Pipeline-Related Residential Benzene Exposure and Groundwater Natural Attenuation Capacity in the Eastern Niger Delta, Nigeria
Abstract
1. Introduction
2. Materials and Methods
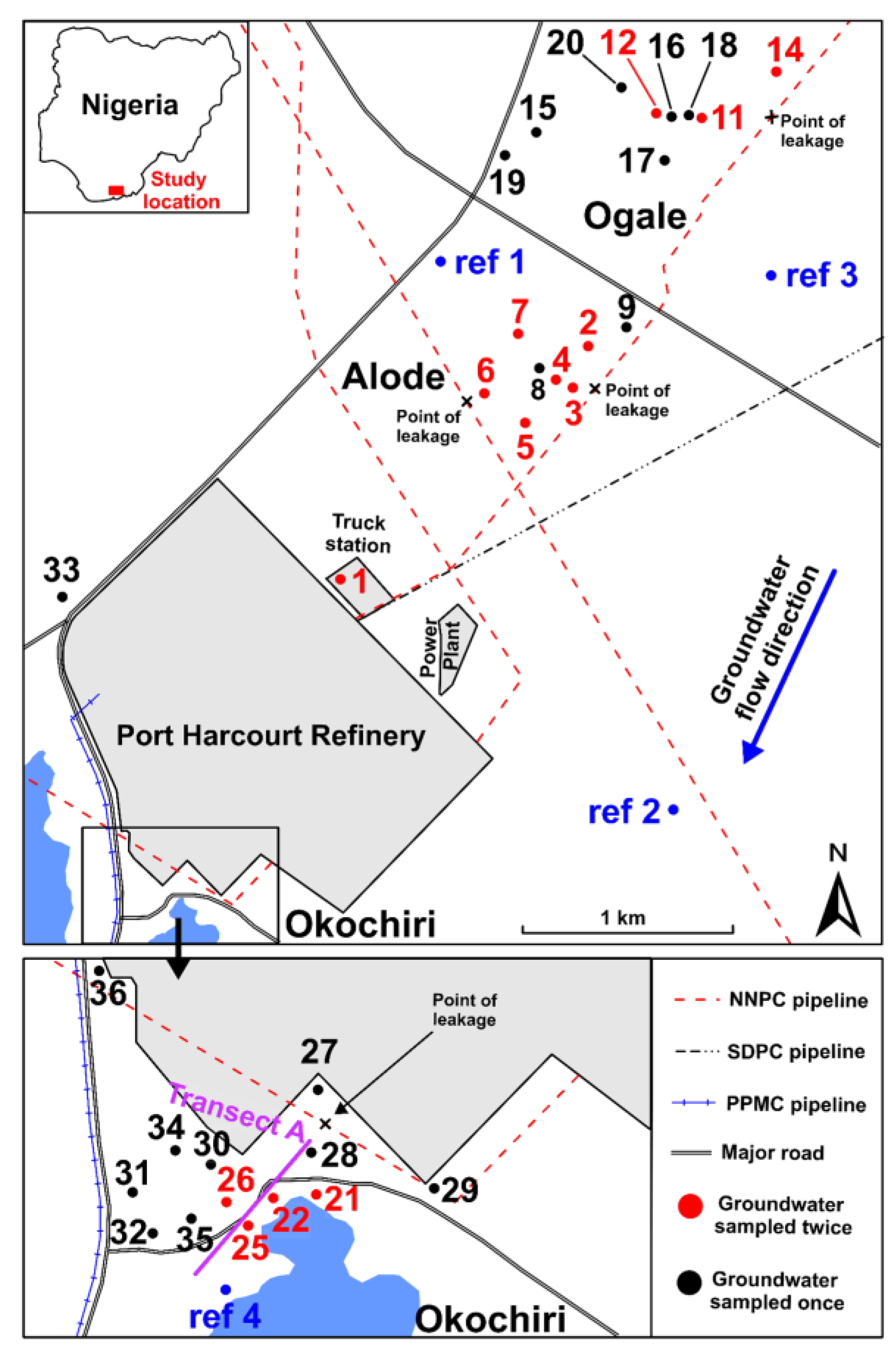
2.1. Site Description, Geology, and Hydrogeology
2.2. Groundwater Sampling
2.3. Analytical Procedures
2.3.1. Determination of BTEX and DOC
2.3.2. Anion and Cation Measurements
2.3.3. Expressed Biodegradation Capacity and Natural Attenuation Rate
3. Results and Discussion
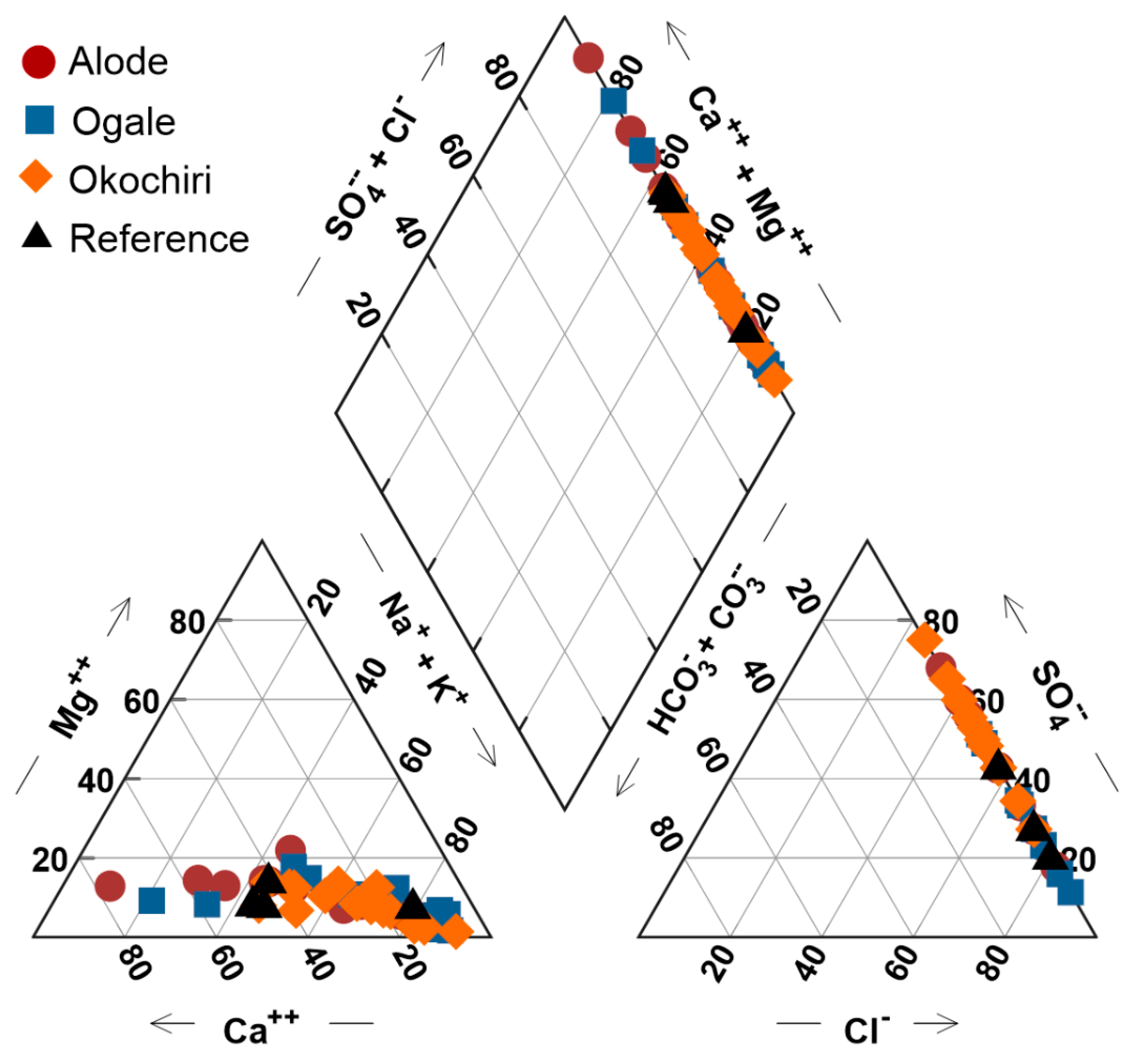
3.1. Results
3.1.1. Field Measurements and Chemical Data
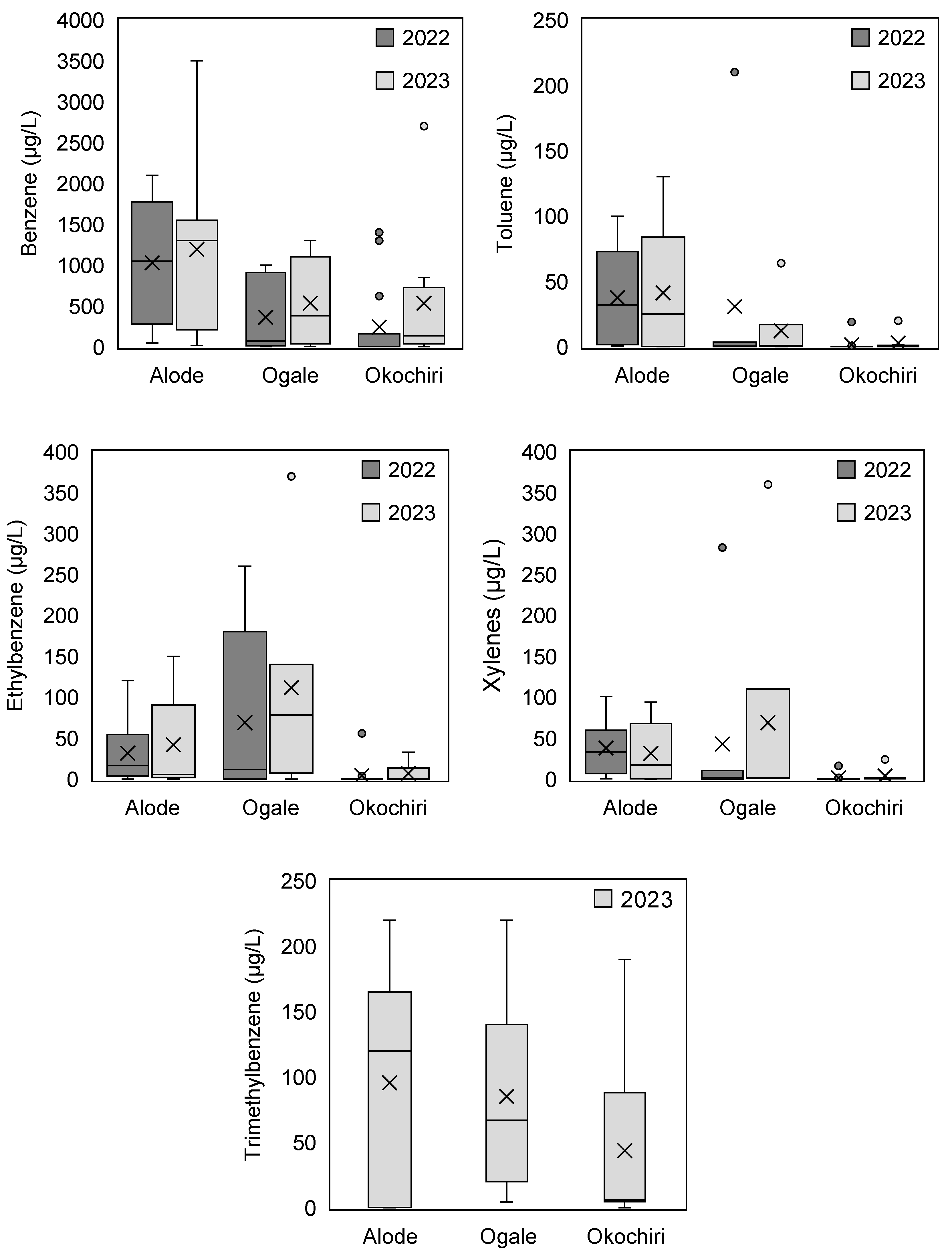
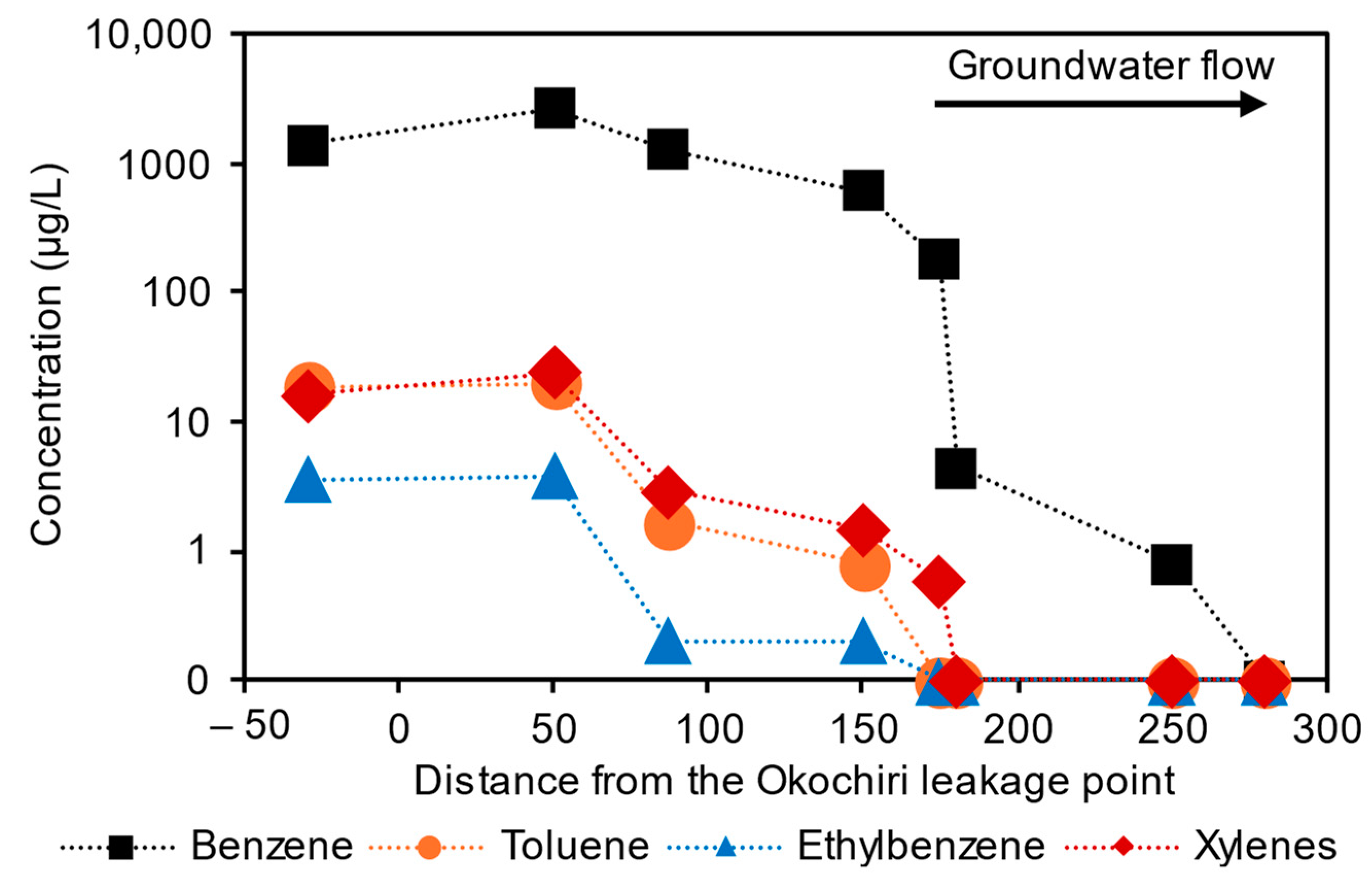
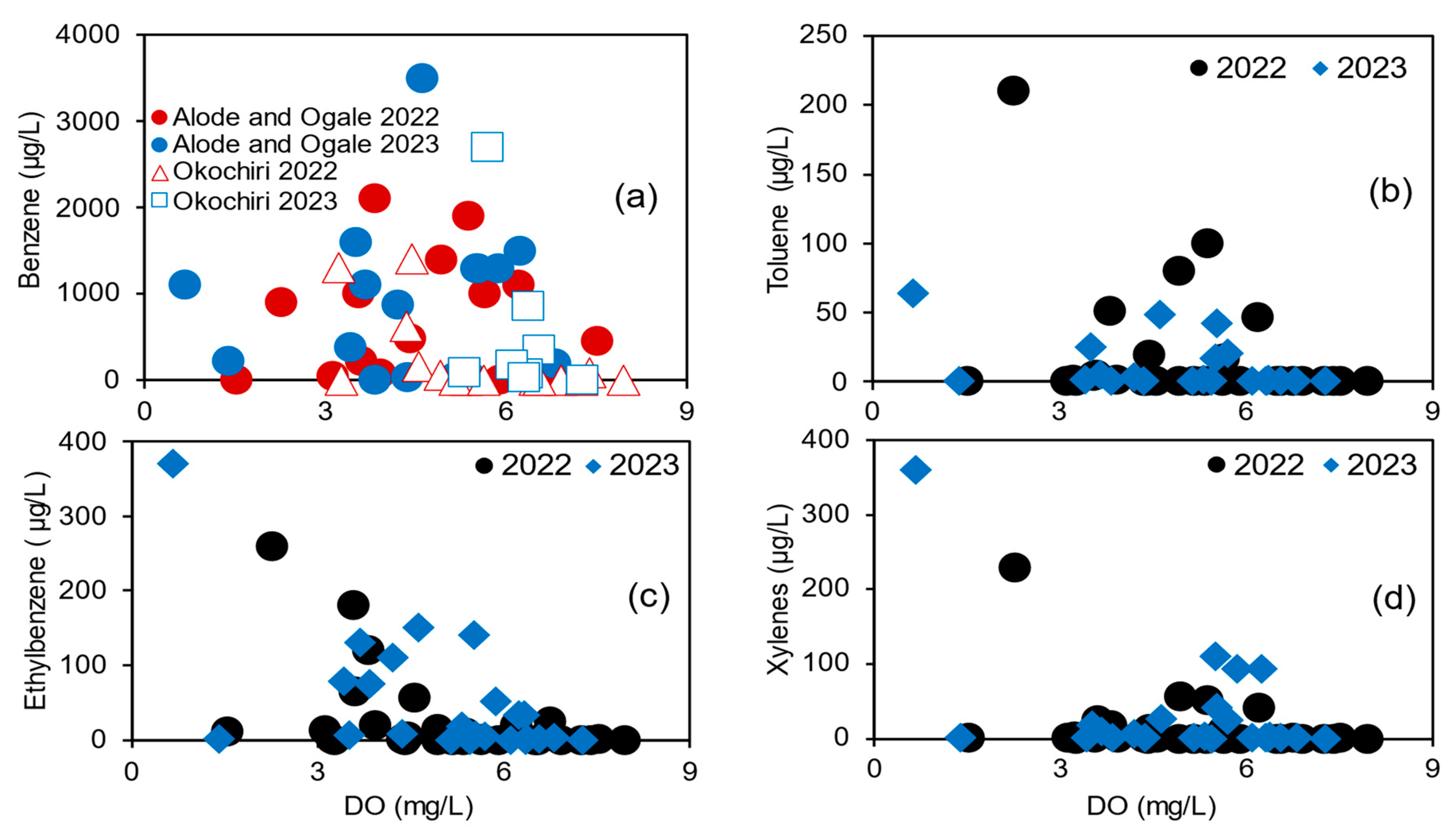
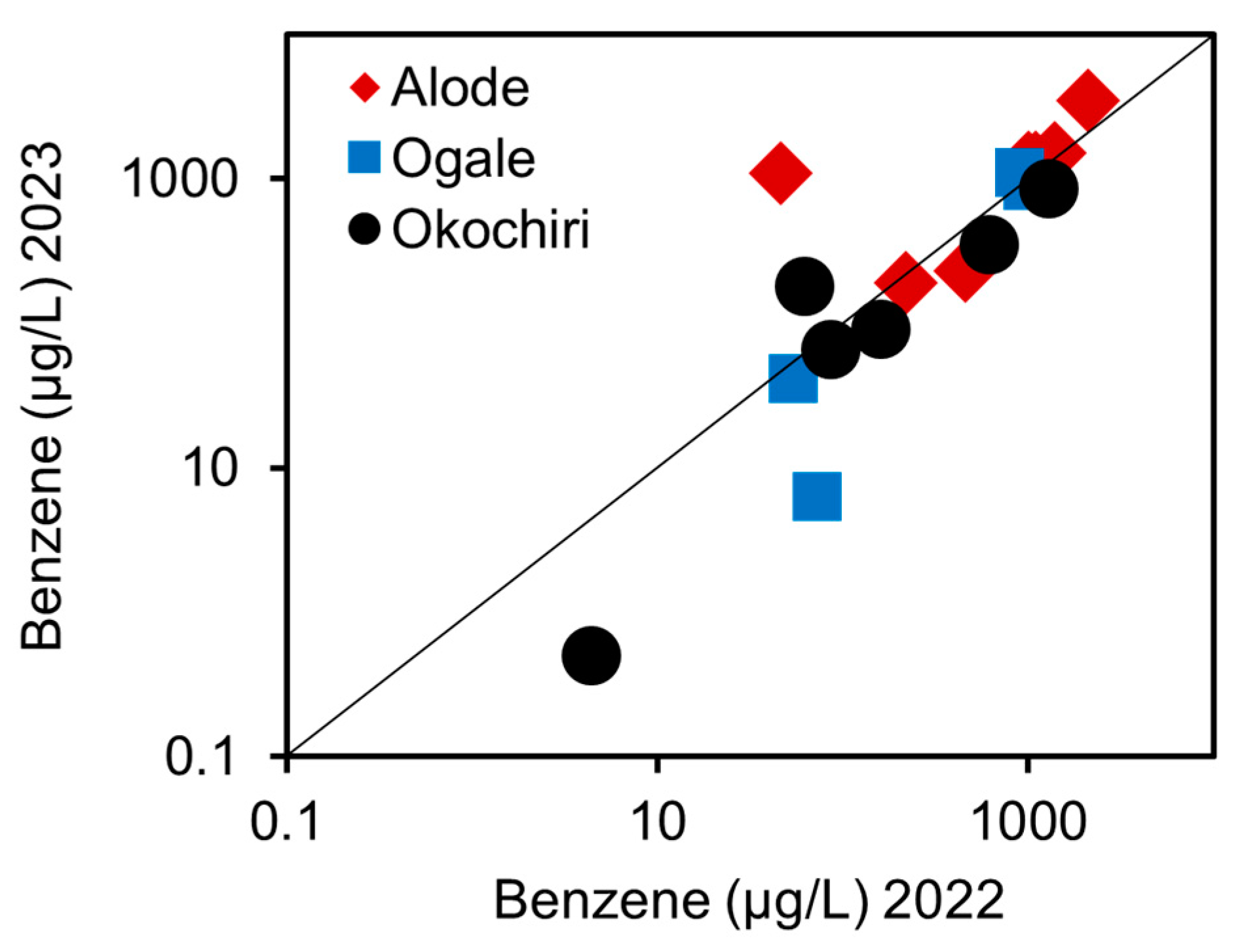
3.1.2. Benzene and TEX Concentration
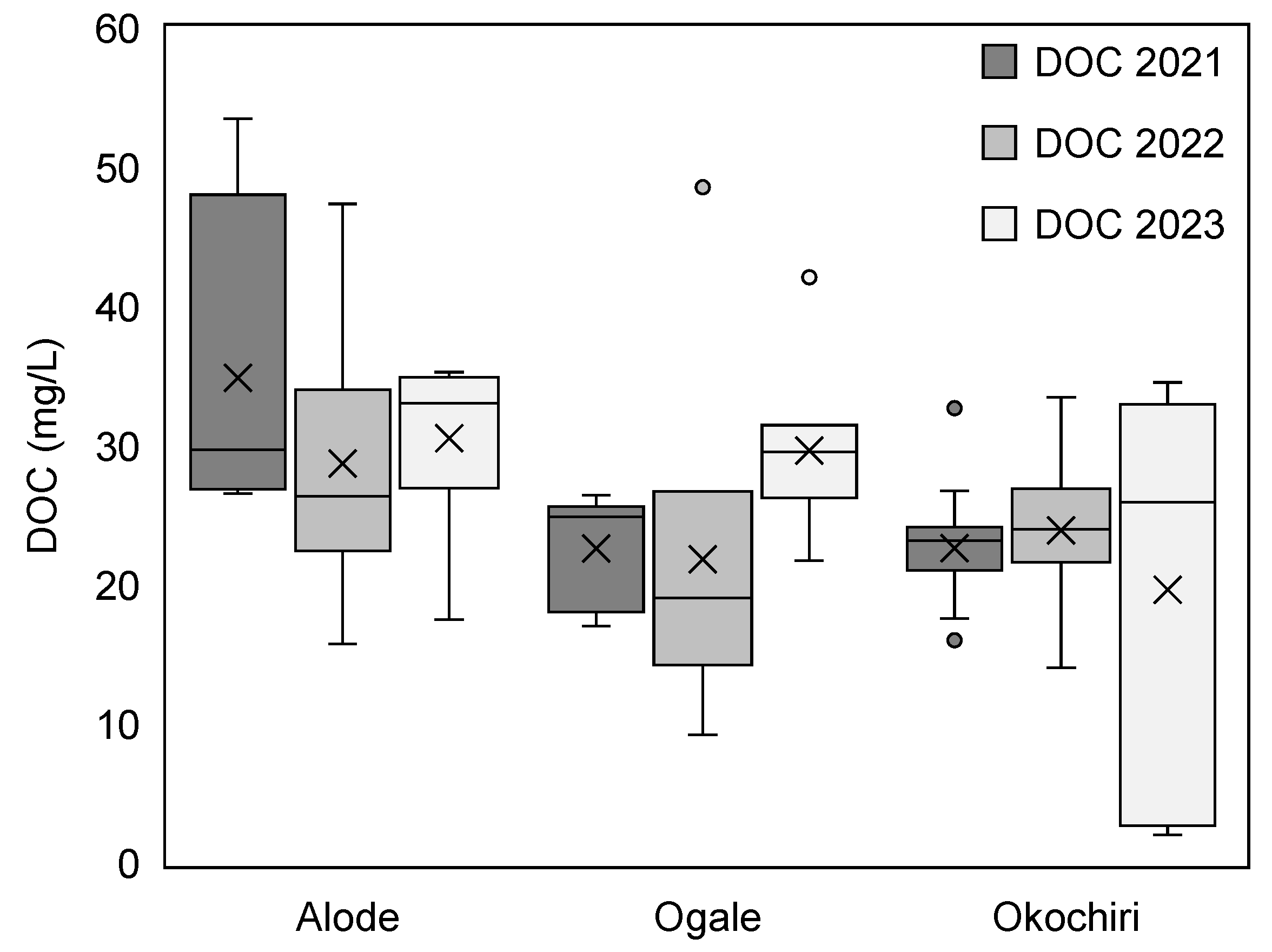
3.1.3. Dissolved Organic Carbon (DOC)
3.2. Discussion
3.2.1. The Source, Transport, and Fate of the Benzene
Source
Benzene Concentrations in Relation to Toluene, Ethylbenzene, Trimethylbenzene, and Xylenes
Dissolved Organic Carbon (DOC) in the Eastern Niger Delta
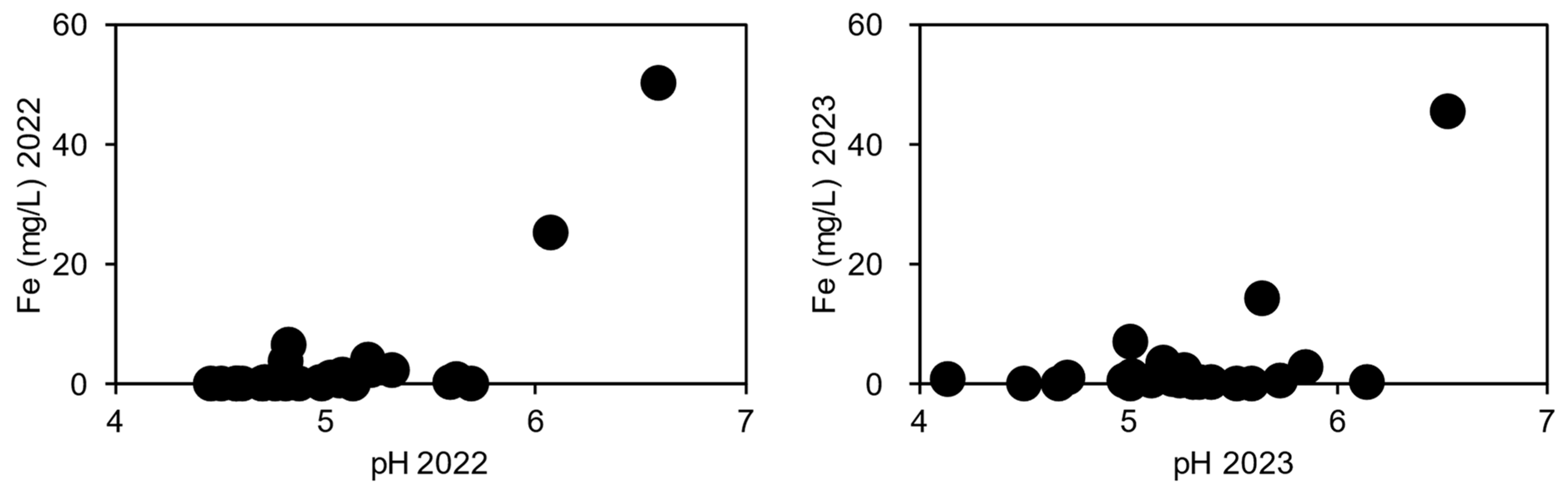
Fe Contamination and Influence on the Fate of Benzene
3.2.2. Potential for Natural Attenuation of Benzene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feo, A.; Pinardi, R.; Scanferla, E.; Celico, F. How to Minimize the Environmental Contamination Caused by Hydrocarbon Releases by Onshore Pipelines: The Key Role of a Three-Dimensional Three-Phase Fluid Flow Numerical Model. Water 2023, 15, 1900. [Google Scholar] [CrossRef]
- Ahmed, S.; Le Mouël, F.; Stouls, N.; Lipeme Kouyi, G. Development and Analysis of a Distributed Leak Detection and Localisation System for Crude Oil Pipelines. Sensors 2023, 23, 4298. [Google Scholar] [CrossRef] [PubMed]
- Unueroh, U.; Omonria, G.; Efosa, O.; Awotunde, M. Pipeline corrosion control in oil and gas industry: A case study of NNPC/PPMC system 2A pipeline. Niger. J. Technol. 2016, 35, 317–320. [Google Scholar] [CrossRef]
- Umar, H.; Abdul Khanan, M.; Magashi, S.; Ja’afar, M.; Sani, M. Fractal Analysis for Oil Spills Clustering in Ahoada Communities of the Niger Delta Region of Nigeria. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2023, 48, 371–377. [Google Scholar] [CrossRef]
- PHMSA. Pipeline Failure Causes; Technical Report; U.S. Department of Transportation: Washington, DC, USA, 2019.
- Ekong, A.P.; James, G.G.; Ohaeri, I. Oil and Gas Pipeline Leakage Detection using IoT and Deep Learning Algorithm. J. Inf. Syst. Inform. 2024, 6, 421–434. [Google Scholar] [CrossRef]
- Ambituuni, A.; Hopkins, P.; Amezaga, J.; Werner, D.; Wood, J. Risk assessment of a petroleum product pipeline in Nigeria: The realities of managing problems of theft/sabotage. In Safety and Security Engineering V; WIT Press: Southampton, UK, 2015; pp. 49–60. [Google Scholar]
- Lu, H.; Iseley, T.; Behbahani, S.; Fu, L. Leakage detection techniques for oil and gas pipelines: State-of-the-art. Tunn. Undergr. Space Technol. 2020, 98, 103249. [Google Scholar] [CrossRef]
- Korlapati, N.V.S.; Khan, F.; Noor, Q.; Mirza, S.; Vaddiraju, S. Review and analysis of pipeline leak detection methods. J. Pipeline Sci. Eng. 2022, 2, 100074. [Google Scholar] [CrossRef]
- Cozzarelli, I.M.; Baedecker, M.J.; Mumford, A.C.; Jaeschke, J.B.; Spencer, T.A. Understanding the Evolution of Groundwater-Contaminant Plume Chemistry Emanating from Legacy Contaminant Sources: An Example from a Long-Term Crude Oil Spill. Groundw. Monit. Remediat. 2022, 42, 30–42. [Google Scholar] [CrossRef]
- McGuire, J.T.; Cozzarelli, I.M.; Bekins, B.A.; Link, H.; Martinović-Weigelt, D. Toxicity assessment of groundwater contaminated by petroleum hydrocarbons at a well-characterized, aged, crude oil release site. Environ. Sci. Technol. 2018, 52, 12172–12178. [Google Scholar] [CrossRef]
- Baedecker, M.J.; Siegel, D.I.; Bennett, P.; Cozzarelli, I.M. The fate and effects of crude oil in a shallow aquifer. In US Geological Survey Toxic Substances Hydrology Program, Proceedings of the Technical Meeting, Phoenix, AZ, USA, 26–30 September 1988; Department of the Interior, US Geological Survey: Washington, DC, USA, 1989. [Google Scholar]
- Baedecker, M.J.; Cozzarelli, I.; Siegel, D.; Bennett, P.; Egan-house, R. Crude oil in a shallow sand and gravel aquifer. III. Biochemical reactions and mass balance modeling in anoxic groundwater. Appl. Geochem. 1993, 8, 569586. [Google Scholar] [CrossRef]
- Bennett, P.; Siegel, D.; Baedecker, M.J.; Hult, M. Crude oil in a shallow sand and gravel aquifer—I. Hydrogeology and inorganic geochemistry. Appl. Geochem. 1993, 8, 529–549. [Google Scholar] [CrossRef]
- Bekins, B.A.; Brennan, J.C.; Tillitt, D.E.; Cozzarelli, I.M.; Illig, J.M.; Martinović-Weigelt, D. Biological effects of hydrocarbon degradation intermediates: Is the total petroleum hydrocarbon analytical method adequate for risk assessment? Environ. Sci. Technol. 2020, 54, 11396–11404. [Google Scholar] [CrossRef] [PubMed]
- Essaid, H.I.; Bekins, B.A.; Godsy, E.M.; Warren, E.; Baedecker, M.J.; Cozzarelli, I.M. Simulation of aerobic and anaerobic biodegradation processes at a crude oil spill site. Water Resour. Res. 1995, 31, 3309–3327. [Google Scholar] [CrossRef]
- Cozzarelli, I.M.; Eganhouse, R.P.; Baedecker, M.J. Transformation of monoaromatic hydrocarbons to organic acids in anoxic groundwater environment. Environ. Geol. Water Sci. 1990, 16, 135–141. [Google Scholar] [CrossRef]
- Poursanidis, K.; Sharanik, J.; Hadjistassou, C. World’s largest natural gas leak from nord stream pipeline estimated at 478,000 tonnes. iScience 2024, 27, 1108772. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, D.; Lv, X.; Song, L.; Li, J.; Chen, F.; Xie, X. Numerical Simulation of Crude Oil Leakage from Damaged Submarine-Buried Pipeline Keywords: Submarine buried pipeline Crude oil leakage Oil spill Numerical simulation Multiphase flow. J. Appl. Fluid Mech. 2024, 17, 75–88. [Google Scholar]
- Lu, H.; Xu, Z.-D.; Song, K.; Cheng, Y.; Dong, S.; Fang, H.; Peng, H.; Fu, Y.; Xi, D.; Han, Z.; et al. Greenhouse gas emissions from U.S. crude oil pipeline accidents: 1968 to 2020. Sci. Data 2023, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Ismailov, N.; Nadjafova, S. Experience in Assessing Environmental Risks of Main Oil Pipelines in Azerbaijan through the Prism of Soil Biogeoresistance to Crude Oil Pollution. Mosc. Univ. Soil Sci. Bull. 2022, 77, 196–202. [Google Scholar] [CrossRef]
- Adewuyi, G.; Olowu, R. Assessment of oil and grease, total petroleum hydrocarbons and some heavy metals in surface and groundwater NNPC oil depot, Apata, Ibadan, Nigeria. Int. J. Aquat. Sci. 2012, 13, 45–76. [Google Scholar]
- Nambi, I.M.; Rajasekhar, B.; Loganathan, V.; RaviKrishna, R. An assessment of subsurface contamination of an urban coastal aquifer due to oil spill. Environ. Monit. Assess. 2017, 189, 148. [Google Scholar] [CrossRef]
- Gross, S.A.; Avens, H.J.; Banducci, A.M.; Sahmel, J.; Panko, J.M.; Tvermoes, B.E. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manag. Assoc. 2013, 63, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Nwankwoala, H.O.; Omofuophu, E. Investigation of hydrocarbon contaminant levels and groundwater quality assessment in parts of Bonny Island, Rivers State of Nigeria. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 61–70. [Google Scholar]
- UNEP. Environmental Assessment of Ogoniland, Nigeria; United Nations Environment Programme: Nairobi, Kenya, 2011. [Google Scholar]
- Sivasankar, V.; Gopalakrishna, G. Quantification of benzene in groundwater sources and risk analysis in a popular South Indian Pilgrimage City—A GIS based approach. Arab. J. Chem. 2017, 10, S2523–S2533. [Google Scholar]
- doRego, E.C.P.; Pereira, N.A.D. PAHs and BTEX in groundwater of gasoline stations from Rio de Janeiro City, Brazil. Bull. Environ. Contam. Toxicol. 2007, 79, 660–664. [Google Scholar] [CrossRef]
- Rao, S.M.; Joshua, R.E.; Arkenadan, L. BTEX contamination of Bengaluru aquifers, Karnataka, India. J. Environ. Eng. Sci. 2017, 12, 56–61. [Google Scholar] [CrossRef]
- Gomes, K.J.M.; Oliva, P.A.C.; da Rocha, H.O.; de Alcantara Mendes, R.; da Costa, A.C.G.; dos Santos Miranda, C.; de Oliveira Almeida, N. Evaluation of the contamination of the subsurface and groundwater by monoaromatic hydrocarbons in an eastern Amazonian town in northern Brazil. Environ. Earth Sci. 2023, 82, 23. [Google Scholar] [CrossRef]
- Joshua, R.E. Evaluation of Btex Contamination in Bengaluru Groundwater and Remediation of Contaminated Water Samples. Ph.D. Dissertation, Indian Institute of Science, Bangalore, India, 2020. [Google Scholar]
- Chen, X.; Zhang, S.; Yi, L.; Liu, Z.; Ye, X.; Yu, B.; Shi, S.; Lu, X. Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China. Int. J. Environ. Res. Public Health 2022, 19, 16449. [Google Scholar] [CrossRef]
- Belpaire, C.; Goemans, G. Eels: Contaminant cocktails pinpointing environmental contamination. ICES J. Mar. Sci. 2007, 64, 1423–1436. [Google Scholar] [CrossRef][Green Version]
- An, Y.-J. Toxicity of benzene, toluene, ethylbenzene, and xylene (BTEX) mixtures to Sorghum bicolor and Cucumis sativus. Bull. Environ. Contam. Toxicol. 2004, 72, 1006–1011. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lv, N.; Wang, H.; Zhang, H. Multiphase migration and transformation of BTEX on groundwater table fluctuation in riparian petrochemical sites. Environ. Sci. Pollut. Res. 2023, 30, 55756–55767. [Google Scholar] [CrossRef]
- Kaur, G.; Lecka, J.; Krol, M.; Brar, S.K. Novel BTEX-degrading strains from subsurface soil: Isolation, identification and growth evaluation. Environ. Pollut. 2023, 335, 122303. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M. Biodegradation and Bioremediation; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- López, E.; Schuhmacher, M.; Domingo, J.L. Human health risks of petroleum-contaminated groundwater. Environ. Sci. Pollut. Res. 2008, 15, 278–288. [Google Scholar] [CrossRef]
- Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Susan, M.A.B.H.; Kyzas, G.Z. Petroleum hydrocarbon removal from wastewaters: A review. Processes 2020, 8, 447. [Google Scholar] [CrossRef]
- Umar, H.; Abdul Khanan, M.; Ogbonnaya, C.; Shiru, M.; Ahmad, A.; Baba, A. Environmental and socioeconomic impacts of pipeline transport interdiction in Niger Delta, Nigeria. Heliyon 2021, 7, e06999. [Google Scholar] [CrossRef]
- Behnami, A.; Jafari, N.; Benis, K.Z.; Fanaei, F.; Abdolahnejad, A. Spatio-temporal variations, ozone and secondary organic aerosol formation potential, and health risk assessment of BTEX compounds in east of Azerbaijan Province, Iran. Urban Clim. 2023, 47, 101360. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Exposure to Benzene: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Volume 120: Benzene; International Agency for Research on Cancer: Lyon, France, 2018; Volume 120. [Google Scholar]
- Kuranchie, F.A.; Angnunavuri, P.N.; Attiogbe, F.; Nerquaye-Tetteh, E.N. Occupational exposure of benzene, toluene, ethylbenzene and xylene (BTEX) to pump attendants in Ghana: Implications for policy guidance. Cogent Environ. Sci. 2019, 5, 1603418. [Google Scholar] [CrossRef]
- Zoleikha, S.; Mirzaei, R.; Roksana, M. Exposure to chemical hazards in petrol pumps stations in Ahvaz City, Iran. Arch. Environ. Occup. Health 2017, 72, 3–9. [Google Scholar] [CrossRef] [PubMed]
- National Environmental Regulations. National Environmental (Surface and Ground Water Quality Control) Regulations of Nigeria; National Environmental Regulations: Lagos, Nigeria, 2011. [Google Scholar]
- Billersjö, S. In-Situ Remediation of Benzene-Contaminated Groundwater—A Bench-Scale Study; TRITA–LWR Degree Project 13:19; Royal Institute of Technology: Stockholm, Sweden, 2013; 37p. [Google Scholar]
- Bedics, A.; Táncsics, A.; Tóth, E.; Banerjee, S.; Harkai, P.; Kovács, B.; Bóka, K.; Kriszt, B. Microaerobic enrichment of benzene-degrading bacteria and description of Ideonella benzenivorans sp. nov., capable of degrading benzene, toluene and ethylbenzene under microaerobic conditions. Antonie Leeuwenhoek 2022, 115, 1113–1128. [Google Scholar] [CrossRef]
- Sohrabi, T.; Shakiba, M.; Mirzaei, F.; Pourbabaee, A. BTEX biodegradation using Bacillus sp. in a synthetic hypoxic aquatic environment: Optimization by Taguchi-based design of experiments. Int. J. Environ. Sci. Technol. 2022, 19, 5571–5578. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Benzene; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007.
- Vaishnav, D.; Babeu, L. Comparison of Occurrence and Rates of Chemical Biodegradation in Natural Waters; Center for Lake Superior Environmental: Superior, WI, USA, 1987. [Google Scholar]
- Christensen, T.H.; Bjerg, P.L.; Banwart, S.A.; Jakobsen, R.; Heron, G.; Albrechtsen, H.-J. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 2000, 45, 165–241. [Google Scholar] [CrossRef]
- Vogt, C.; Kleinsteuber, S.; Richnow, H.H. Anaerobic benzene degradation by bacteria. Microb. Biotechnol. 2011, 4, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, C.; Fillinger, L.; Atashgahi, S.; da Rocha, U.N.; Kuiper, E.; Olivier, B.; Braster, M.; Gottstein, W.; Helmus, R.; Parsons, J.R. High biodiversity in a benzene-degrading nitrate-reducing culture is sustained by a few primary consumers. Commun. Biol. 2021, 4, 530. [Google Scholar]
- Wu, J.; Bian, J.; Wang, Q.; Ruan, D. Degradation of benzene in anaerobic groundwater in the typical cold industrial region: Identification, interactions, and optimization of nitrate-/sulfate-reducing assemblages. Biochem. Eng. J. 2023, 192, 108833. [Google Scholar] [CrossRef]
- Atashgahi, S.; Hornung, B.; van der Waals, M.J.; da Rocha, U.N.; Hugenholtz, F.; Nijsse, B.; Molenaar, D.; van Spanning, R.; Stams, A.J.M.; Gerritse, J.; et al. A benzene-degrading nitrate-reducing microbial consortium displays aerobic and anaerobic benzene degradation pathways. Sci. Rep. 2018, 8, 4490. [Google Scholar] [CrossRef]
- Toth, C.R.A.; Luo, F.; Bawa, N.; Webb, J.; Guo, S.; Dworatzek, S.; Edwards, E.A. Anaerobic Benzene Biodegradation Linked to the Growth of Highly Specific Bacterial Clades. Environ. Sci. Technol. 2021, 55, 7970–7980. [Google Scholar] [CrossRef] [PubMed]
- Wiedemeier, T.H.; Rifai, H.S.; Newell, C.J.; Wilson, J.T. Natural Attenuation of Fuels and Chlorinated Solvents in the Subsurface; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Kao, C.; Prosser, J. Evaluation of natural attenuation rate at a gasoline spill site. J. Hazard. Mater. 2001, 82, 275–289. [Google Scholar] [CrossRef]
- Scow, K.M.; Hicks, K.A. Natural attenuation and enhanced bioremediation of organic contaminants in groundwater. Curr. Opin. Biotechnol. 2005, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Cozzarelli, I.M.; Bekins, B.A.; Eganhouse, R.P.; Warren, E.; Essaid, H.I. In situ measurements of volatile aromatic hydrocarbon biodegradation rates in groundwater. J. Contam. Hydrol. 2010, 111, 48–64. [Google Scholar] [CrossRef]
- Choi, H.-M.; Lee, J.-Y. Groundwater contamination and natural attenuation capacity at a petroleum spilled facility in Korea. J. Environ. Sci. 2011, 23, 1650–1659. [Google Scholar] [CrossRef]
- Ordinioha, B.; Brisibe, S. The human health implications of crude oil spills in the Niger delta, Nigeria: An interpretation of published studies. Niger. Med. J. 2013, 54, 10–16. [Google Scholar] [CrossRef]
- Nriagu, J.; Udofia, E.A.; Ekong, I.; Ebuk, G. Health risks associated with oil pollution in the Niger Delta, Nigeria. Int. J. Environ. Res. Public Health 2016, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Nanadeinboemi, O.A.; Uju, M.L.; Christopher, C.N.; Hakeem, O.O.; David, D.S. Environmental and Health Influences of Crude Oil Spills in Niger Delta, Nigeria: Case Study Oporoma Community. J. Health Environ. Res. 2024, 8, 29–40. [Google Scholar] [CrossRef]
- Howard, I.C.; Okpara, K.E.; Techato, K. Toxicity and risks assessment of polycyclic aromatic hydrocarbons in river bed sediments of an artisanal crude oil refining area in the Niger Delta, Nigeria. Water 2021, 13, 3295. [Google Scholar] [CrossRef]
- Enuneku, A.; Ogbeide, O.; Okpara, B.; Kubeyinje, B.F.; Job, O.; Asemota, C.O.; Imoobe, T.; Ezemonye, L.I. Ingestion and dermal cancer risk via exposure to polycyclic aromatic hydrocarbon–contaminated soils in an oil-producing community, Niger Delta, Nigeria. Environ. Toxicol. Chem. 2021, 40, 261–271. [Google Scholar] [CrossRef]
- Kalnas, J.; Teitelbaum, D.T. Dermal Absorption of Benzene: Implications for Work Practices and Regulations. Int. J. Occup. Environ. Health 2000, 6, 114–121. [Google Scholar] [CrossRef]
- Obaje, N.G. Geology and Mineral Resources of Nigeria; Springer: Berlin/Heidelberg, Germany, 2009; Volume 120. [Google Scholar]
- Short, K.; Stäuble, A. Outline of geology of Niger Delta. AAPG Bull. 1967, 51, 761–779. [Google Scholar]
- Avbovbo, A.A. Tertiary lithostratigraphy of Niger Delta. AAPG Bull. 1978, 62, 295–300. [Google Scholar]
- Tuttle, M.L.; Charpentier, R.R.; Brownfield, M.E. The Niger Delta Petroleum System: Niger Delta Province, Nigeria, Cameroon, and Equatorial Guinea, Africa; US Department of the Interior, US Geological Survey: Washington, DC, USA, 1999.
- Ajaegwu, N.; Ozumba, B.; Anomneze, D.; Ugwueze, C. The impact of global drawdown of sea-level on the Messinian deposits of shallow offshore, Niger Delta, Nigeria. Basic Phys. Res. 2017, 7, 68–86. [Google Scholar]
- Akujieze, C.N.; Coker, S.; Oteze, G. Groundwater in Nigeria–a millennium experience–distribution, practice, problems and solutions. Hydrogeol. J. 2003, 11, 259–274. [Google Scholar] [CrossRef]
- Adelana, S.M.A.; Olasehinde, P.I.; Bale, R.B.; Vrbka, P.; Edet, A.E.; Goni, I.B. An Overview of the Geology and Hydrogeology of Nigeria; Taylor & Francis Group eBooks: London, UK, 2008; pp. 171–197. [Google Scholar]
- Akpokodje, E.; Etu-Efeotor, J.; Mbeledogu, I. A Study of Environmental Effects of Deep Subsurface Injection of Drilling Waste on Water Resources of the Niger Delta; CORDEC, University of Port Harcourt: Port Harcourt, Nigeria, 1996. [Google Scholar]
- Ogbe, O.; Opatola, O.; Idjerhe, W.; Ocheli, A. Reservoir quality evaluation of sand bodies of K-field, onshore Niger Delta, using wireline logs. Int. J. Sci. Emerg. Technol. Latest Trends 2013, 13, 46–64. [Google Scholar]
- Adagunodo, T.A.; Sunmonu, L.A.; Adabanija, M.A. Reservoir characterization and seal integrity of Jemir field in Niger Delta, Nigeria. J. Afr. Earth Sci. 2017, 129, 779–791. [Google Scholar] [CrossRef]
- Diab, A.I.; Sanuade, O.; Radwan, A.E. An integrated source rock potential, sequence stratigraphy, and petroleum geology of (Agbada-Akata) sediment succession, Niger Delta: Application of well logs aided by 3D seismic and basin modeling. J. Pet. Explor. Prod. Technol. 2023, 13, 237–257. [Google Scholar] [CrossRef]
- Adiela, U.; Odiri, N. Depositional Enviroment and Reservoir Characterization of the Z10 Reservoir sand, Niger Delta, Nigeria. Int. J. Pure Appl. Sci. Technol. 2018, 38, 008–012. [Google Scholar]
- Ogbe, O.B. Sequence stratigraphic controls on reservoir characterization and architectural analysis: A case study of Tovo field, coastal swamp depobelt, Niger Delta Basin, Nigeria. Mar. Pet. Geol. 2020, 121, 104579. [Google Scholar] [CrossRef]
- Onyeagocha, A. Petrography and depositional environment of the Benin Formation. J. Min. Geol. 1980, 17, 147–150. [Google Scholar]
- Ohwoghere-Asuma, O.; Oteng, F.M.; Ophori, D. Simulation of Saltwater Intrusion into Coastal Aquifer of the Western Niger Delta. In Recent Research on Hydrogeology, Geoecology and Atmospheric Sciences; Springer Nature Switzerland: Cham, Switzerland, 2023. [Google Scholar]
- Abam, T.; Nwankwoala, H. Hydrogeology of Eastern Niger Delta: A Review. J. Water Resour. Prot. 2020, 12, 741–777. [Google Scholar] [CrossRef]
- Richard, G.; Izah, S.C.; Morufu, O.R.; Austin-Asomeji, I. Public and environmental health implications of artisanal petroleum refining and risk reduction strategies in the Niger Delta region of Nigeria. Bio-Research 2023, 21, 1896–1910. [Google Scholar] [CrossRef]
- Ewim, D.R.E.; Orikpete, O.F.; Scott, T.O.; Onyebuchi, C.N.; Onukogu, A.O.; Uzougbo, C.G.; Onunka, C. Survey of wastewater issues due to oil spills and pollution in the Niger Delta area of Nigeria: A secondary data analysis. Bull. Natl. Res. Cent. 2023, 47, 116. [Google Scholar] [CrossRef]
- Sam, K.S.; Onyena, A.P.; Erieegha, O.J.; Eze, F. Water quality evaluation using water quality index and pollution model in selected communities in Gbaramatu Kingdom, Niger Delta, Nigeria. Afr. J. Environ. Sci. Technol. 2023, 17, 118–134. [Google Scholar]
- Adeniran, M.A.; Oladunjoye, M.A.; Doro, K.O. Soil and groundwater contamination by crude oil spillage: A review and implications for remediation projects in Nigeria. Front. Environ. Sci. 2023, 11, 1137496. [Google Scholar] [CrossRef]
- USEPA. Method 5021A: Volatile Organic Compounds in Various Sample Matrices Using Equilibrium Headspace Analysis; Revision 1; US Environmental Protection Agency: Washington, DC, USA, 2003.
- Newell, C.J. Calculation and Use of First-Order Rate Constants for Monitored Natural Attenuation Studies; US Environmental Protection Agency, National Risk Management Research Laboratory: San Francisco, CA, USA, 2002.
- McAllister, P.M.; Chiang, C.Y. A practical approach to evaluating natural attenuation of contaminants in ground water. Groundw. Monit. Remediat. 1994, 14, 161–173. [Google Scholar] [CrossRef]
- Bockelmann, A.; Zamfirescu, D.; Ptak, T.; Grathwohl, P.; Teutsch, G. Quantification of mass fluxes and natural attenuation rates at an industrial site with a limited monitoring network: A case study. J. Contam. Hydrol. 2003, 60, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chaocheng, Z.; Qiyou, L.; Yunbo, Z.; Chunshuang, L.; Jianliang, X. Optimization for microbial degradation of dibenzothiophene by Pseudomonas sp. Lky-5 using response surface methodology. China Pet. Process. Petrochem. Technol. 2014, 16, 19–26. [Google Scholar]
- Aweto, K.; Ohwohere-Asuma, O.; Ovwamuedo, G.; Atiti, P. Hydro-geochemical characterization and Groundwater modelling of the subsurface around Ughelli West Engineered Dumpsite in the Western Niger Delta, Nigeria. Niger. J. Technol. Dev. 2023, 20, 62–72. [Google Scholar] [CrossRef]
- Eyankware, M.; Akakuru, O.; Ulakpa, R.; Eyankware, E. Hydrogeochemical approach in the assessment of coastal aquifer for domestic, industrial, and agricultural utilities in Port Harcourt urban, southern Nigeria. Int. J. Energy Water Resour. 2023, 7, 401–419. [Google Scholar] [CrossRef]
- Nwankwoala, H.O.; Walter, I. Assessment of groundwater quality in shallow coastal aquifers of Okrika Island, Eastern Niger Delta, Nigeria. Ife J. Sci. 2012, 14, 297–304. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Zhang, P.; Aagaard, P.; Gottschalk, L. Probability method used in predicting contaminant risk in groundwater adjacent to airport. Water Air Soil Pollut. 2010, 211, 323–339. [Google Scholar] [CrossRef]
- Freeze, R.; Cherry, J. Groundwater; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Pantazidou, M.; Sitar, N. Emplacement of nonaqueous liquids in the vadose zone. Water Resour. Res. 1993, 29, 705–722. [Google Scholar] [CrossRef]
- Wadge, A.; Salisbury, J. Benzene, National Environmental Health Forum Monographs; Air Series No. 2; South Australian Health Commission, Rundle Mall: Adelaide, SA, Australia, 1997. [Google Scholar]
- Baedecker, M.J.; Eganhouse, R.P.; Bekins, B.A.; Delin, G.N. Loss of volatile hydrocarbons from an LNAPL oil source. J. Contam. Hydrol. 2011, 126, 140–152. [Google Scholar] [CrossRef]
- Baedecker, M.J.; Eganhouse, R.P.; Qi, H.; Cozzarelli, I.M.; Trost, J.J.; Bekins, B.A. Weathering of oil in a surficial aquifer. Groundwater 2018, 56, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Weidemeier, T.; Swanson, M.; Wilson, J.; Kampbell, D.; Miller, R.; Hansen, J. Approximation of Biodegradation Rate Constants for Monoaromatic Hydrocarbons (BTEX) in Ground Water; US Environmental Protection Agency Papers; U.S. Environmental Protection Agency: Washington, DC, USA, 1996; p. 26.
- Johnson, S.J.; Woolhouse, K.J.; Prommer, H.; Barry, D.A.; Christofi, N. Contribution of anaerobic microbial activity to natural attenuation of benzene in groundwater. Eng. Geol. 2003, 70, 343–349. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Acio, J.A.; El Telib, A.E. Aerobic biodegradation of BTEX: Progresses and prospects. J. Environ. Chem. Eng. 2014, 2, 1104–1122. [Google Scholar] [CrossRef]
- Eziuzor, S.; Schmidt, M.; Vogt, C. Anaerobic benzene mineralization by natural microbial communities from Niger Delta. Biodegradation 2021, 32, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.; Hynds, P.; Flynn, R. An overview of dissolved organic carbon in groundwater and implications for drinking water safety. Hydrogeol. J. 2017, 25, 959. [Google Scholar] [CrossRef]
- Bekins, B.A.; Cozzarelli, I.M.; Erickson, M.L.; Steenson, R.A.; Thorn, K.A. Crude oil metabolites in groundwater at two spill sites. Groundwater 2016, 54, 681–691. [Google Scholar] [CrossRef]
- Podgorski, D.C.; Zito, P.; McGuire, J.T.; Martinovic-Weigelt, D.; Cozzarelli, I.M.; Bekins, B.A.; Spencer, R.G. Examining natural attenuation and acute toxicity of petroleum-derived dissolved organic matter with optical spectroscopy. Environ. Sci. Technol. 2018, 52, 6157–6166. [Google Scholar] [CrossRef]
- Chinago, A. Analysis of rainfall trend, fluctuation and pattern over Port Harcourt, Niger Delta coastal environment of Nigeria. Biodivers. Int. J. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- McDonough, L.K.; Santos, I.R.; Andersen, M.S.; O’Carroll, D.M.; Rutlidge, H.; Meredith, K.; Oudone, P.; Bridgeman, J.; Gooddy, D.C.; Sorensen, J.P. Changes in global groundwater organic carbon driven by climate change and urbanization. Nat. Commun. 2020, 11, 1279. [Google Scholar] [CrossRef]
- Rajendiran, T.; Sabarathinam, C.; Panda, B.; Elumalai, V. Influence of Dissolved Oxygen, Water Level and Temperature on Dissolved Organic Carbon in Coastal Groundwater. Hydrology 2023, 10, 85. [Google Scholar] [CrossRef]
- Chapelle, F.H.; Bradley, P.M.; McMahon, P.B.; Kaiser, K.; Benner, R. Dissolved oxygen as an indicator of bioavailable dissolved organic carbon in groundwater. Groundwater 2012, 50, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.N.; Gschwend, P.M. Effect of iron diagenesis on the transport of colloidal clay in an unconfined sand aquifer. Geochim. Cosmochim. Acta 1992, 56, 1507–1521. [Google Scholar] [CrossRef]
- White, A.F. Heterogeneous electrochemical reactions associated with oxidation of ferrous oxide and silicate surfaces. Rev. Mineral. Geochem. 1990, 23, 467–509. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Castro, A.R.; Martins, G.; Salvador, A.F.; Cavaleiro, A.J. Iron Compounds in anaerobic degradation of petroleum hydrocarbons: A review. Microorganisms 2022, 10, 2142. [Google Scholar] [CrossRef]
- Van Leeuwen, J.A.; Gerritse, J.; Hartog, N.; Ertl, S.; Parsons, J.R.; Hassanizadeh, S.M. Anaerobic degradation of benzene and other aromatic hydrocarbons in a tar-derived plume: Nitrate versus iron reducing conditions. J. Contam. Hydrol. 2022, 248, 104006. [Google Scholar] [CrossRef]
- Botton, S.; Parsons, J. Degradation of BTEX compounds under iron-reducing conditions in contaminated aquifer microcosms. Environ. Toxicol. Chem. 2006, 25, 2630–2638. [Google Scholar] [CrossRef]
- Marić, N.; Štrbački, J.; Polk, J.; Slavković Beškoski, L.; Avdalović, J.; Lješević, M.; Joksimović, K.; Žerađanin, A.; Beškoski, V.P. Spatial–temporal assessment of hydrocarbon biodegradation mechanisms at a contaminated groundwater site in Serbia. Chem. Ecol. 2022, 38, 95–107. [Google Scholar] [CrossRef]
- Jindrová, E.; Chocová, M.; Demnerová, K.; Brenner, V. Bacterial aerobic degradation of benzene, toluene, ethylbenzene and xylene. Folia Microbiol. 2002, 47, 83–93. [Google Scholar] [CrossRef] [PubMed]
- NRC. Alternatives for Managing the Nation’s Complex Contaminated Groundwater Sites; National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Finneran, K.T.; Housewright, M.E. Enhanced anaerobic bioremediation of BTEX and TCE in groundwater. In Situ and On-Site Bioremediation; Battelle Press: Columbus, OH, USA, 2001; Volume 7. [Google Scholar]
- Singh, R.; Celin, S.M. Biodegradation of BTEX (benzene, toluene, ethyl benzene and xylene) compounds by bacterial strain under aerobic conditions. J. Ecobiotechnol. 2010, 2, 27–32. [Google Scholar]
- Seeger, E.M. Treatment of Groundwater Contaminated with Benzene, MTBE, and Ammonium by Constructed Wetlands. Ph.D. Thesis, Universität Tübingen, Tübingen, Germany, 2013. [Google Scholar]
- Ali, M.; Song, X.; Wang, Q.; Zhang, Z.; Zhang, M.; Chen, X.; Tang, Z.; Liu, X. Thermally enhanced biodegradation of benzo[a]pyrene and benzene co-contaminated soil: Bioavailability and generation of ROS. J. Hazard. Mater. 2023, 455, 131494. [Google Scholar] [CrossRef]
- Eze, M.O. Metagenome analysis of a hydrocarbon-degrading bacterial consortium reveals the specific roles of BTEX biodegraders. Genes 2021, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Pfeffer, F.M.; Weaver, J.W.; Kampbell, D.H.; Wiedemeier, T.H.; Hansen, J.E.; Miller, R.N. Intrinsic bioremediation of JP-4 jet fuel. In Proceedings of the Symposium on Intrinsic Bioremediation of Ground Water, EPA/540/R-94/515, Denver, CO, USA, 30 August–1 September 1994. [Google Scholar]


| Parameter | Units | Okochiri (n = 15) | Ogale (n = 7) | Alode (n = 8) |
|---|---|---|---|---|
| pH | 4.5–6.1 (5.4) | 4.1–6.6 (5.2) | 4.7–6.1 (5.1) | |
| DO | mg/L | 3.2–7.4 (6.3) | 0.7–5.9 (4.2) | 1.4–7.5 (5.5) |
| DO saturation | % | 41.4–97 (85) | 9–74.5 (55.4) | 19–95 (76) |
| Eh | mV | 238–801 (652) | 118–561 (427) | 113–596 (401) |
| EC | µS/cm | 21–207 (59) | 20–364 (67) | 20–106 (49) |
| TDS | mg/L | 11–105 (30) | 10–183 (33) | 10–53 (25) |
| Temperature | °C | 26.6–32.5 (29.3) | 25.12–30.7 (29.4) | 26.45–31 (30.8) |
| Salinity | PSU | 0.01–0.1 (0.03) | 0.01–0.17 (0.03) | 0.01–0.1 (0.02) |
| Alkalinity | mg/L | 0–25 (1) | 0–100 (0) | 0–30 (3) |
| DIC | mg/L | 0–31 (1.3) | 0–122 (0) | 0–37 (4) |
| F− | mg/L | <0.01 | <0.01–0.2 (<0.01) | <0.01–3 (0.1) |
| Cl− | mg/L | 1–6 (5) | 3–17 (8) | <0.01–12 (4) |
| NO2− | mg/L | <0.01 | <0.01–1 (<0.01) | <0.01–3 (<0.01) |
| NO3− | mg/L | <0.01–3 (2) | <0.01–39 (2) | <0.01–2 (<0.01) |
| SO42− | mg/L | 1–14 (9) | 1–23 (6) | 1–20 (6) |
| Ca | mg/L | 0.5–1 (0.6) | 0.2–13 (1) | 0.5–12 (2) |
| Na | mg/L | 0.6–15 (4) | 1–14 (4) | 0.6–10 (3) |
| K | mg/L | 0.2–1 (0.4) | 0.1–12 (1) | 0.1–7 (1) |
| Mg | mg/L | 0.04–0.4 (0.2) | 0.02–4 (0.1) | 0.1–1 (0.3) |
| Si | mg/L | 0.5–5 (4) | 0.3–4 (3) | 1–4 (3.6) |
| Fe | mg/L | 0.01–25 (0.2) | 0.01–50 (2) | 0.01–7 (1) |
| Mn | mg/L | 0.01–0.3 (0.1) | 0.01–0.2 (0.02) | 0.01–0.2 (0.03) |
| Sr | mg/L | 0.002–0.01 (0.003) | 0.001–0.02 (0.003) | 0.001–0.03 (0.01) |
| DOC | mg/L | 3–33 (24) | 9–49 (30) | 16–47 (32) |
| Location | Benzene | Toluene | Ethylbenzene | Xylenes | Trimethylbenzene | Source |
|---|---|---|---|---|---|---|
| Bragança, Brazil 1 | <0.1–0.6 | <0.1–10.4 | <0.1 | <0.1–0.5 | NA | UST at gas station |
| Bengaluru, India 2 | <0.1–485 | <0.1–153 | <0.1–80 | <0.1–2620 | NA | UST at gas station |
| China | <0.1–644 | <0.1–16.7 | <0.1–209 | <0.1–181 | NA | Petrochemical site |
| Bonny, Nigeria 3 | <0.1–660 | <0.1–800 | <0.1–250 | <0.1–4200 | NA | Petroleum spillage |
| Eleme, Nigeria 4 | 161–9280 | NA | NA | NA | NA | Petroleum spillage |
| Minnesota, USA 5 | <0.1–2550 | <0.1–10.37 | 0.3–3.26 | <0.1–1230.7 | <0.1–678.23 | Pipeline rupture |
| Utah, USA 6 | <0.1–5600 | <0.1–5870 | 2–950 | 36–9050 | 2–650 | Hydrocarbon storage facility |
| Eleme/Okrika, Nigeria 7 | <0.1–3500 | <0.1–210 | <0.1–370 | <0.1–360 | <0.1–220 | Pipeline leakage |
| Year | Parameter | Alode | Ogale | Okochiri |
|---|---|---|---|---|
| 2022 | CP (mg/L, DO) | 3.6 | 1.53 | 3.23 |
| CB (mg/L, DO) | 8.89 | 8.17 | 8.44 | |
| F | 3.14 | 3.14 | 3.14 | |
| EBCDO (mg/L) | 1.68 | 2.11 | 1.66 | |
| 2023 | CP (mg/L, DO) | 1.4 | 0.66 | 5.32 |
| CB (mg/L, DO) | 7.86 | 7.28 | 7.27 | |
| F | 3.14 | 3.14 | 3.14 | |
| EBCDO (mg/L) | 2.06 | 2.11 | 0.62 | |
| Mean | EBCDO (mg/L) | 1.87 | 2.11 | 1.14 |
| Sample | Site | Contaminant | Point Attenuation (day−1) | Half-Life (yr) | Remediation Goal a | Remediation Time (yr) b |
|---|---|---|---|---|---|---|
| W-21 | Okochiri | Benzene | 0.425 | 7.1 | 0.0002 | 20.7 |
| BTEX | 0.383 | 7.7 | 0.0008 | 19.3 | ||
| W-22 | Okochiri | Benzene | 0.572 | 4.6 | 0.0002 | 14.1 |
| BTEX | 0.556 | 4.5 | 0.0008 | 12 | ||
| W-12 | Ogale | Benzene | 0.128 | 32.8 | 0.0002 | 66.5 |
| BTEX | 0.086 | 51.7 | 0.0008 | 85 | ||
| W-1 | Alode | Benzene | 0.693 | 3.5 | 0.0002 | 11.2 |
| BTEX | 0.4609 | 4.8 | 0.0008 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleku, D.L.; Biester, H.; Pichler, T. Pipeline-Related Residential Benzene Exposure and Groundwater Natural Attenuation Capacity in the Eastern Niger Delta, Nigeria. Environments 2024, 11, 221. https://doi.org/10.3390/environments11100221
Aleku DL, Biester H, Pichler T. Pipeline-Related Residential Benzene Exposure and Groundwater Natural Attenuation Capacity in the Eastern Niger Delta, Nigeria. Environments. 2024; 11(10):221. https://doi.org/10.3390/environments11100221
Chicago/Turabian StyleAleku, Dogo Lawrence, Harald Biester, and Thomas Pichler. 2024. "Pipeline-Related Residential Benzene Exposure and Groundwater Natural Attenuation Capacity in the Eastern Niger Delta, Nigeria" Environments 11, no. 10: 221. https://doi.org/10.3390/environments11100221
APA StyleAleku, D. L., Biester, H., & Pichler, T. (2024). Pipeline-Related Residential Benzene Exposure and Groundwater Natural Attenuation Capacity in the Eastern Niger Delta, Nigeria. Environments, 11(10), 221. https://doi.org/10.3390/environments11100221










