Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece
Abstract
1. Introduction
2. Materials and Methods
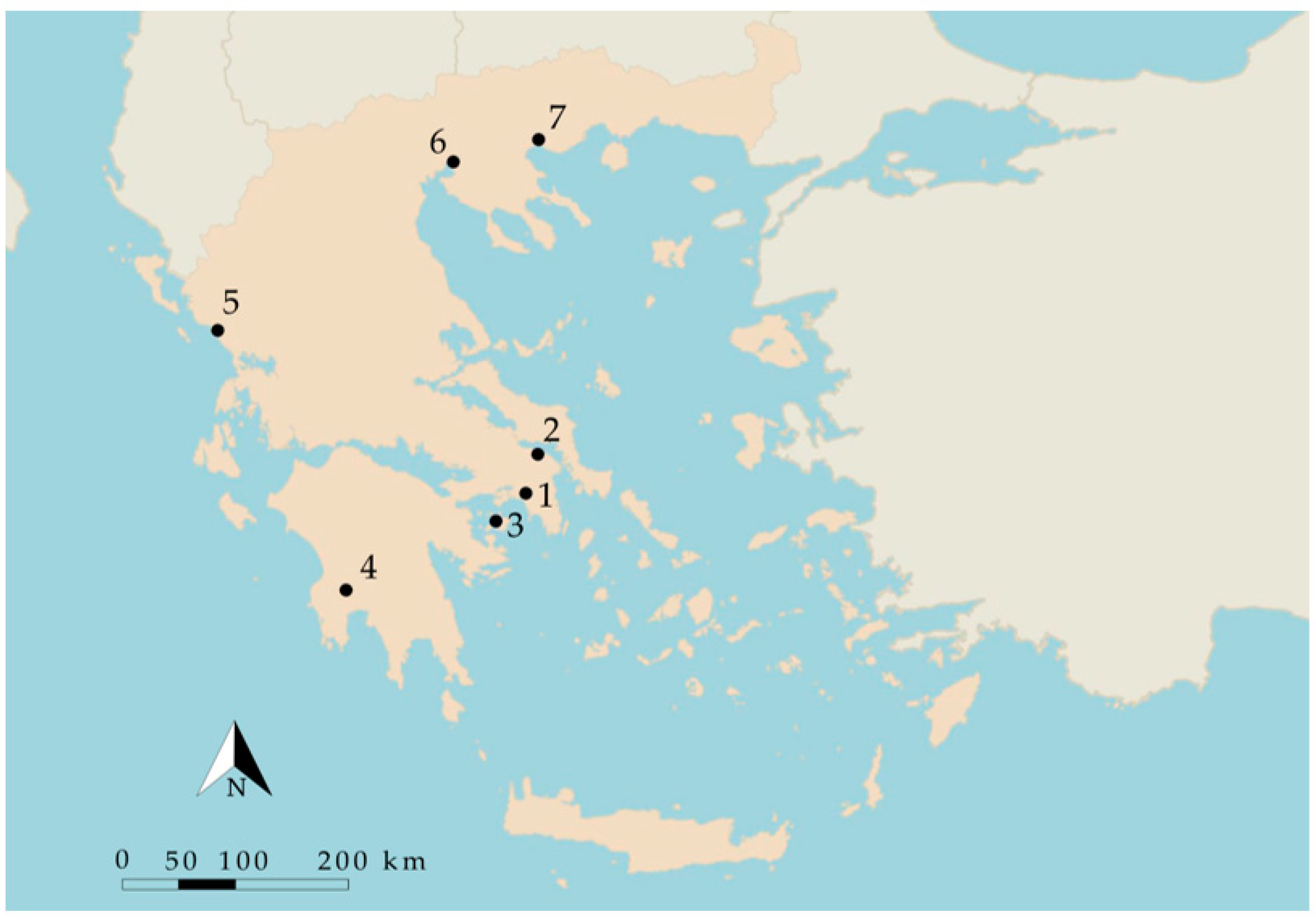
2.1. Sites Description
2.2. Data Collection
2.3. Data Processing
3. Results
3.1. Species Distribution to Biological Forms
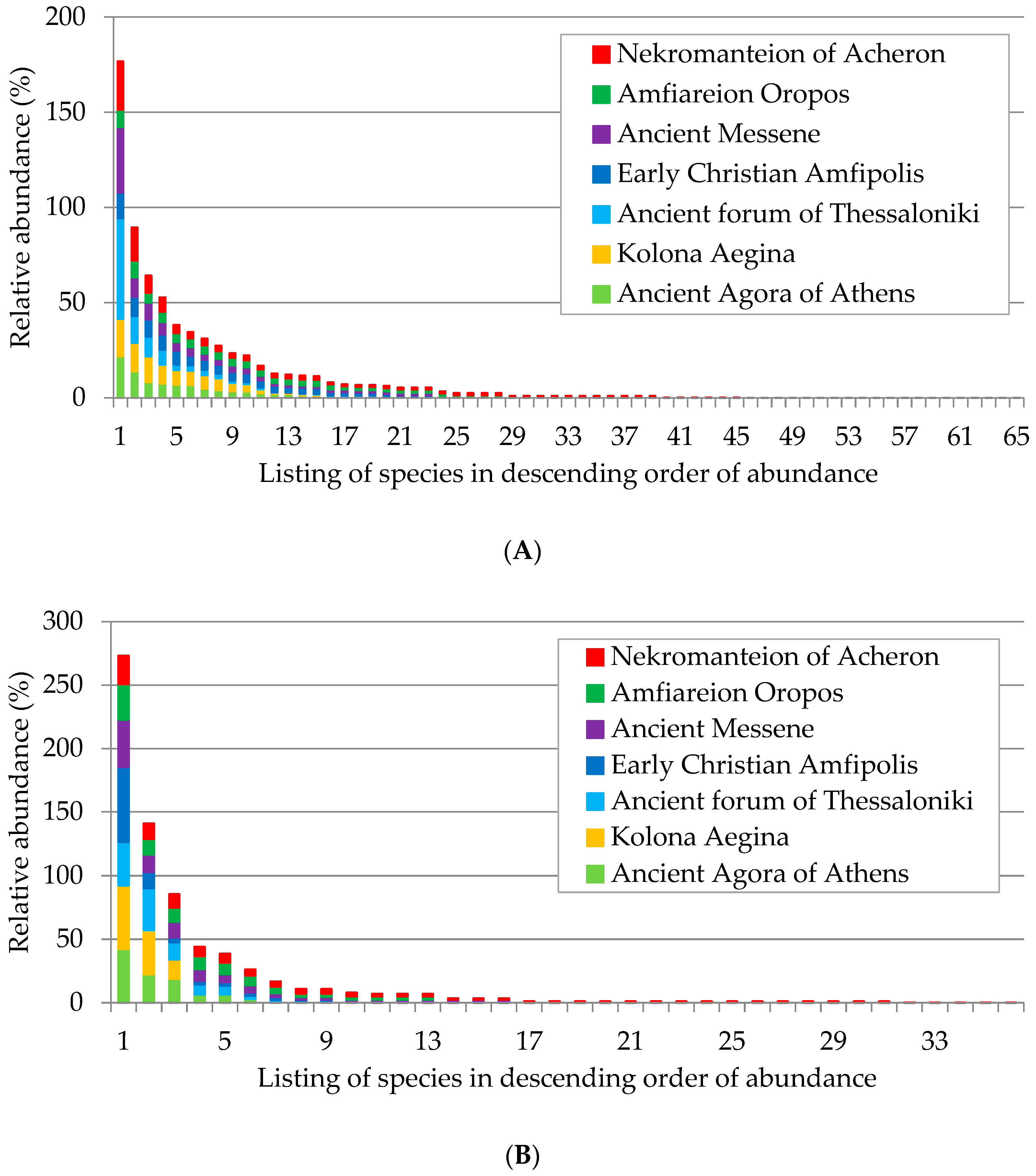
3.2. Species Distribution to Botanical Families
3.3. Species Richness and Diversity Indices
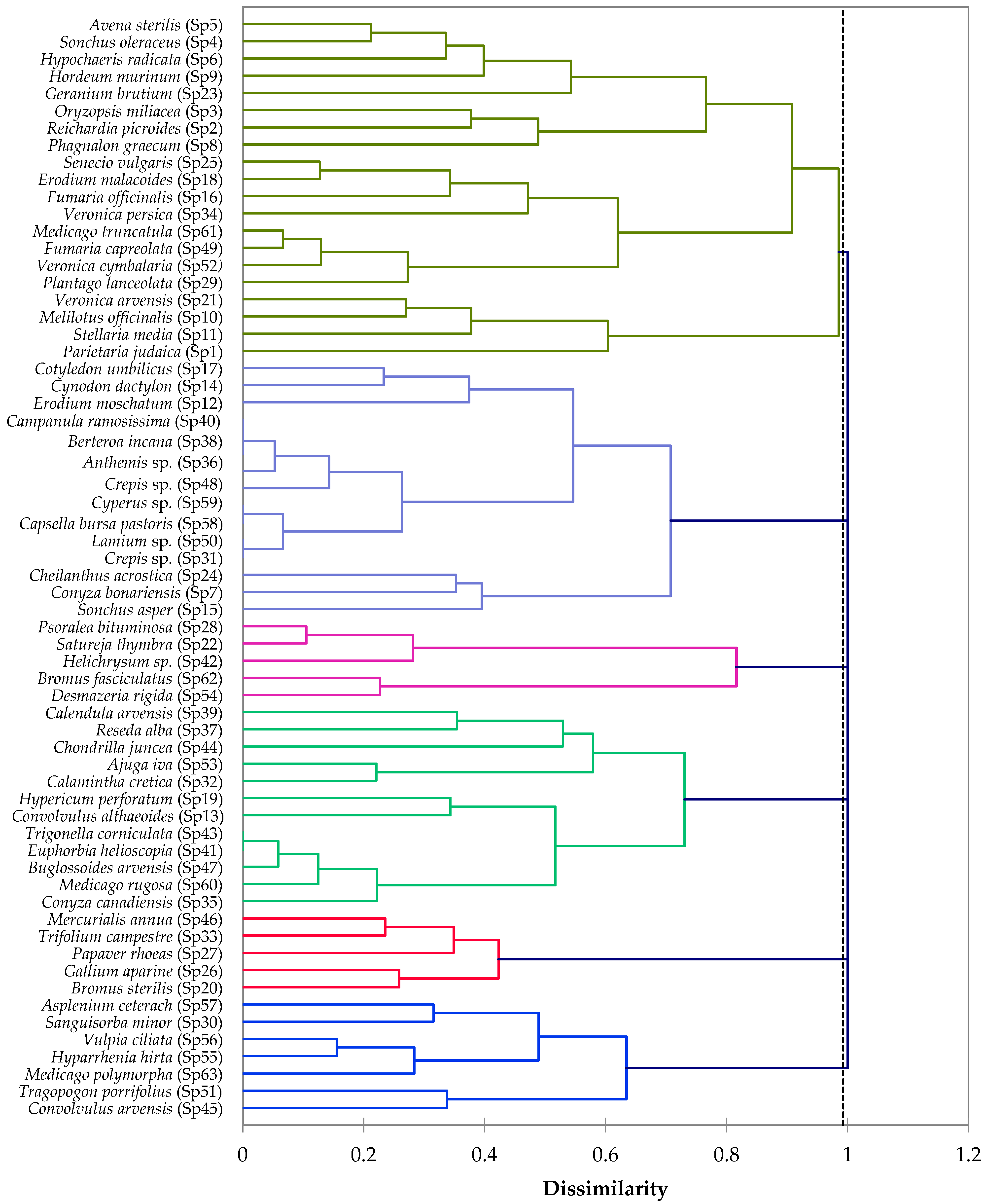
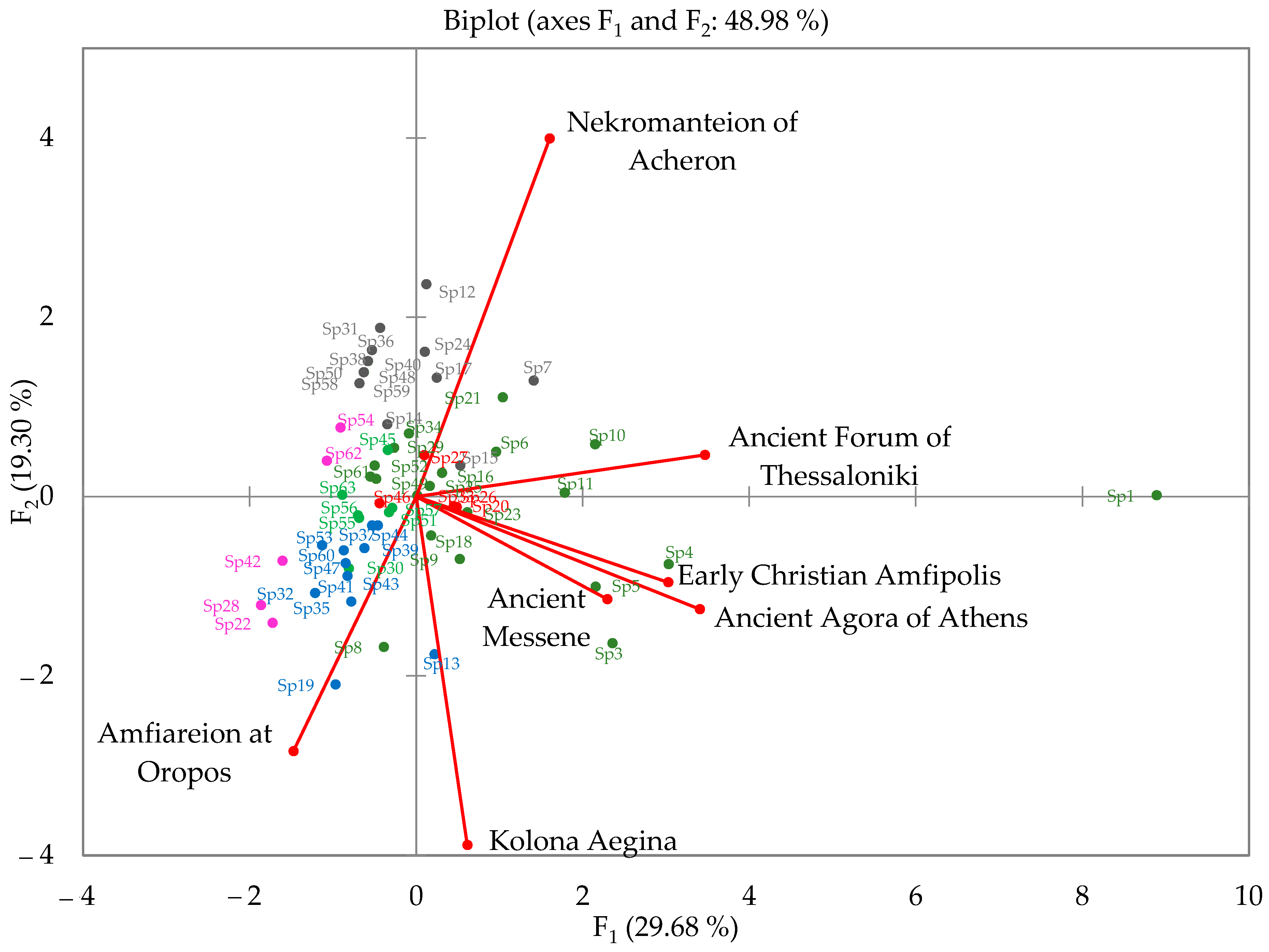
3.4. Cluster and Principal Component Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caneva, G.; Pacini, A. Problems of biodeterioration. In Plant Biology for Cultural Heritage. Biodeterioration and Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 222–237. [Google Scholar]
- Motti, R.; Bonanomi, G. Vascular plant colonization of four castles in southern Italy: Effects of substrate bioreceptivity, local environment factors and current management. Int. Biodeterior. Biodegrad. 2018, 133, 26–33. [Google Scholar] [CrossRef]
- Cicinelli, E.; Benelli, F.; Bartoli, F.; Traversetti, L.; Caneva, G. Trends of plant communities growing on the Etruscan tombs (Cerveteri, Italy) related to different management practices. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2020, 154, 158–164. [Google Scholar] [CrossRef]
- Caneva, G.; Ceschin, S. Ecology of biodeterioration. In Plant Biology for Cultural Heritage; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 35–58. [Google Scholar]
- Caneva, G.; Benelli, F.; Bartoli, F.; Cicinelli, E. Safeguarding natural and cultural heritage on Etruscan tombs (La Banditaccia, Cerveteri, Italy). Rend. Fis. Acc. Lincei 2018, 29, 891–907. [Google Scholar] [CrossRef]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and higher plants on stone: A review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Pacini, E.; Signorini, M. Vascular plants. In Plant Biology for Cultural Heritage; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 87–96. [Google Scholar]
- Cicinelli, E.; Salerno, G.; Caneva, G. An assessment methodology to combine the preservation of biodiversity and cultural heritage: The San Vincenzo al Volturno historical site (Molise, Italy). Biodivers. Conserv. 2018, 27, 1073–1093. [Google Scholar] [CrossRef]
- Ceschin, S.; Bartoli, F.; Salerno, G.; Zuccarello, V.; Caneva, G. Natural habitats of typical plants growing on ruins of Roman archaeological sites (Rome, Italy). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 866–875. [Google Scholar] [CrossRef]
- Minissale, P.; Trigilia, A.; Brogna, F.; Sciandrello, S. Plants and Vegetation in the Archaeological Park of Neapolis of Syracuse (Sicily, Italy): A Management Effort and also an Opportunity for Better Enjoyment of the Site. Conserv. Manag. Archaeol. Sites 2015, 17, 340–369. [Google Scholar] [CrossRef]
- Touloupa, E. Introductory note. In Proceedings of the Symposium on Spontaneous Vegetation in Archaeological Sites, Athens, Greece, 22–23 May 1998; Acropolis Friends Association: Athens, Greece, 1998; pp. 3–4. [Google Scholar]
- Pinna, D.; Salvadori, O. Processes of biodeterioration: General mechanisms. In Plant Biology for Cultural Heritage; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 15–34. [Google Scholar]
- Zahos, K. The Spontaneous Flora in Archaeological Sites: The Greek Experience. In Proceedings of the Symposium on Spontaneous Vegetation in Archaeological Sites, Athens, Greece, 22–23 May 1998; Acropolis Friends Association: Athens, Greece, 1998; pp. 9–17. [Google Scholar]
- Stratou, F. Introductory note. In Proceedings of the Symposium on Spontaneous Vegetation in Archaeological Sites, Athens, Greece, 22–23 May 1998; Acropolis Friends Association: Athens, Greece, 1998; p. 5. [Google Scholar]
- Baumann, H. Greek Wild Flowers and Plant Lore in Ancient Greece; The Greek Foundation for the Protection of Nature: Athens, Greece, 1999; pp. 14–22; 169–192. [Google Scholar]
- Venice Charter for the Conservation and Restoration of Monuments and Sites; International Council on Monuments and Sites (ICOMOS): Venice, Italy, 1964.
- The ICOMOS Charter for the Interpretation and Presentation of Cultural Heritage Sites; International Council on Monuments and Sites (ICOMOS): Quebec, QC, Canada, 2008.
- Quebec Declaration on the Preservation of the Spirit of Place; International Council on Monuments and Sites (ICOMOS): Quebec, QC, Canada, 2008.
- Tsaravopoulos, A.; Fragou, G. Archaeological sites as self-sustained resources for economic regeneration: Towards the creation of living archaeological parks on the islands of kythera and antikythera. Conserv. Manag. Archaeol. Sites 2013, 15, 94–108. [Google Scholar] [CrossRef]
- Hosseini, Z.; Bartoli, F.; Cicinelli, E.; Lucchese, F. First floristic investigation in archaeological sites of Iran: Features and plant richness of the Pasargadae World Heritage Site. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2023, 157, 605–621. [Google Scholar] [CrossRef]
- Croce, A.; Biagioli, A.; Bella, V.D.; Topi, C. Archaeological sites as refugia and conservation areas of native orchids a case study from Northern Campania. GIROS Orch. Spont. Eur. 2019, 62, 70–89. [Google Scholar]
- Heneidy, S.Z.; Al-Sodany, Y.A.; Bidak, L.M.; Fakhry, A.M.; Hamouda, S.K.; Halmy, M.W.A.; Alrumman, S.A.; Al-Bakre, D.A.; Eid, E.M.; Toto, S.M. Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt. Sustainability 2022, 14, 2416. [Google Scholar] [CrossRef]
- DeBerry, D.A. Vegetation Sampling Concepts for Compensatory Mitigation Sites. Wetl. Sci. Pract. 2020, 37, 174–182. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Plant Sociology. In The Study of Plant Communities, 1st ed.; McGraw-Hill: New York, NY, USA, 1932; p. 539. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Heltshe, J.; Forrester, N. Estimating species richness using the Jackknife procedure. Biometrics 1983, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bottcher, L.; Chou, T. Diversity in biology: Definitions, quantification and models. Phys. Biol. 2020, 17, 031001. [Google Scholar] [CrossRef] [PubMed]
- Washington, H. Diversity, biotic and similarity indices. A review with special relevance to aquatic ecosystems. Water Res. 1984, 18, 653–694. [Google Scholar] [CrossRef]
- Mauzy, G. Agora Excavations, 1931–2006: A Pictorial History; American School of Classical Studies at Athens: Athens, Greece, 2006. [Google Scholar]
- Motti, R.; Stinca, A. Analysis of the Biodeteriogenic Vascular Flora at the Royal Palace of Portici in Southern Italy. Int. Biodeterior. Biodegrad. 2011, 65, 1256–1265. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.; Salvadori, O. Methodological aspects of the treatments. In Plant Biology for Cultural Heritage; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 309–311. [Google Scholar]
- Kanellou, E.; Economou, G.; Papafotiou, M.; Ntoulas, N.; Lyra, D.; Knezevic, S. Flame weeding at archaeological sites of the Mediterranean region. Weed Technol. 2017, 31, 396–403. [Google Scholar] [CrossRef]
- Kanellou, E.; Papafotiou, M.; Economou, G.; Ntoulas, N. Testing soil solarization as an alternative weed control method for archaeological sites of the Mediterranean region. Sustainability 2023, 15, 11324. [Google Scholar] [CrossRef]
- Kanellou, E.; Papafotiou, M.; Economou, G.; Paraskevopoulou, A.; Kartsonas, E.; Ntoulas, N. Response of sowed herbaceous forb mixtures suitable for aesthetic improvement and vegetation management at archaeological sites of the Mediterranean region. Ecol. Eng. 2021, 167, 106256. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Dupre, C.; Diekman, M. Differences in species richness and life history traits between grazed and abandoned grasslands in southern Sweden. Ecography 2001, 24, 275–286. [Google Scholar] [CrossRef]
- Noy-Meir, I.; Gutman, M.; Kaplan, Y. Responses of Mediterranean grassland plants to grazing and protection. J. Ecol. 1989, 77, 290–310. [Google Scholar] [CrossRef]
- Hadar, L.; Noy-Meir, I.P.A. The effect of shrub clearing and grazing on the composition of a Mediterranean plant community: Functional groups versus species. J. Veg. Sci. 1999, 10, 673–682. [Google Scholar] [CrossRef]
- Hawkins, B.; Field, R.; Cornell, H.V.; Currie, D.J.; Guegan, J.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Osman, R.W. The Intermediate Disturbance Hypothesis. In Encyclopedia of Ecology, 2nd ed.; Fath, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 441–450. [Google Scholar] [CrossRef]
- Bakker, J.P.; de Leeuw, J.; Dijkema, K.S.; Leendertse, P.C.; Prins, H.H.T.; Rozema, J. Salt marshes along the coast of the Netherlands. Hydrobiologia 1993, 265, 73–79. [Google Scholar] [CrossRef]
- Tremont, R. Life history attributes of plants in grazed and ungrazed grasslands on the Northern Table grasslands of New South Wales. Aust. J. Bot. 1994, 42, 511–530. [Google Scholar] [CrossRef]
- Smith, R.; Rushton, S. The effects of grazing management on the vegetation of mesotrophic (meadow) grassland in Northern England. J. Appl. Ecol. 1994, 31, 13–24. [Google Scholar] [CrossRef]
- Petit, N.; Froend, R.; Ladd, P. Grazing in remnant woodland vegetation: Changes in species composition and life form groups. J. Veg. Sci. 1995, 6, 121–130. [Google Scholar] [CrossRef]
- Baumann, H. Plants on Ancient Greek Coins; Ilivaton: Athens, Greece, 2004. [Google Scholar]



| Archaeological Site * | January | February | March | April | May | June | July | August | September | October | November | December |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air temperature (°C) | ||||||||||||
| AAA | 9.36 | 9.51 | 11.47 | 15.65 | 21.31 | 26.02 | 28.55 | 27.87 | 23.79 | 19.07 | 14.00 | 10.58 |
| AO | 8.14 | 8.7 | 10.9 | 14.07 | 19.18 | 24.43 | 26.5 | 26.68 | 22.52 | 17.71 | 12.6 | 9.93 |
| KA | 10.29 | 9.83 | 12.15 | 15.77 | 20.78 | 25.47 | 28.12 | 27.49 | 24.27 | 19.47 | 14.97 | 11.35 |
| AM | 9.27 | 9.02 | 11.42 | 14.66 | 19.48 | 24.26 | 26.97 | 26.01 | 23.04 | 18.23 | 13.86 | 10.48 |
| NA | 8.43 | 8.69 | 11.02 | 13.76 | 19.12 | 23.06 | 24.7 | 24.53 | 21.6 | 17.43 | 12.88 | 9.42 |
| AFT | 5.18 | 6.62 | 9.39 | 13.99 | 19.91 | 24.59 | 26.76 | 26.15 | 21.78 | 16.45 | 10.77 | 6.16 |
| ECA | 5.60 | 6.83 | 9.17 | 14.01 | 19.31 | 23.87 | 26.07 | 25.24 | 21.74 | 16.43 | 10.63 | 6.40 |
| Precipitation (mm) | ||||||||||||
| AAA | 37.29 | 47.38 | 55.79 | 31.92 | 17.87 | 7.06 | 7.30 | 7.21 | 9.16 | 35.23 | 51.05 | 66.17 |
| AO | 68.51 | 49.50 | 62.67 | 33.62 | 22.34 | 9.41 | 8.41 | 8.04 | 15.03 | 53.53 | 90.34 | 112.29 |
| KA | 45.68 | 37.83 | 46.15 | 31.19 | 12.27 | 5.44 | 7.55 | 6.76 | 8.24 | 37.14 | 71.40 | 74.37 |
| AM | 129.44 | 96.29 | 80.79 | 67.46 | 29.12 | 8.02 | 8.59 | 14.93 | 35.75 | 83.88 | 159.16 | 170.88 |
| NA | 102.41 | 113.96 | 82.35 | 69.24 | 38.88 | 17.67 | 12.55 | 19.07 | 55.59 | 113.98 | 192.06 | 173.51 |
| AFT | 25.00 | 38.81 | 36.99 | 43.71 | 39.91 | 29.00 | 26.35 | 24.06 | 19.92 | 26.43 | 35.85 | 40.00 |
| ECA | 34.90 | 37.22 | 47.27 | 37.11 | 42.04 | 34.60 | 29.11 | 19.67 | 20.03 | 41.97 | 59.76 | 63.47 |
| Around Monuments Spring | Around Monuments Autumn | On Monuments Spring | On Monuments Autumn | |
|---|---|---|---|---|
| Therophytes | 66.1 | 41.2 | 55.4 | 31.6 |
| Hemicryptophytes | 23.0 | 36.2 | 22.3 | 27.4 |
| Geophytes | 5.7 | 15.0 | 4.3 | 8.4 |
| Chamaephytes | 4.6 | 6.3 | 6.0 | 9.4 |
| Phanerophytes | 0.6 | 1.3 | 12.0 | 23.2 |
| Botanical Family | Around Monuments Spring | Around Monuments Autumn | On Monuments Spring | On Monuments Autumn |
|---|---|---|---|---|
| Fabaceae | 21.0 | 5.8 | 14.4 | 7.4 |
| Poaceae | 20.6 | 13.9 | 16.9 | 10.8 |
| Asteraceae | 17.7 | 19.7 | 16.3 | 17.6 |
| Plantaginaceae | 4.5 | 0.0 | 4.1 | 0.0 |
| Geraniaceae | 3.9 | 0.0 | 4.1 | 0.0 |
| Brassicaceae | 3.9 | 0.0 | 0.0 | 0.0 |
| Malvaceae | 0.0 | 5.1 | 0.0 | 0.0 |
| Lamiaceae | 0.0 | 4.4 | 4.1 | 4.1 |
| Euphorbiaceae | 0.0 | 4.4 | 0.0 | 0.0 |
| Moraceae | 0.0 | 0.0 | 0.0 | 4.1 |
| Urticaceae | 0.0 | 0.0 | 0.0 | 4.1 |
| Other | 28.4 | 46.7 | 40.1 | 51.9 |
| Archaeological Site | Sobs | Se |
|---|---|---|
| Amfiareion Oropos | 124 | 152 |
| Nekromanteion of Acheron | 98 | 123 |
| Ancient Messene | 92 | 122 |
| Ancient Agora of Athens | 77 | 95 |
| Early Christian Amfipolis | 72 | 90 |
| Kolona Aegina | 59 | 64 |
| Ancient Forum of Thessaloniki | 53 | 41 |
| Index | ||||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | |||||||
| Archaeological Site | S * | H′ | D | Ε | S | H′ | D | Ε |
| Ancient Forum of Thessaloniki | 21 | 1.61 | 0.68 | 0.53 | 10 | 1.56 | 0.74 | 0.68 |
| Kolona Aegina | 33 | 2.36 | 0.89 | 0.67 | 3 | 1.00 | 0.61 | 0.91 |
| Ancient Messene | 48 | 2.50 | 0.85 | 0.65 | 29 | 2.08 | 0.81 | 0.62 |
| Nekromanteion of Archeron | 54 | 2.68 | 0.88 | 0.67 | 39 | 2.84 | 0.91 | 0.78 |
| Ancient Agora of Athens | 43 | 2.73 | 0.90 | 0.72 | 7 | 1.48 | 0.73 | 0.76 |
| Early Christian Amfipolis | 38 | 2.89 | 0.93 | 0.79 | 28 | 1.65 | 0.63 | 0.50 |
| Amfiareion Oropos | 82 | 3.42 | 0.96 | 0.78 | 26 | 2.27 | 0.86 | 0.70 |
| Archaeological Site | AAA * | AFT | AM | AO | ECA | KA | NA | Sum of Sites |
|---|---|---|---|---|---|---|---|---|
| Species | Relative Abundance | |||||||
| Parietaria judaica L. | 8.39 | 17.71 | 7.01 | 0.00 | 13.56 | 3.52 | 7.07 | 7.28 |
| Reichardia picroides (L.) Roth | 9.01 | 0.00 | 2.55 | 7.69 | 0.00 | 9.05 | 0.00 | 4.63 |
| Oryzopsis miliacea (L.) Asch. & Schweinf. | 12.42 | 0.00 | 3.18 | 1.36 | 0.00 | 2.01 | 0.67 | 3.68 |
| Sonchus oleraceus L. | 4.04 | 3.13 | 5.10 | 0.00 | 4.52 | 4.52 | 3.37 | 3.47 |
| Avena sterilis L. | 3.73 | 1.04 | 3.82 | 1.36 | 5.65 | 2.51 | 2.02 | 2.93 |
| Hypochaeris radicata L. | 4.04 | 1.04 | 0.00 | 0.45 | 1.69 | 2.51 | 3.70 | 2.31 |
| Conyza bonariensis (L.) Cronquist | 0.93 | 3.13 | 5.10 | 0.00 | 0.56 | 0.00 | 4.04 | 1.84 |
| Phagnalon graecum Boiss. & Heldr. | 4.35 | 0.00 | 0.00 | 3.17 | 0.00 | 3.02 | 0.00 | 1.84 |
| Hordeum murinum L. | 2.48 | 0.00 | 1.27 | 0.00 | 1.69 | 4.02 | 1.35 | 1.70 |
| Melilotus officinalis (L.) Lam. | 2.17 | 18.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.70 |
| Stellaria media (L.) Vill. | 2.17 | 9.38 | 1.27 | 0.00 | 3.39 | 0.00 | 0.00 | 1.63 |
| Erodium moschatum (L.) L’Hér. | 0.31 | 0.00 | 0.64 | 0.45 | 0.56 | 0.00 | 6.06 | 1.50 |
| Convolvulus althaeoides L. | 0.00 | 0.00 | 0.00 | 0.00 | 4.52 | 6.53 | 0.00 | 1.43 |
| Cynodon dactylon (L.) Pers. | 0.00 | 0.00 | 1.27 | 2.26 | 1.13 | 0.00 | 3.37 | 1.29 |
| Sonchus asper (L.) Hill | 0.00 | 0.00 | 6.37 | 0.90 | 0.00 | 0.00 | 2.36 | 1.29 |
| Fumaria officinalis L. | 4.97 | 1.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.67 | 1.29 |
| Cotyledon umbilicus Britten | 1.24 | 0.00 | 1.27 | 0.00 | 1.13 | 0.00 | 3.37 | 1.23 |
| Erodium malacoides (L.) L’Hér. | 3.73 | 2.08 | 0.00 | 0.00 | 0.00 | 2.01 | 0.00 | 1.23 |
| Hypericum perforatum L. | 0.00 | 0.00 | 0.00 | 1.81 | 0.00 | 7.04 | 0.00 | 1.23 |
| Bromus sterilis L. | 3.11 | 0.00 | 0.00 | 0.00 | 3.95 | 0.00 | 0.00 | 1.16 |
| Veronica arvensis L. | 0.31 | 12.50 | 0.00 | 0.00 | 0.00 | 0.00 | 1.35 | 1.16 |
| Satureja thymbra L. | 0.93 | 0.00 | 0.00 | 6.33 | 0.00 | 0.00 | 0.00 | 1.16 |
| Geranium brutium Gasp. | 2.17 | 1.04 | 2.55 | 0.00 | 2.26 | 0.00 | 0.00 | 1.09 |
| Cheilanthes acrostica (Balb.) Tod. | 0.00 | 0.00 | 2.55 | 0.00 | 0.00 | 0.00 | 4.04 | 1.09 |
| Senecio vulgaris L. | 3.73 | 2.08 | 0.00 | 0.00 | 0.00 | 0.50 | 0.34 | 1.09 |
| Gallium aparine L. | 1.24 | 0.00 | 0.64 | 0.00 | 5.08 | 0.00 | 0.00 | 0.95 |
| Papaver rhoeas L. | 0.31 | 0.00 | 0.00 | 0.00 | 3.95 | 0.50 | 1.35 | 0.88 |
| Psoralea bituminosa L. | 0.00 | 0.00 | 0.00 | 5.88 | 0.00 | 0.00 | 0.00 | 0.88 |
| Plantago lanceolata L. | 2.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.01 | 0.82 |
| Sanguisorba minor Scop. | 0.00 | 0.00 | 3.18 | 3.17 | 0.00 | 0.00 | 0.00 | 0.82 |
| Crepis sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.04 | 0.82 |
| Calamintha cretica (L.) Lam. | 0.00 | 0.00 | 0.00 | 2.26 | 0.00 | 3.02 | 0.00 | 0.75 |
| Trifolium campestre Schreb. | 0.31 | 0.00 | 1.27 | 0.45 | 3.39 | 0.00 | 0.34 | 0.75 |
| Veronica persica Poir. | 1.55 | 3.13 | 0.00 | 0.00 | 0.00 | 0.00 | 1.01 | 0.75 |
| Conyza canadiensis (L.) Cronquist | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.53 | 0.00 | 0.75 |
| Anthemis sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.37 | 0.68 |
| Reseda alba L. | 1.86 | 0.00 | 0.00 | 0.00 | 0.00 | 2.01 | 0.00 | 0.68 |
| Berteroa incana (L.) DC. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.03 | 0.61 |
| Calendula arvensis L | 0.62 | 0.00 | 0.64 | 0.00 | 0.00 | 3.02 | 0.00 | 0.61 |
| Campanula ramosissima Sm. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.03 | 0.61 |
| Euphorbia helioscopia L. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.52 | 0.00 | 0.61 |
| Helichrysum sp. | 0.00 | 0.00 | 0.00 | 4.07 | 0.00 | 0.00 | 0.00 | 0.61 |
| Trigonella corniculata (L.) L. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.52 | 0.00 | 0.61 |
| Chondrilla juncea L. | 0.00 | 0.00 | 0.00 | 0.00 | 2.26 | 2.01 | 0.00 | 0.54 |
| Convolvulus arvensis L. | 0.62 | 0.00 | 1.91 | 0.00 | 0.00 | 0.00 | 1.01 | 0.54 |
| Mercurialis annua L. | 0.00 | 0.00 | 0.00 | 0.90 | 3.39 | 0.00 | 0.00 | 0.54 |
| Buglossoides arvensis (L.) I. M. Johnst. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.02 | 0.00 | 0.54 |
| Crepis sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.69 | 0.54 |
| Fumaria capreolata L. | 2.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 |
| Lamium sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.69 | 0.54 |
| Tragopogon porrifolius L. | 0.62 | 0.00 | 1.91 | 0.00 | 0.56 | 1.01 | 0.00 | 0.54 |
| Veronica cymbalaria Bodard | 2.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.54 |
| Ajuga iva (L.) Schreb. | 0.00 | 0.00 | 0.00 | 1.36 | 0.00 | 2.01 | 0.00 | 0.48 |
| DDesmazeria rigida (L.) Tutin | 0.00 | 0.00 | 0.00 | 0.90 | 0.00 | 0.00 | 1.68 | 0.48 |
| Hyparrhenia hirta (L.) Stapf. | 0.00 | 0.00 | 2.55 | 1.36 | 0.00 | 0.00 | 0.00 | 0.48 |
| Vulpia ciliata Dumort. | 0.00 | 0.00 | 1.91 | 1.36 | 0.56 | 0.00 | 0.00 | 0.48 |
| Asplenium ceterach L. | 0.00 | 0.00 | 3.82 | 0.45 | 0.00 | 0.00 | 0.00 | 0.48 |
| Capsella bursa subsp. pastoris (L.) Medik. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.36 | 0.48 |
| Cyperus sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.36 | 0.48 |
| Medicago rugosa Desr. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.52 | 0.00 | 0.48 |
| Medicago truncatula Gaerth. | 2.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 |
| Bromus fasciculatus C. Presl | 0.00 | 0.00 | 0.00 | 1.36 | 0.00 | 0.00 | 1.01 | 0.41 |
| Medicago polymorpha L. | 0.00 | 0.00 | 1.27 | 0.90 | 0.00 | 0.00 | 0.00 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellou, E.; Papafotiou, M.; Saitanis, C.; Economou, G. Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece. Environments 2024, 11, 16. https://doi.org/10.3390/environments11010016
Kanellou E, Papafotiou M, Saitanis C, Economou G. Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece. Environments. 2024; 11(1):16. https://doi.org/10.3390/environments11010016
Chicago/Turabian StyleKanellou, Electra, Maria Papafotiou, Costas Saitanis, and Garifalia Economou. 2024. "Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece" Environments 11, no. 1: 16. https://doi.org/10.3390/environments11010016
APA StyleKanellou, E., Papafotiou, M., Saitanis, C., & Economou, G. (2024). Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece. Environments, 11(1), 16. https://doi.org/10.3390/environments11010016








