gómez, X. Anaerobic Co-Digestion of Sheep Manure and Waste from a Potato Processing Factory: Techno-Economic Analysis. Fermentation 2021, 7, 235. [Google Scholar] [CrossRef]
- Kaur, H.; Kommalapati, R.R. Optimizing anaerobic co-digestion of goat manure and cotton gin trash using biochemical methane potential (BMP) test and mathematical modeling. SN Appl. Sci. 2021, 3, 724. [Google Scholar] [CrossRef]
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563. [Google Scholar] [CrossRef]
- Akyürek, Z. Synergetic Effects During Co-Pyrolysis of Sheep Manure and Recycled Polyethylene Terephthalate. Polymers 2021, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, V.; Pecchi, M.; Baratieri, M. Combustion kinetics of hydrochar from cow-manure digestate via thermogravimetric analysis and peak deconvolution. Bioresour. Technol. 2022, 353, 127142. [Google Scholar] [CrossRef] [PubMed]
- Bartocci, P.; Tschentscher, R.; Stensrød, R.E.; Barbanera, M.; Fantozzi, F. Kinetic Analysis of Digestate Slow Pyrolysis with the Application of the Master-Plots Method and Independent Parallel Reactions Scheme. Molecules 2019, 24, 1657. [Google Scholar] [CrossRef]
- Tsai, W.T.; Fang, Y.Y.; Cheng, P.H.; Lin, Y.Q. Characterization of mesoporous biochar produced from biogas digestate implemented in an anaerobic process of large-scale hog farm. Biomass Convers. Bioref. 2018, 8, 945–951. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Sourdon, L.; Pérémé, M.; Guilayn, F.; Steyer, J.; Patureau, D.; Jimenez, J. Retention time and organic loading rate as anaerobic co-digestion key-factors for better digestate valorization practices: C and N dynamics in soils. Waste Manag. 2024, 181, 1–10. [Google Scholar] [CrossRef]
- González-Rojo, S.; Carrillo-Peña, D.; González, R.G.; Gómez, X. Assessing Digestate at Different Stabilization Stages: Application of Thermal Analysis and FTIR Spectroscopy. Eng 2024, 5, 1499–1512. [Google Scholar] [CrossRef]
- Provenzano, M.R.; Malerba, A.D.; Pezzolla, D.; Gigliotti, G. Chemical and spectroscopic characterization of organic matter during the anaerobic digestion and successive composting of pig slurry. Waste Manag. 2014, 34, 653–660. [Google Scholar] [CrossRef]
- Kandel, T.P.; Gislum, R.; Jørgensen, U.; Lærke, P.E. Prediction of biogas yield and its kinetics in reed canary grass using near infrared reflectance spectroscopy and chemometrics. Bioresour. Technol. 2013, 146, 282–287. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Zhen, F.; Xu, Y.; Liu, J.; Li, N.; Sun, Y.; Luo, L.; Wang, M.; Zhang, L. Biochemical methane potential prediction for mixed feedstocks of straw and manure in anaerobic co-digestion. Bioresour. Technol. 2021, 326, 124745. [Google Scholar] [CrossRef] [PubMed]
- Mortreuil, P.; Baggio, S.; Lagnet, C.; Schraauwers, B.; Monlau, F. Fast prediction of organic wastes methane potential by near infrared reflectance spectroscopy: A successful tool for farm-scale biogas plant monitoring. Waste Manag. Res. 2018, 36, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Araújo, N.R.S.; Amaral, L.V.; Pujatti, F.J.P.; Freitas-Marques, M.B.; Mussel, W.N.; Sebastião, R.C. Kinetic study of domestic sewage sludge combustion using Hopfield neural network. J. Therm. Anal. Calorim. 2022, 147, 14371–14380. [Google Scholar] [CrossRef]
- Gómez, X.; Cuetos, M.; García, A.; Morán, A. Evaluation of digestate stability from anaerobic process by thermogravimetric analysis. Thermochim. Acta 2005, 426, 179–184. [Google Scholar] [CrossRef]
- Sánchez, M.; Gomez, X.; Barriocanal, G.; Cuetos, M.; Morán, A. Assessment of the stability of livestock farm wastes treated by anaerobic digestion. Int. Biodeterior. Biodegrad. 2008, 62, 421–426. [Google Scholar] [CrossRef]
- Jimenez, J.; Lei, H.; Steyer, J.; Houot, S.; Patureau, D. Methane production and fertilizing value of organic waste: Organic matter characterization for a better prediction of valorization pathways. Bioresour. Technol. 2017, 241, 1012–1021. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Yan, X.; Dong, B.; Dai, X.; Yu, L.; Wang, Y.; Ding, G.; Yu, F.; Zhou, J. Molecular characteristics of the refractory organic matter in the anaerobic and aerobic digestates of sewage sludge. RSC Adv. 2018, 8, 33138–33148. [Google Scholar] [CrossRef]
- Kataki, S.; Hazarika, S.; Baruah, D. Investigation on by-products of bioenergy systems (anaerobic digestion and gasification) as potential crop nutrient using FTIR, XRD, SEM analysis and phyto-toxicity test. J. Environ. Manag. 2017, 196, 201–216. [Google Scholar] [CrossRef]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Wei, Y.; Hong, J.; Ji, W. Thermal characterization and pyrolysis of digestate for phenol production. Fuel 2018, 232, 141–146. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Patureau, D.; Houot, S.; Sertillanges, N.; Zennaro, B.; Jimenez, J. Prediction of organic matter accessibility and complexity in anaerobic digestates. Waste Manag. 2021, 136, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; Gómez, X.; Morán, A.; García-González, M.C. Anaerobic co-digestion of livestock and vegetable processing wastes: Fibre degradation and digestate stability. Waste Manag. 2013, 33, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of swine manure and crude glycerine: Increasing glycerine ratio results in preferential degradation of labile compounds. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef]
- Kumar, V.K.; Mahendiran, R.; Subramanian, P.; Karthikeyan, S.; Surendrakumar, A.; Kumargouda, V.; Ravi, Y.; Choudhary, S.; Singh, R.; Verma, A.K. Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion. Open Life Sci. 2023, 18, 2020721. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Morán, A.; Otero, M.; Gómez, X. Anaerobic co-digestion of poultry blood with OFMSW: FTIR and TG–DTG study of process stabilization. Environ. Technol. 2009, 30, 571–582. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Gómez, X.; Blanco, D.; Cuetos, M.J.; Fernández, B.; Flotats, X. Study of thermal pre-treatment on anaerobic digestion of slaughterhouse waste by TGA-MS and FTIR spectroscopy. Waste Manag. Res. 2013, 31, 1195–1202. [Google Scholar] [CrossRef]
- De Oliveira Silva, J.; Filho, G.R.; Da Silva Meireles, C.; Ribeiro, S.D.; Vieira, J.G.; Da Silva, C.V.; Cerqueira, D.A. Thermal analysis and FTIR studies of sewage sludge produced in treatment plants. The case of sludge in the city of Uberlândia-MG, Brazil. Thermochim. Acta 2012, 528, 72–75. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Guilayn, F.; Patureau, D.; Jimenez, J. Characterising the stability of the organic matter during anaerobic digestion: A selective review on the major spectroscopic techniques. Rev. Environ. Sci. Bio/Technol. 2022, 21, 691–726. [Google Scholar] [CrossRef]
- Dziedzic, K.; Łapczyńska-Kordon, B.; Jurczyk, M.; Arczewska, M.; Wróbel, M.; Jewiarz, M.; Mudryk, K.; Pająk, T. Solid Digestate—Physicochemical and Thermal Study. Energies 2021, 14, 7224. [Google Scholar] [CrossRef]
- Martínez, E.J.; González, R.; Ellacuriaga, M.; Gómez, X. Valorization of Fourth-Range Wastes: Evaluating Pyrolytic Behavior of Fresh and Digested Wastes. Fermentation 2022, 8, 744. [Google Scholar] [CrossRef]
- González, R.; Smith, R.; Blanco, D.; Fierro, J.; Gómez, X. Application of thermal analysis for evaluating the effect of glycerine addition on the digestion of swine manure. J. Therm. Anal. Calorim. 2019, 135, 2277–2286. [Google Scholar] [CrossRef]
- Fernandez, A.; Palacios, C.; Echegaray, M.; Mazza, G.; Rodriguez, R. Pyrolysis and combustion of regional agro-industrial wastes: Thermal behavior and kinetic parameters comparison. Combust. Sci. Technol. 2018, 190, 114–135. [Google Scholar] [CrossRef]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 2015, 156, 71–80. [Google Scholar] [CrossRef]
- Otero, M.; Sanchez, M.; Gómez, X.; Morán, A. Thermogravimetric analysis of biowastes during combustion. Waste Manag. 2010, 30, 1183–1187. [Google Scholar] [CrossRef]
- Mphahlele, K.; Matjie, R.H.; Osifo, P.O. Thermodynamics, kinetics and thermal decomposition characteristics of sewage sludge during slow pyrolysis. J. Environ. Manag. 2021, 284, 112006. [Google Scholar] [CrossRef]
- Ming, X.; Xu, F.; Jiang, Y.; Zong, P.; Wang, B.; Li, J.; Qiao, Y.; Tian, Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Giwa, A.S.; Maurice, N.J.; Luoyan, A.; Liu, X.; Yunlong, Y.; Hong, Z. Advances in sewage sludge application and treatment: Process integration of plasma pyrolysis and anaerobic digestion with the resource recovery. Heliyon 2023, 9, e19765. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Gnanendra, P.M.; Ramesha, D.K.; Dasappa, S. Preliminary investigation on the use of biogas sludge for gasification. Int. J. Sustain. Energy 2012, 31, 251–267. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- González-Arias, J.; Fernández, C.; Rosas, J.G.; Bernal, M.P.; Clemente, R.; Sanchez, M.E.; Gómez, X. Integrating anaerobic digestion of pig slurry and thermal valorisation of biomass. Waste Biomass Valori. 2020, 11, 6125–6137. [Google Scholar] [CrossRef]
- González, R.; Ellacuriaga, M.; Aguilar-Pesantes, A.; Carrillo-Peña, D.; García-Cascallana, J.; Smith, R.; Gómez, X. Feasibility of Coupling Anaerobic Digestion and Hydrothermal Carbonization: Analyzing Thermal Demand. Appl. Sci. 2021, 11, 11660. [Google Scholar] [CrossRef]
- Ferrentino, R.; Langone, M.; Mattioli, D.; Fiori, L.; Andreottola, G. Investigating the Enhancement in Biogas Production by Hydrothermal Carbonization of Organic Solid Waste and Digestate in an Inter-Stage Treatment Configuration. Processes 2022, 10, 777. [Google Scholar] [CrossRef]
- Guilayn, F.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Valorization of digestates from urban or centralized biogas plants: A critical review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 419–462. [Google Scholar] [CrossRef]
- Szymańska, M.; Ahrends, H.E.; Srivastava, A.K.; Sosulski, T. Anaerobic Digestate from Biogas Plants—Nuisance Waste or Valuable Product? Appl. Sci. 2022, 12, 4052. [Google Scholar] [CrossRef]
- The European Biochar Certificate (EBC). Available online: https://www.european-biochar.org/en/home (accessed on 10 September 2024).
- Garcia, B.; Alves, O.; Rijo, B.; Lourinho, G.; Nobre, C. Biochar: Production, Applications, and Market Prospects in Portugal. Environments 2022, 9, 95. [Google Scholar] [CrossRef]
- Arbelaez Breton, L.; Mahdi, Z.; Pratt, C.; El Hanandeh, A. Modification of Hardwood Derived Biochar to Improve Phosphorus Adsorption. Environments 2021, 8, 41. [Google Scholar] [CrossRef]
- Breault, R.W. Gasification Processes Old and New: A Basic Review of the Major Technologies. Energies 2010, 3, 216–240. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation 2023, 9, 898. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2023, 1, 140–165. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Bao, W.; Huang, A.; Zhang, S.; Zhang, H. Syngas production from biomass gasification: Influences of feedstock properties, reactor type, and reaction parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Frolov, S.M. Organic Waste Gasification: A Selective Review. Fuels 2021, 2, 556–650. [Google Scholar] [CrossRef]
- Safavi, A.; Richter, C.; Unnthorsson, R. Dioxin Formation in Biomass Gasification: A Review. Energies 2022, 15, 700. [Google Scholar] [CrossRef]
- Barahmand, Z.; Eikeland, M.S. A Scoping Review on Environmental, Economic, and Social Impacts of the Gasification Processes. Environments 2022, 9, 92. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage sludge gasification in a fluidized bed: Experimental investigation and modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Gil, M.; Arauzo, I. Hammer mill operating and biomass physical conditions effects on particle size distribution of solid pulverized biofuels. Fuel Process. Technol. 2014, 127, 80–87. [Google Scholar] [CrossRef]
- Petersen, I.; Werther, J. Experimental investigation and modeling of gasification of sewage sludge in the circulating fluidized bed. Chem. Eng. Process. 2005, 44, 717–736. [Google Scholar] [CrossRef]
- Gil-Lalaguna, N.; Sánchez, J.L.; Murillo, M.B.; Rodríguez, E.; Gea, G. Air–steam gasification of sewage sludge in a fluidized bed. Influence of some operating conditions. Chem. Eng. J. 2014, 248, 373–382. [Google Scholar] [CrossRef]
- Mallick, D.; Sharma, S.D.; Kushwaha, A.; Brahma, H.S.; Nath, R.; Bhowmik, R. Emerging commercial opportunities for conversion of waste to energy: Aspect of gasification technology. In Waste-To-Energy Approaches Towards Zero Waste; Hussain, C.M., Singh, S., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 105–127. [Google Scholar] [CrossRef]
- Elbl, P.; Baláš, M.; Lisý, M.; Lisá, H. Sewage sludge and digestate gasification in an atmospheric fluidized bed gasifier. Biomass Convers. Biorefin. 2023, 14, 21821–21829. [Google Scholar] [CrossRef]
- Nilsson, S.; Gómez-Barea, A.; Cano, D.F. Gasification reactivity of char from dried sewage sludge in a fluidized bed. Fuel 2012, 92, 346–353. [Google Scholar] [CrossRef]
- Manyà, J.J.; Sánchez, J.L.; Ábrego, J.; Gonzalo, A.; Arauzo, J. Influence of gas residence time and air ratio on the air gasification of dried sewage sludge in a bubbling fluidised bed. Fuel 2006, 85, 2027–2033. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, Y.; Lu, Q.; Gao, M.; Ouyang, Z. Experimental investigation of gasification and incineration characteristics of dried sewage sludge in a circulating fluidized bed. Fuel 2015, 150, 441–447. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Li, H.; Mehmood, D.; Thorin, E.; Yu, Z. Biomethane Production Via Anaerobic Digestion and Biomass Gasification. Energy Procedia 2017, 105, 1172–1177. [Google Scholar] [CrossRef]
- Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. The pyrolysis and gasification of digestate from agricultural biogas plant. Arch. Environ. Prot. 2015, 41, 70–75. [Google Scholar] [CrossRef]
- Salamat, R.; Scaar, H.; Weigler, F.; Berg, W.; Mellmann, J. Drying of biogas digestate: A review with a focus on available drying techniques, drying kinetics, and gaseous emission behavior. Dry. Technol. 2022, 40, 5–29. [Google Scholar] [CrossRef]
- Nylen, J.; Sheehan, M. Review of the Integration of Drying and Thermal Treatment Processes for Energy Efficient Reduction of Contaminants and Beneficial Reuse of Wastewater Treatment Plant Biosolids. Energies 2023, 16, 1964. [Google Scholar] [CrossRef]
- Sanaye, S.; Alizadeh, P.; Yazdani, M. Thermo-economic analysis of syngas production from wet digested sewage sludge by gasification process. Renew. Energy 2022, 190, 524–539. [Google Scholar] [CrossRef]
- Mabalane, P.N.; Oboirien, B.O.; Sadiku, E.R.; Masukume, M. A techno-economic analysis of anaerobic digestion and gasification hybrid system: Energy recovery from municipal solid waste in South Africa. Waste Biomass Valori. 2021, 12, 1167–1184. [Google Scholar] [CrossRef]
- Werle, S.; Sobek, S. Gasification of sewage sludge within a circular economy perspective: A Polish case study. Environ. Sci. Pollut. Res. 2019, 26, 35422–35432. [Google Scholar] [CrossRef]
- Ayol, A.; Tezer Yurdakos, O.; Gurgen, A. Investigation of municipal sludge gasification potential: Gasification characteristics of dried sludge in a pilot-scale downdraft fixed bed gasifier. Int. J. Hydrogen Energy 2019, 44, 17397–17410. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Ayol, A. Syngas production from municipal sewage sludge by gasification Process: Effects of fixed bed reactor types and gasification agents on syngas quality. Sustain. Energy Technol. Assess. 2023, 56, 103042. [Google Scholar] [CrossRef]
- Nam, H.; Maglinao, A.L.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-air fluidized bed gasification using bench and pilot scale reactors of dairy manure with sand bedding based on response surface methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air gasification of biogas-derived digestate in a downdraft fixed bed gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef]
- Li, W.; Lu, C.; An, G.; Zhang, Y.; Tong, Y.W. Integration of high-solid digestion and gasification to dispose horticultural waste and chicken manure. Chin. J. Chem. Eng. 2018, 26, 1145–1151. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Cao, L.; Ma, L.; You, S.; Li, W. Co-gasification of digestate and lignite in a downdraft fixed bed gasifier: Effect of temperature. Energy Convers. Manag. 2020, 213, 112798. [Google Scholar] [CrossRef]
- Mu, Q.; Aleem, R.D.; Liu, C.; Elendu, C.C.; Cao, C.; Duan, P.-G. Oxygen blown steam gasification of different kinds of lignocellulosic biomass for the production of hydrogen-rich syngas. Renew. Energy 2024, 232, 121132. [Google Scholar] [CrossRef]
- Raizada, A.; Shukla, A.; Yadav, S.; Katiyar, P.; Kumar, R. Enhancement of H2 production via catalytic steam gasification of food waste as feedstock and its ash as a support and promoter for Ni catalyst. Int. J. Hydrogen Energy 2024, 60, 272–281. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; De Jong, W. Supercritical Water Gasification of Biomass: A Literature and Technology Overview. Energies 2015, 8, 859–894. [Google Scholar] [CrossRef]
- Dutzi, J.; Boukis, N.; Sauer, J. Process Effluent Recycling in the Supercritical Water Gasification of Dry Biomass. Processes 2023, 11, 797. [Google Scholar] [CrossRef]
- Boukis, N.; Stoll, I.K. Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant. Processes 2021, 9, 455. [Google Scholar] [CrossRef]
- Sawai, O.; Nunoura, T.; Yamamoto, K. Supercritical water gasification of sewage sludge using bench-scale batch reactor: Advantages and drawbacks. J. Mater. Cycles Waste Manag. 2014, 16, 82–92. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Characteristics of Liquid Products in Supercritical Water Gasification of Municipal Sewage Sludge by Continuous Flow Tubular Reactor. Waste Biomass Valori. 2020, 11, 6321–6335. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Weng, Z.; Mubeen, I.; Zhang, C.; Yan, M.; Fauziah, S.H. Alkali-catalyzed supercritical water gasification of sewage sludge: Effect of liquid residue reuse as homogenous catalyst. Int. J. Environ. Sci. Technol. 2020, 17, 2845–2852. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Scheirs, J.; Camino, G.; Tumiatti, W. Overview of water evolution during the thermal degradation of cellulose. Eur. Polym. J. 2001, 37, 933–942. [Google Scholar] [CrossRef]
- Inari, G.N.; Mounguengui, S.; Dumarçay, S.; Pétrissans, M.; Gérardin, P. Evidence of char formation during wood heat treatment by mild pyrolysis. Polym. Degrad. Stab. 2007, 92, 997–1002. [Google Scholar] [CrossRef]
- Igliński, B.; Kujawski, W.; Kiełkowska, U. Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review. Energies 2023, 16, 1829. [Google Scholar] [CrossRef]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of torrefaction on biomass physicochemical characteristics and the resulting pyrolysis behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Garba, M.U.; Gambo, S.U.; Musa, U.; Tauheed, K.; Alhassan, M.; Adeniyi, O.D. Impact of torrefaction on fuel property of tropical biomass feedstocks. Biofuels 2018, 9, 369–377. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Patel, A.; Agrawal, B.; Rawal, B.R. Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources Part A 2019, 42, 1649–1661. [Google Scholar] [CrossRef]
- Bridgwater, T. Challenges and opportunities in fast pyrolysis of biomass: Part I. Johns. Matthey Technol. Rev. 2018, 62, 118–130. [Google Scholar] [CrossRef]
- Waheed, Q.M.K.; Nahil, M.A.; Williams, P.T. Pyrolysis of waste biomass: Investigation of fast pyrolysis and slow pyrolysis process conditions on product yield and gas composition. J. Energy Inst. 2013, 86, 233–241. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability 2023, 15, 11238. [Google Scholar] [CrossRef]
- Okolie, J.A.; Jimoh, T.; Akande, O.; Okoye, P.U.; Ogbaga, C.C.; Adeleke, A.A.; Ikubanni, P.P.; Güleç, F.; Amenaghawon, A.N. Pathways for the Valorization of Animal and Human Waste to Biofuels, Sustainable Materials, and Value-Added Chemicals. Environments 2023, 10, 46. [Google Scholar] [CrossRef]
- Bai, L.; Sun, W.; Yang, Z.; Ouyang, Y.; Wang, M.; Yuan, F. Laboratory Research on Design of Three-Phase AC Arc Plasma Pyrolysis Device for Recycling of Waste Printed Circuit Boards. Processes 2022, 10, 1031. [Google Scholar] [CrossRef]
- Bhatt, K.P.; Patel, S.; Upadhyay, D.S.; Patel, R.N. A critical review on solid waste treatment using plasma pyrolysis technology. Chem. Eng. Process. 2022, 177, 108989. [Google Scholar] [CrossRef]
- Rana, T.; Kar, S. Assessment of energy consumption and environmental safety measures in a plasma pyrolysis plant for eco-friendly waste treatment. J. Energy Inst. 2024, 114, 101617. [Google Scholar] [CrossRef]
- Zitouni-Petrogianni, A.; Voutsas, E. Modeling, optimization and cost analysis of municipal solid waste treatment with plasma gasification. Environ. Process. 2021, 8, 747–767. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Lu, G.; Dang, Z. Effects of pyrolysis temperature and holding time on physicochemical properties of swine-manure-derived biochar. Waste Biomass Valori. 2020, 11, 613–624. [Google Scholar] [CrossRef]
- Chen, H.; Zhai, Y.; Xu, B.; Xiang, B.; Zhu, L.; Qiu, L.; Liu, X.; Li, C.; Zeng, G. Characterization of bio-oil and biochar from high-temperature pyrolysis of sewage sludge. Environ. Technol. 2015, 36, 470–478. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2016, 59, 211–221. [Google Scholar] [CrossRef]
- Shahraki, S.; Miri, M.; Motahari-Nezhad, M. Experimental analysis of pyrolysis of sewage sludge. Energy Source Part A 2018, 40, 2037–2043. [Google Scholar] [CrossRef]
- Neumann, J.; Binder, S.; Apfelbacher, A.; Gasson, J.R.; García, P.R.; Hornung, A. Production and characterization of a new quality pyrolysis oil, char and syngas from digestate–Introducing the thermo-catalytic reforming process. J. Anal. Appl. Pyrolysis 2015, 113, 137–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Karatzos, S.; van Dyk, J.S.; McMillan, J.D.; Saddler, J. Drop-in biofuel production via conventional (lipid/fatty acid) and advanced (biomass) routes. Part I. Biofuels Bioprod. Biorefining 2017, 11, 344–362. [Google Scholar] [CrossRef]
- Chavando, A.; Silva, V.B.; Tarelho, L.A.C.; Cardoso, J.S.; Eusebio, D. Simulation of a Continuous Pyrolysis Reactor for a Heat Self-Sufficient Process and Liquid Fuel Production. Energies 2024, 17, 3526. [Google Scholar] [CrossRef]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Predictive modelling and simulation of integrated pyrolysis and anaerobic digestion process. Energy Proc. 2017, 105, 850–857. [Google Scholar] [CrossRef]
- Cong, H.; Meng, H.; Mašek, O.; Yao, Z.; Li, L.; Yu, B.; Qin, C.; Zhao, L. Comprehensive Analysis of Industrial-Scale Heating Plants Based on Different Biomass Slow Pyrolysis Technologies: Product Property, Energy Balance, and Ecological Impact. Clean. Eng. Technol. 2022, 6, 100391. [Google Scholar] [CrossRef]
- Jeder, A.; Sanchez-Sanchez, A.; Gadonneix, P.; Masson, E.; Ouederni, A.; Celzard, A.; Fierro, V. The severity factor as a useful tool for producing hydrochars and derived carbon materials. Environ. Sci. Pollut. Res. 2018, 25, 1497–1507. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2020, 13, 11061. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Li, C.; Zhang, S.; Wang, Y.; Niu, S.; Li, Y.; Hu, X. Progress of the development of reactors for pyrolysis of municipal waste. Sustain. Energy Fuels 2020, 4, 5885–5915. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Daud, W.W.; Sahu, J.N. Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes. BioEnergy Res. 2013, 6, 830–840. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Production and analysis of fast pyrolysis oils from proteinaceous biomass. BioEnergy Res. 2011, 4, 303–311. [Google Scholar] [CrossRef]
- Seyedi, S.; Venkiteshwaran, K.; Benn, N.; Zitomer, D. Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge. Sustainability 2020, 12, 3441. [Google Scholar] [CrossRef]
- Alghashm, S.; Song, L.; Liu, L.; Ouyang, C.; Zhou, J.L.; Li, X. Improvement of Biogas Production Using Biochar from Digestate at Different Pyrolysis Temperatures during OFMSW Anaerobic Digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- Fabbri, D.; Torri, C. Linking pyrolysis and anaerobic digestion (Py-AD) for the conversion of lignocellulosic biomass. Current Opin. Biotechnol. 2016, 38, 167–173. [Google Scholar] [CrossRef]
- Wen, C.; Moreira, C.M.; Rehmann, L.; Berruti, F. Feasibility of anaerobic digestion as a treatment for the aqueous pyrolysis condensate (APC) of birch bark. Bioresour. Technol. 2020, 307, 123199. [Google Scholar] [CrossRef]
- Hübner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion—Use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Catalán, N.I.; Pérez-Fabiel, S.; Mejía-González, G.; Herrera-López, D.; Castro-Chan, R.; Cruz-Salomón, A.; Sebastian, P.J. Power generation from cheese whey treatment by anaerobic digestion and microbial fuel cell. Waste Biomass Valorization 2022, 13, 3221–3231. [Google Scholar] [CrossRef]
- Fernández, C.; Cuetos, M.; Martínez, E.; Gómez, X. Thermophilic anaerobic digestion of cheese whey: Coupling H2 and CH4 production. Biomass Bioenergy 2015, 81, 55–62. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of municipal sewage sludge for biofuel production: A review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Li, J.; Yan, B.; Chen, G. Pyrolysis of sewage sludge digestate with different degrees of digestion: Thermodynamics, kinetics and product characterization. Energy Convers. Manag. 2024, 315, 118756. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of food waste and its digestate as feedstock for thermochemical processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Abdeljaoued, E.; Brulé, M.; Tayibi, S.; Manolakos, D.; Oukarroum, A.; Monlau, F.; Barakat, A. Bibliometric analysis of the evolution of biochar research trends and scientific production. Clean Technol. Environ. Policy 2020, 22, 1967–1997. [Google Scholar] [CrossRef]
- Kamali, M.; Jahaninafard, D.; Mostafaie, A.; Davarazar, M.; Gomes, A.P.D.; Tarelho, L.A.; Dewil, R.; Aminabhavi, T.M. Scientometric analysis and scientific trends on biochar application as soil amendment. Chem. Eng. J. 2020, 395, 125128. [Google Scholar] [CrossRef]
- Rodrigues, L.; Budai, A.; Elsgaard, L.; Hardy, B.; Keel, S.G.; Mondini, C.; Plaza, C.; Leifeld, J. The importance of biochar quality and pyrolysis yield for soil carbon sequestration in practice. Eur. J. Soil Sci. 2023, 74, e13396. [Google Scholar] [CrossRef]
- Document 32021R2088: Commission Delegated Regulation (EU) 2021/2088 of 7 July 2021 Amending Annexes II, III and IV to Regulation (EU) 2019/1009 of the European Parliament and of the Council for the Purpose of Adding Pyrolysis and Gasification Materials as a Component Material Category in EU Fertilising Products (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32021R2088 (accessed on 13 September 2024).
- Guo, S.; Xiong, X.; Che, D.; Liu, H.; Sun, B. Effects of sludge pyrolysis temperature and atmosphere on characteristics of biochar and gaseous products. Korean J. Chem. Eng. 2021, 38, 55–63. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, J.; He, Y.; Evrendilek, F.; Buyukada, M.; Xie, W.; Sun, S. Co-pyrolytic mechanisms, kinetics, emissions and products of biomass and sewage sludge in N2, CO2 and mixed atmospheres. Chem. Eng. J. 2020, 397, 125372. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Kwon, H. Thermo-chemical process with sewage sludge by using CO2. J. Environ. Manag. 2013, 128, 435–440. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]
- Zuo, L.; Lin, R.; Shi, Q.; Xu, S. Evaluation of the bioavailability of heavy metals and phosphorus in biochar derived from manure and manure digestate. Water Air Soil Pollut. 2020, 231, 1–11. [Google Scholar] [CrossRef]
- Mikusińska, J.; Kuźnia, M.; Czerwińska, K.; Wilk, M. Hydrothermal Carbonization of Digestate Produced in the Biogas Production Process. Energies 2023, 16, 5458. [Google Scholar] [CrossRef]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-scale pyrolysis and hydrothermal carbonization of biomass digestate: Characterization of solid products and compliance with biochar standards. Biomass Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Sun, K.; Sun, H.; Wang, Z.; Libra, J.A.; Xing, B. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefinery 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Ischia, G.; Berge, N.D.; Bae, S.; Marzban, N.; Román, S.; Farru, G.; Wilk, M.; Kulli, B.; Fiori, L. Advances in Research and Technology of Hydrothermal Carbonization: Achievements and Future Directions. Agronomy 2024, 14, 955. [Google Scholar] [CrossRef]
- TerraNova Energy. Available online: https://www.terranova-energy.com/en/technology/ (accessed on 13 September 2024).
- BIATEX GMBH, Biological Manure and Digestate Treatment. Available online: https://www.biatex.net/en/products/htc-coal (accessed on 13 September 2024).
- The Ingelia Patented HTC Plant. Available online: https://ingelia.com/index.php/planta-htc/carbonizacion-de-biomasa/?lang=en (accessed on 13 September 2024).
- Ipiales, R.; Lelli, G.; Diaz, E.; Diaz-Portuondo, E.; Mohedano, A.; De la Rubia, M. Study of two approaches for the process water management from hydrothermal carbonization of swine manure: Anaerobic treatment and nutrient recovery. Environ. Res. 2024, 246, 118098. [Google Scholar] [CrossRef]
- Ipiales, R.P.; de La Rubia, M.A.; Diaz, E.; Mohedano, A.F.; Rodriguez, J.J. Integration of hydrothermal carbonization and anaerobic digestion for energy recovery of biomass waste: An overview. Energy Fuels 2021, 35, 17032–17050. [Google Scholar] [CrossRef]
- Kakar, F.L.; Tadesse, F.; Elbeshbishy, E. Comprehensive Review of Hydrothermal Pretreatment Parameters Affecting Fermentation and Anaerobic Digestion of Municipal Sludge. Processes 2022, 10, 2518. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Xiao, S.; Yang, Q.; Wang, L.; Hao, J. Review of Melanoidins as By-Product from Thermal Hydrolysis of Sludge: Properties, Hazards, and Removal. Processes 2024, 12, 135. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Grasham, O.; Ross, A.; Dupont, V.; Camargo-Valero, M. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling hydrothermal carbonization and anaerobic digestion for sewage digestate management: Influence of hydrothermal treatment time on dewaterability and bio-methane production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of hydrochars obtained by hydrothermal carbonization of manure-based digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Correa, C.; Bernardo, M.; Ribeiro, R.P.; Esteves, I.A.; Kruse, A. Evaluation of hydrothermal carbonization as a preliminary step for the production of functional materials from biogas digestate. J. Anal. Appl. Pyrolysis 2017, 124, 461–474. [Google Scholar] [CrossRef]
- Aragon-Briceño, C.; Pożarlik, A.; Bramer, E.; Brem, G.; Wang, S.; Wen, Y.; Yang, W.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Urbanowska, A.; et al. Integration of hydrothermal carbonization treatment for water and energy recovery from organic fraction of municipal solid waste digestate. Renew. Energy 2022, 184, 577–591. [Google Scholar] [CrossRef]
- Reza, M.T.; Coronella, C.; Holtman, K.M.; Franqui-Villanueva, D.; Poulson, S.R. Hydrothermal carbonization of autoclaved municipal solid waste pulp and anaerobically treated pulp digestate. ACS Sustain. Chem. Eng. 2016, 4, 3649–3658. [Google Scholar] [CrossRef]
- Ferrentino, R.; Merzari, F.; Fiori, L.; Andreottola, G. Coupling Hydrothermal Carbonization with Anaerobic Digestion for Sewage Sludge Treatment: Influence of HTC Liquor and Hydrochar on Biomethane Production. Energies 2020, 13, 6262. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef]
- Weiner, B.; Poerschmann, J.; Wedwitschka, H.; Koehler, R.; Kopinke, F.D. Influence of process water reuse on the hydrothermal carbonization of paper. ACS Sustain. Chem. Eng. 2014, 2, 2165–2171. [Google Scholar] [CrossRef]
- Feng, H.; Cui, J.; Xu, Z.; Hantoko, D.; Zhong, L.; Xu, D.; Yan, M. Sewage sludge treatment via hydrothermal carbonization combined with supercritical water gasification: Fuel production and pollution degradation. Renew. Energy 2023, 210, 822–831. [Google Scholar] [CrossRef]
- Riedel, G.; Koehler, R.; Poerschmann, J.; Kopinke, F.; Weiner, B. Combination of hydrothermal carbonization and wet oxidation of various biomasses. Chem. Eng. J. 2015, 279, 715–724. [Google Scholar] [CrossRef]
- Reza, M.T.; Freitas, A.; Yang, X.; Coronella, C.J. Wet air oxidation of hydrothermal carbonization (HTC) process liquid. ACS Sustain. Chem. Eng. 2016, 4, 3250–3254. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Belete, Y.Z.; Mau, V.; Yahav Spitzer, R.; Posmanik, R.; Jassby, D.; Iddya, A.; Kassem, N.; Tester, J.W.; Gross, A. Hydrothermal carbonization of anaerobic digestate and manure from a dairy farm on energy recovery and the fate of nutrients. Bioresour. Technol. 2021, 333, 125164. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Blach, T.; Engelhart, M. Electrochemical oxidation of refractory compounds from hydrothermal carbonization process waters. Chemosphere 2024, 352, 141310. [Google Scholar] [CrossRef]
- González-Arias, J.; De la Rubia, M.; Sánchez, M.; Gómez, X.; Cara-Jiménez, J.; Martínez, E. Treatment of hydrothermal carbonization process water by electrochemical oxidation: Assessment of process performance. Environ. Res. 2022, 216, 114773. [Google Scholar] [CrossRef]
- Stutzenstein, P.; Bacher, M.; Rosenau, T.; Pfeifer, C. Optimization of nutrient and carbon recovery from anaerobic digestate via hydrothermal carbonization and investigation of the influence of the process parameters. Waste Biomass Valorization 2018, 9, 1303–1318. [Google Scholar] [CrossRef]
| Material | Methane Yield (mL CH4/g VS) | Ref. | Proximate and Ultimate Analysis (%) | HHV 2 (MJ/kg) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vol. 1 | Ash | C | H | N | S | |||||
| Fresh SS | 118–214 3 143–249 | [27,28,29] | 49.7–72.0 | 26.7–38.9 | 32.9–33.6 | 4.5–4.7 | 3.9–4.5 | 0.9–1.4 | 15.8–16.2 | [13,30,31] |
| Digested SS | - | - | 29.6–53.9 | 38.0–59.5 | 19.3–30.1 | 2.9–5.0 | 3.2–4.7 | 0.6–3.4 | 12.2–14.0 | [13,32,33,34,35] |
| Fresh FW | 212.0–675.2 | [28,29] | 73.4–82.8 | 3.3–17.0 | 41.4–46.1 | 3.9–5.9 | 1.7–4.4 | 0.2–0.5 | 14.4–16.1 | [31,36,37,38,39] |
| Digested FW | - | - | 53.5–61.8 | 25.6–42.1 | 23.1–42.1 | 3.6–6.0 | 3.0–5.8 | 0.6–0.9 | 13.3–29.1 | [38,39,40,41] |
| Fresh CM | 110–230 | [42,43,44] | 59.1–75.5 | 14.7–27.4 | 33.0–50.0 | 3.1–7.1 | 2.1–6.6 | 0.3–1.0 | 16.0–17.8 | [45,46,47,48,49] |
| Fresh pig/SM | 323–568 | [42,43,50] | 51.9–77.7 | 7.1–34.7 | 33.5–57.0 | 5.3–7.7 | 2.8–4.8 | 0.2–1.8 | 13.0–17.6 | [51,52,53,54] |
| Chicken/poultry manure | 140–259 | [43,55,56] | 43.6–71.6 | 16.6–61.6 | 25.6–39.7 | 3.3–4.8 | 2.2–6.0 | 0.4–0.8 | 12.0–14.2 | [56,57,58,59] |
| Goat/sheep manure | 159–309 | [43,60,61,62] | 48.1–84.7 | 11.1–40.7 | 22.5–43.9 | 1.5–6.1 | 2.3–3.1 | 0.1–0.6 | 10.4–17.5 | [51,61,62,63] |
| Digested manure | - | - | 55.0–72.6 | 12.4–40.8 | 34.1–42.5 | 4.4–5.9 | 1.8–4.3 | 0.3–1.9 | 10.4–19.7 | [16,44,64,65,66] |
| Material | Techniques | Main Findings | References |
|---|---|---|---|
| SS | FTIR 1, solid-phase fluorescence excitation–emission matrix (SPF EEM), Py-GC–MS 2, X-ray photoelectron spectroscopy (XPS). | Raw sewage sludge and stabilized material derived from AD and aerobic digestion were analyzed. The degradation of proteins was higher than that of compounds containing phenolic groups, carboxylic acids, or cellulose. Digestates had a higher percentage of aromatics. | [78] |
| Pig manure | FTIR, fluorescence spectroscopy as excitation–emission matrix (EEM). | Pig slurry. Raw sample and digested and composted material. Spectra of digested material was characterized by complex structures derived from lignocellulosic material recalcitrant to anaerobic degradation. | [69] |
| Cow manure | FTIR, X-ray diffraction (XRD). | Co-digestion of cow dung with Ipomoea carnea and rice straw: FTIR indicated the presence in the digestate of lignin/cellulose materials (C-H stretching, 1381 cm−1). Quartz was the main mineral component of digestate ash. | [79] |
| Swine manure | FTIR, Py-GC–MS, SEM 3. | Swine manure digestion: Reduction in aliphatic and protein content after digestion with an increase in aromatic signals. | [80] |
| Lignocellulosic biomass | Thermal analysis. | Fresh and digested Sargassum horneri: Digestate showed a high content in cellulose and lignin. | [81] |
| Group of different digestates | Fractionation of the sample. Fluorescence spectroscopy. | Prediction of the organic quality of digestates. The operational conditions of the digester could not be well correlated. | [82] |
| Digested FW | Thermal analysis, TG–MS 4, TG-FTIR. | Digestate ash mainly contained CaCO3. A large amount of water was produced during digestate pyrolysis. Pyrolysis products contain ketones, aldehydes, and carboxylic acids. | [40] |
| Configuration | Characteristics | Reference |
|---|---|---|
| AD + gasification. | Gasification of digestate. Main products are syngas (for energy production or to be used as fuel) and char. Gasification can use air, steam or CO2 as gasification agent. | [102,103] |
| AD + pyrolysis. | Multiple fuel products obtained (biogas, syngas, pyro-oil and char) increases energy gain. | [104,105] |
| AD + HTC. | Hydorchar is obtained as the main product. Drying is not required before thermal processing. The high thermal demand of the process makes the full valorization of digestate by HTC unfeasible. HTC has also been proposed as an intermediary stage between a two-phase digestion system, with the second stage treating HTC slurry. | [106,107] |
| Material | Type of Gasifier and Conditions | Main Results | Reference |
|---|---|---|---|
| SS | Rotary kiln. Mass rate input: 170–260 g/h. Temperature: 800–850 °C. ER: 0.15–0.24. | HHV of Syngas (dry): 6–9 MJ/m3. Gas yield of 1 m3/kg SS. Low tar production (4–6 g/m3 dry gas). Char with HHV of 14.9–15.3 MJ/kg dry. Ash content of 67.6–74%. | [129] |
| Digested SS | Circulating fluidized bed gasifier. Gasification agent: air, CO2-N2 mixture and N2. Temperature: 750–850 °C. ER: 0.3–0.6. | Better results were obtained at an ER of 0.3 and with a syngas with an LHV of 4.7 MJ/m3. Increasing the air proportion caused a detriment in performance. The carbon conversion was 85% at an ER of 0.3. Operating temperature was restricted to 750 °C due to ash agglomeration problems when ER was set at 0.3. | [122] |
| Digested SS | Low-temperature circulating fluidized bed (LT-CFB). | Sludge co-gasification with cereal straw was considered for avoiding sludge drying. The mixture with dehydrated sludge showed good performance, avoiding accumulation of inorganics in the bed. No bed agglomeration or ash sintering was observed. High tar production was obtained when testing the sludge–straw mixture due to the high water content affecting process temperature. | [34] |
| Digested SS | Fixed bed gasifier. Evaluating ER from 0.12–0.27. | Low CH4 content in syngas. The energy content of syngas was low (below 5 MJ/m3). Phosphorus recovery from char was proposed. | [136] |
| Digested SS | Down-draft fixed bed gasifier. Temperature: 1100–1150 °C. | Solid char residue and a glassy material was obtained. Syngas was used to feed a CHP engine. There was an estimated electricity production of 1 kWh per 1.2 kg of dried sludge. | [137] |
| Digested SS | Fixed by gasifiers (up-draft and down-draft) using air or oxygen as gasifying agent. Temperature: 700, 800 and 900 °C. | High content of hydrogen, 40–46% and even higher. When using pure oxygen, the HHV of syngas was 12.7–14 MJ/m3. | [138] |
| Fresh manure | Fluidized bed laboratory scale gasifier. Parameters studied were temperature, ER and O2 concentration using oxygen enriched air. | The effect of temperature was the most significant. Higher temperatures favor hydrogen formation. The maximum energy content of syngas was 8.0 MJ/m3 at 800 °C with an ER of 0.25 using air enriched up to 40% in oxygen. | [139] |
| Fresh manure | Fixed bed gasification. ER: 0.23–0.47 1. Steam addition as gasification agent. | Syngas with low HHV (1.7–4.3 MJ/kg). | [47] |
| Digested manure | Downdraft fixed bed gasifier. Temperature: 750–850 °C. ER: 0.14–0.34. | Digestate was a mixture derived from the digestion of pig and cow manure with maize–triticale silage and cereal bran. The mixture was characterized by a very low ash content (ash: 9.5%, volatiles: 89.5%). Gas yield at 850 °C was 65.5% (wt%) with an LHV of 2.88 MJ/m3. | [102] |
| Digested manure | Downdraft fixed bed gasifier. Temperature: 600–800 °C. ER: 0.25–0.3. | Digestate was obtained from a mixture of manure and straw. Increased temperature resulted in syngas with higher energy content (from 3.4 to 4.78 MJ/m3). Tar production decreased with temperature increase. | [140] |
| Digested manure | Laboratory scale gasifier. Temperature: 700, 900 and 1000 °C. | Digestate was derived from high solid digestion of chicken manure and grass. Co-gasification with wood chips was performed. Gasification temperature had a positive effect on syngas yield, with higher temperatures improving syngas production. A higher proportion of digestate in the feeding mixture led to a syngas with lower values of LHV. | [141] |
| Digestate | Fluidized bed reactor. Maximum operating temperature was 750 °C to avoid slagging and fouling. | Digestate and SS were used as raw material. No information was given regarding the type of digestate. Sludge had a high ash content (44.5%), whereas digestate had an ash content of 11.7%. Syngas had similar LHV (about 4.0 MJ/m3 for both materials). Steam addition favored digestate gasification but not SS gasification. | [125] |
| Digestate | Downdraft fixed bed gasifier. CO2 was used as gasification agent. Temperature: 650–950 °C. | Digestate was derived from a digester treating a mixture of corn straw, sludge and cattle manure. Ash content was 41.5%. Gasification was carried out in 1:1 mass ratio with lignite. Maximum LHV of syngas was 6.52. MJ/m3 at 950 °C. | [142] |
| Classification | Main Characteristics | Products | Reference |
|---|---|---|---|
| Torrefaction | Energy densification process. Reduces hemicellulose content. Main reactions are dehydration, deacetylation and cleavage of ether linkages. Torrefaction temperatures (200–300 °C). | High energy content biomass. Weight loss is achieved by releasing hydrogen and oxygen atoms. | [155,156] |
| Slow pyrolysis | Low temperature. Low heating rates favor char formation, whereas higher heating rates favor liquid products. | Higher oil and char yields, favoring char formation. | [157] |
| Fast pyrolysis | Temperature between 700–900 K. High heating rates. | Higher gas and oil yield. Increasing temperature favors H2 yield. Temperature control (heating rate) and residence time allows controlling product distribution. | [158,159,160] |
| Flash pyrolysis | Temperature > 1000 K. High heating rates. | Higher liquid fuel yield, low residence time (bio-oil yield between 60 and 75%). The low residence time and high temperature reduce secondary reactions. | [158,161,162] |
| Plasma pyrolysis | High (106–108 K) and low (2000–40,000 K) plasma temperature. Non-thermal plasma. | Under plasma conditions molecules change into atomic, ionic or excited states. | [163,164] |
| Material | Temperature (°C) | Main Results | Reference |
|---|---|---|---|
| SS | 300–500 | Increasing the pyrolysis temperature decreased the C, N, and H contents and the H/C atomic ratio while increasing the C/N ratio. | [200] |
| SS | 400–700 | Temperature increase reduces biochar yield while increasing syngas production, which is also promoted by the increase in the heating rate. | [170] |
| SS | 250–700 | Increased pyrolysis temperature increases metal stability in biochar. | [201] |
| SS | 550–850 | Temperature increase reduces bio-oil yield. | [168] |
| Freshly digested manure | 350, 450, 550 | The effect of pyrolysis temperature on manure (chicken and dairy)-derived char and its digestates was studied. Zn and Cu must be removed before biochar can be considered for land application. High pyrolysis temperature reduces phosphorus bioavailability. | [202] |
| Digested hog manure | 300–800 | Production of char. The resulting biochar was suitable for use as a soil amendment. Surface area and porosity were analyzed. Char was rich in Ca, P, Mg, Si, Fe and K. High temperature increased porosity and surface area. | [66] |
| Mixture of digested SS and digested cattle manure | 525 | Manure was treated in a co-digestion plant that also treats food and agro-industry wastes. Experiments were carried out in a laboratory reactor. Between 30–50% of the material can be transformed into char. Manure showed a lower energy recovery when considering the oil phase. | [169] |
| Mixture of digested SS and cattle manure | 550 | Demo pyrolysis plant where only two fractions were obtained. Char and gas containing condensable gases were sent to a combustion unit for energy production. Char fraction was 34%. The gas had an LHV of 21.7 MJ/m3, thanks to the low content of N2 and the presence of condensable gases. | [49] |
| Material | Temperature (°C) | Main Results | Reference |
|---|---|---|---|
| Digested SS | 250 | Process time: 30 min. Process water was tested in BMP 1 tests. Net energy balance was carried out based on experimental results. A positive balance was obtained when digested sludge was treated in the HTC system with a solid content greater than 10% and by considering the use of hydrochar as fuel. | [216] |
| Digested SS | 190 | Process time was between 30 min and 3 h. HTC treatment for 1 h improved the dewaterability of the treated material, although the biogas production of the process water was lower than for the shorter duration treatment. | [217] |
| Digestate from a mixture of components | 190, 220, 250 | Digestate was obtained from a plant treating maize silage, liquid cattle manure and grass silage. Hydrochar was produced to recover phosphate and obtain activated carbon. Due to the composition of the feed, digestate resembles lignocellulosic biomass with a higher ash content (27.7%). Acid leaching of hydrochar allowed phosphate recovery and produced a product with high microporosity and better adsorption capacity after submitting it to an activation protocol. | [206] |
| Cow manure digestate | 180, 220, 250 | Hydrochar and water extracts from hydrochar were tested. Process temperature had more influence on hydrochar characteristics. The carbon content of hydrochar increased with increasing temperature. Phytotoxicity was detected in seed germination tests. | [218] |
| Digestate from a mixture of components | 190, 220, 250 | Digestate derived from a plant treating a mixture of corn and grass silage with cattle manure. Increasing the process temperature and duration increased hydrochar stability and reduced its carbon content. Process water was analyzed. At higher temperatures, its content in organics was higher. Phenol, lignin derivatives (guaiacol and syringol), cyclic ketones, cyclopentanones and N-containing compounds (pyrazines and pyrinidols) were measured in the process water. | [219] |
| Digested MSW | 180, 200, 230 | Process time was between 15 and 120 min. Liquid volume increased after treatment due to solid solubilization. The solid fraction was reduced from 29% (in the digestate) to 21.4% in the carbonized slurry. The energy content of this type of hydrochar is lower than that obtained from fresh equivalent material, as carbon extraction has already taken place during digestion. | [220] |
| Digested MSW | 200, 250, 300 | Process time: 30 min and 2 h. Increasing temperature produced a hydrochar with higher mineral content. | [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelfatah-Aldayyat, E.; González-Rojo, S.; Gómez, X. Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water. Environments 2024, 11, 239. https://doi.org/10.3390/environments11110239
Abdelfatah-Aldayyat E, González-Rojo S, Gómez X. Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water. Environments. 2024; 11(11):239. https://doi.org/10.3390/environments11110239
Chicago/Turabian StyleAbdelfatah-Aldayyat, Ebtihal, Silvia González-Rojo, and Xiomar Gómez. 2024. "Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water" Environments 11, no. 11: 239. https://doi.org/10.3390/environments11110239
APA StyleAbdelfatah-Aldayyat, E., González-Rojo, S., & Gómez, X. (2024). Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water. Environments, 11(11), 239. https://doi.org/10.3390/environments11110239






