Impaired Soil Health in Agricultural Areas Close to Fe-Ni Mines on Euboea Island, Greece, Caused by Increased Concentrations of Potentially Toxic Elements, and the Associated Impacts on Human Health
Abstract
:1. Introduction
2. Materials and Methods
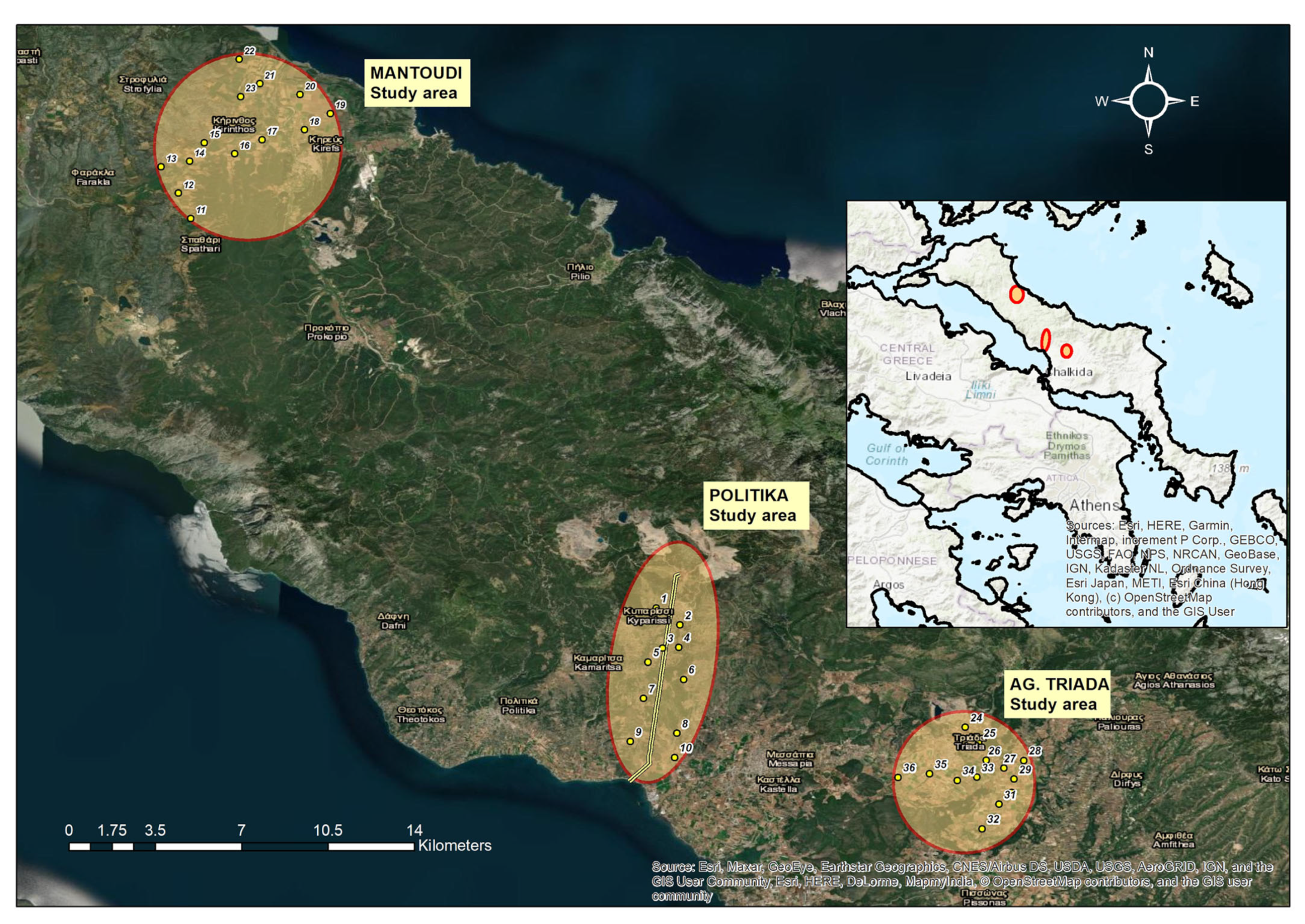
2.1. Description of Study Area
Soils
2.2. Sample Collection, Preparation, and Analysis
2.2.1. Sequential Extraction Procedure
2.2.2. Analytical Determinations
2.2.3. Statistics
2.2.4. GIS
2.3. Single Pollution Index (PI)
2.4. Potential Ecological Risk Index (RI)
| Risk Classification | RI | Risk Classification | ||
|---|---|---|---|---|
| < 40 | Low | RI < 50 | Low | Ni: 5 |
| 40 ≤ < 80 | Moderate | 50 ≤ RI < 200 | Moderate | Cr: 2 |
| 80 ≤ < 160 | Considerable | 200 ≤ RI < 300 | Considerable | Co: 5 |
| 160 ≤ < 320 | High | RI ≥ 300 | High | Zn: 1 |
| ≥ 320 | Very high | Cu: 5 | ||
| Pb: 5 | ||||
| Mn: 1 |
2.5. Human Health Assessment
| Parameter | Value |
|---|---|
| IngR | 100 mg day−1 (adult), 200 mg day −1 (child) |
| EF | 350 days year−1 |
| ED | 24 years (adult), 6 years (child) |
| BW | 70 kg (adult), 15 kg (child) |
| AT | 365 × ED adult/child (days) |
| CF | 1 × 10−6 kg mg−1 |
| InhR | 20 mg cm−2 |
| PEF | 1.36 × 109 m3 kg−1 |
| SA | 5800 cm2 event−1 (adult), 2100 cm2 event−1 (child) |
| AFsoil | 0.07 mg cm−2 |
| ABS | 0.001 (adult and child) |
| Element | RfDing | RfDinh | RfDderm | CSFing | CSFinh |
|---|---|---|---|---|---|
| Ni | 2 × 10−2 | 2.5 × 10−2 | 5.6 × 10−3 | 0.91 | 0.0421 |
| Cr | 1.5 | 3 × 10−5 | 3 × 10−3 | 47.6 | |
| Co | 2 × 10−2 | 5.76 × 10−6 | 5.76 × 10−6 | 9.8 | |
| Mn | 1.4 × 10−1 | 1.4 × 10−1 | 5.6 × 10−2 | ||
| Pb | 3.5 × 10−3 | 3.25 × 10−3 | 5.25 × 10−4 | 8.5 × 10−3 | 4.2 × 10−2 |
| Cu | 4 × 10−2 | 4.02 × 10−2 | 1.2 × 10−2 | ||
| Zn | 3 × 10−1 | 3.5 × 10−1 | 3 × 10−1 |
3. Results
3.1. Soil Properties
3.2. Aqua Regia
3.2.1. Manganese
3.2.2. Nickel
3.2.3. Chromium
3.2.4. Cobalt
3.2.5. Lead, Zinc, and Copper
3.3. Diethylenetriamine Pentaacetic Acid (DTPA)
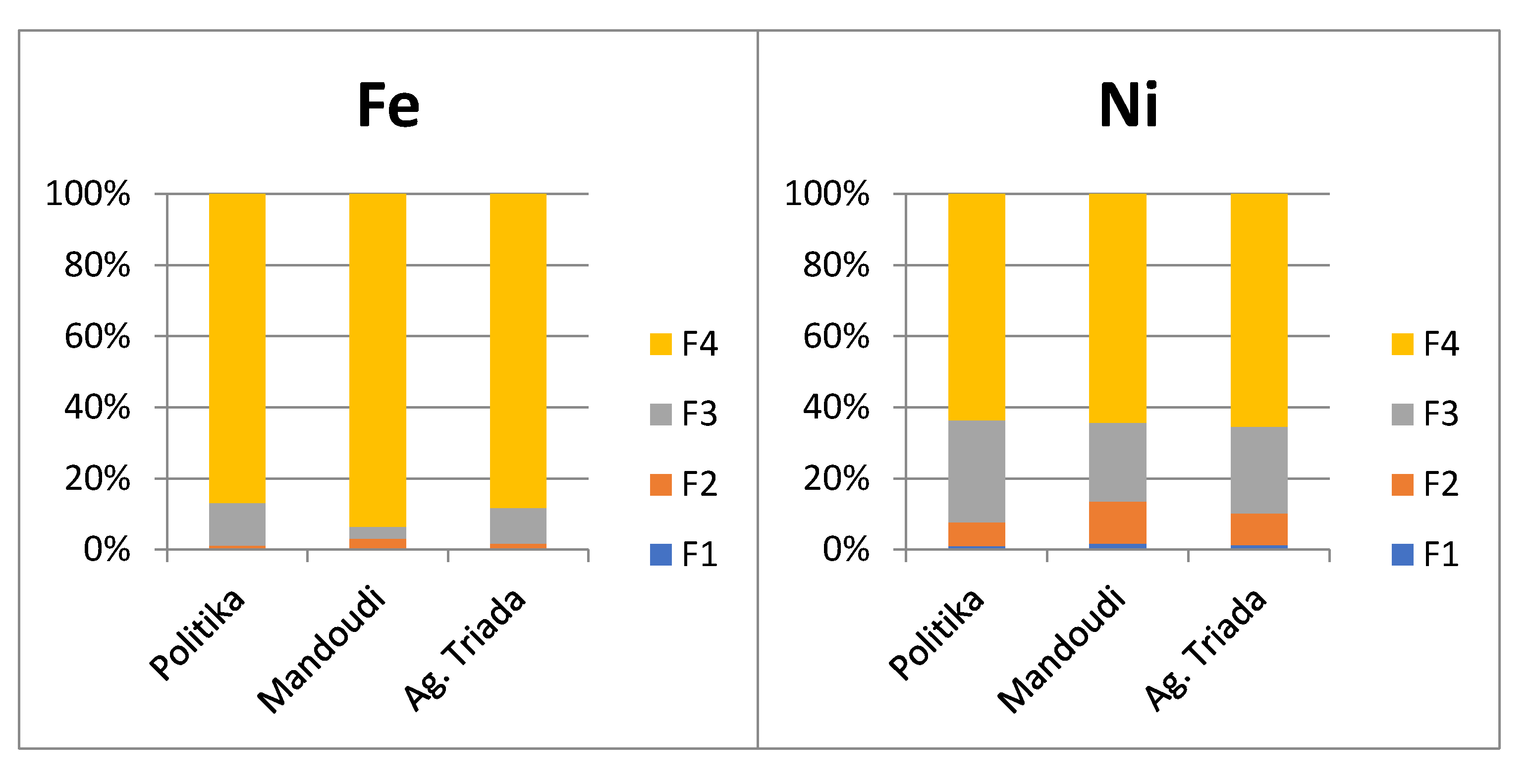
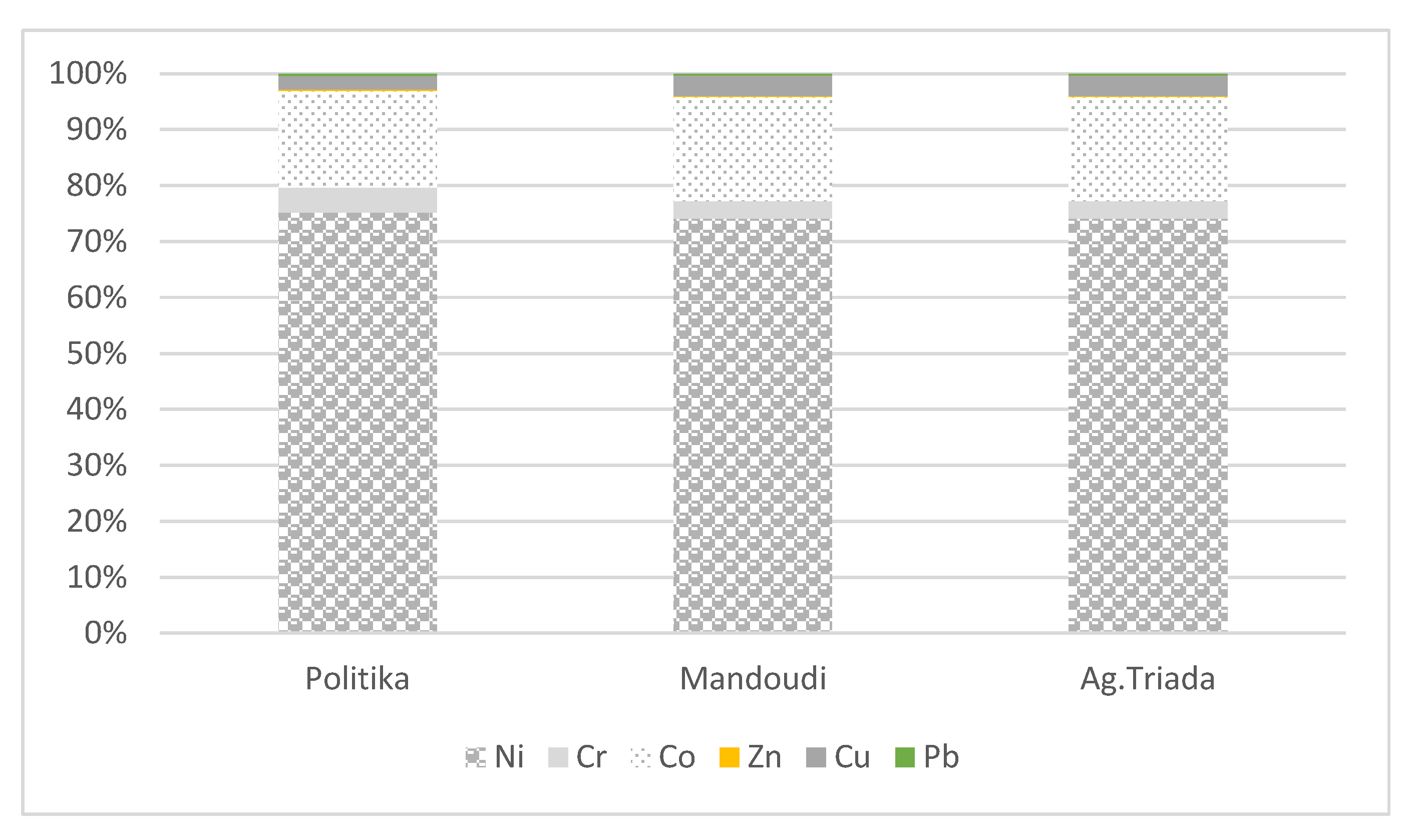
3.4. Chemical Partitioning of Fe, Mn, Ni, Cr, and Co
3.4.1. Iron
3.4.2. Manganese
3.4.3. Nickel
3.4.4. Chromium
3.4.5. Cobalt
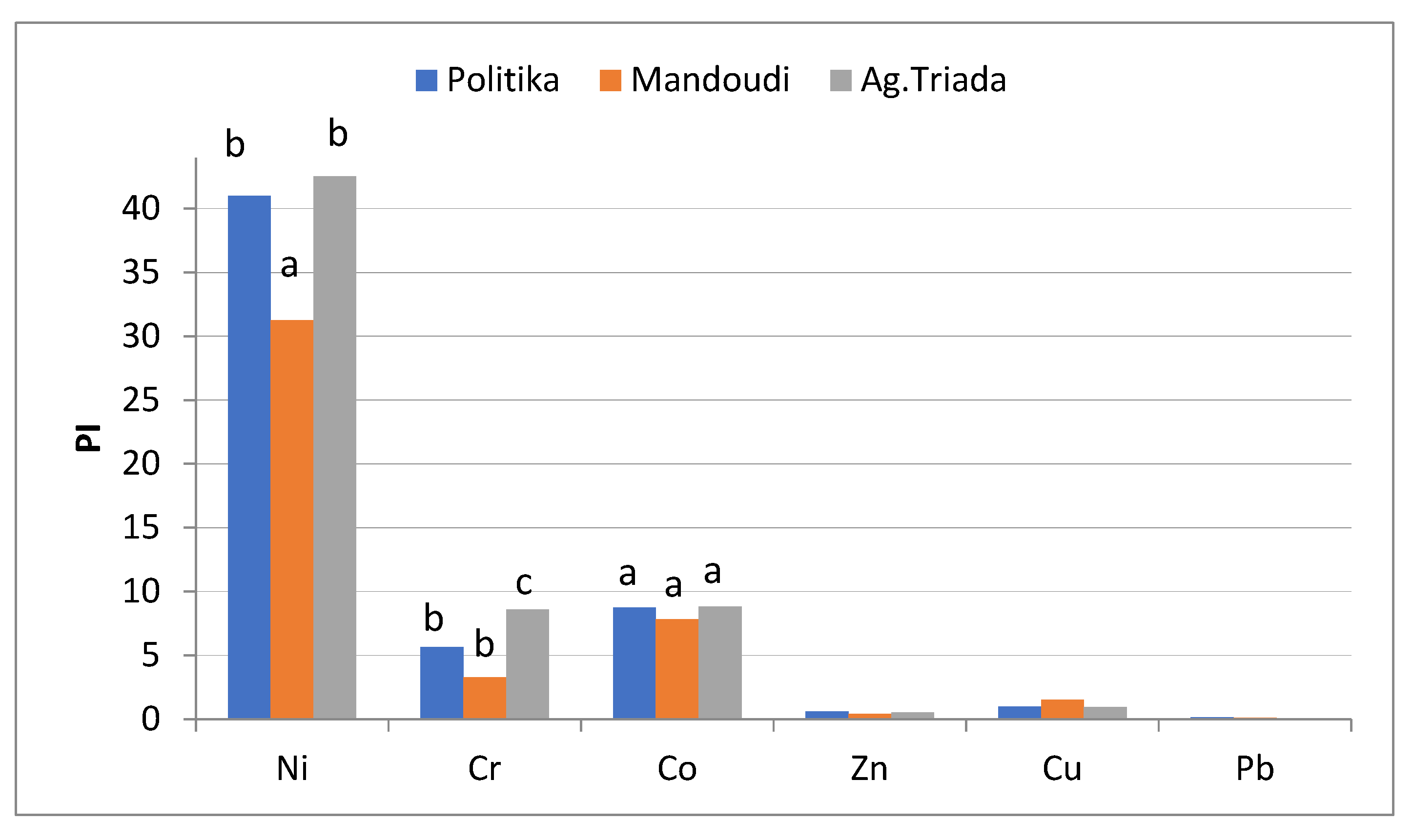
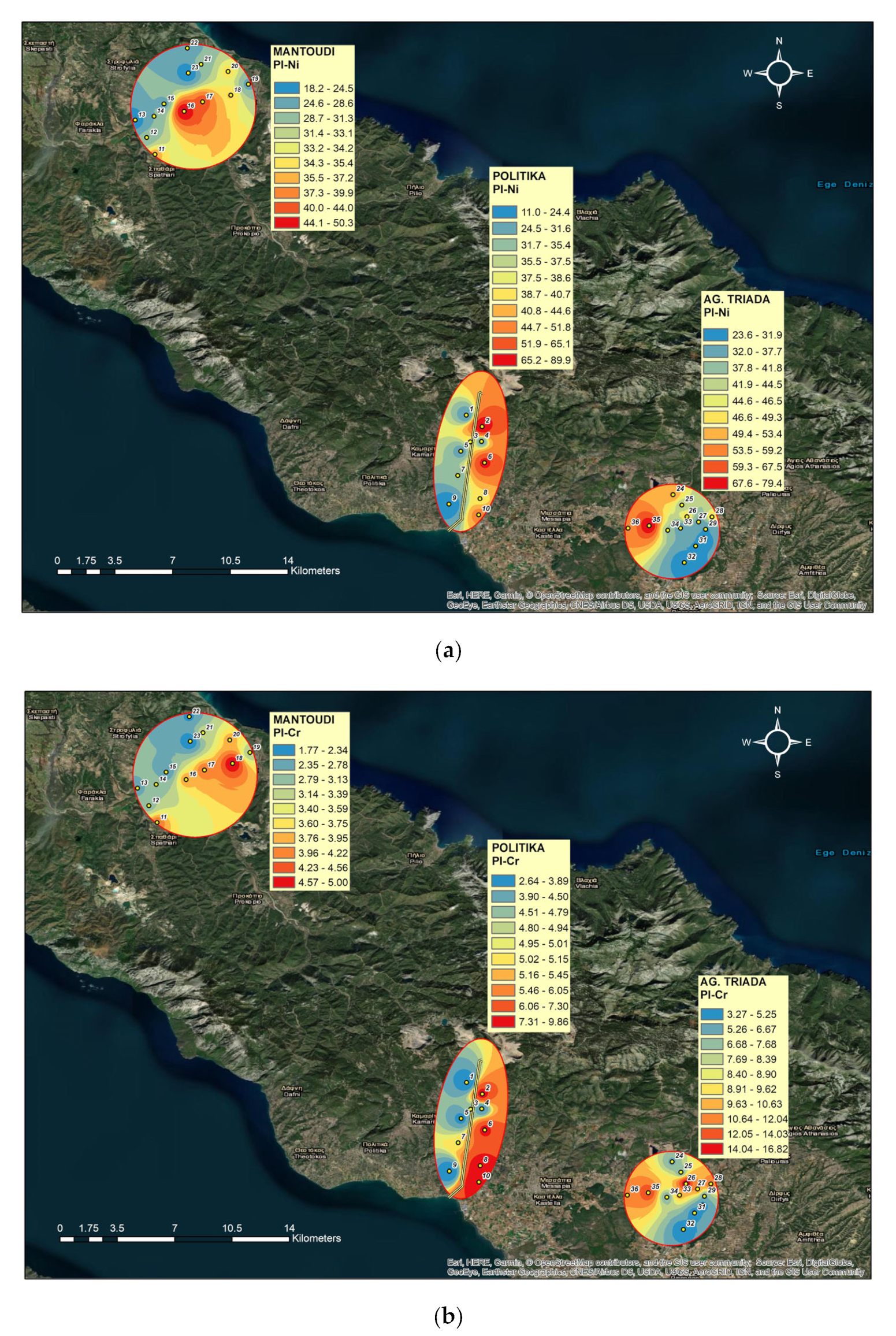
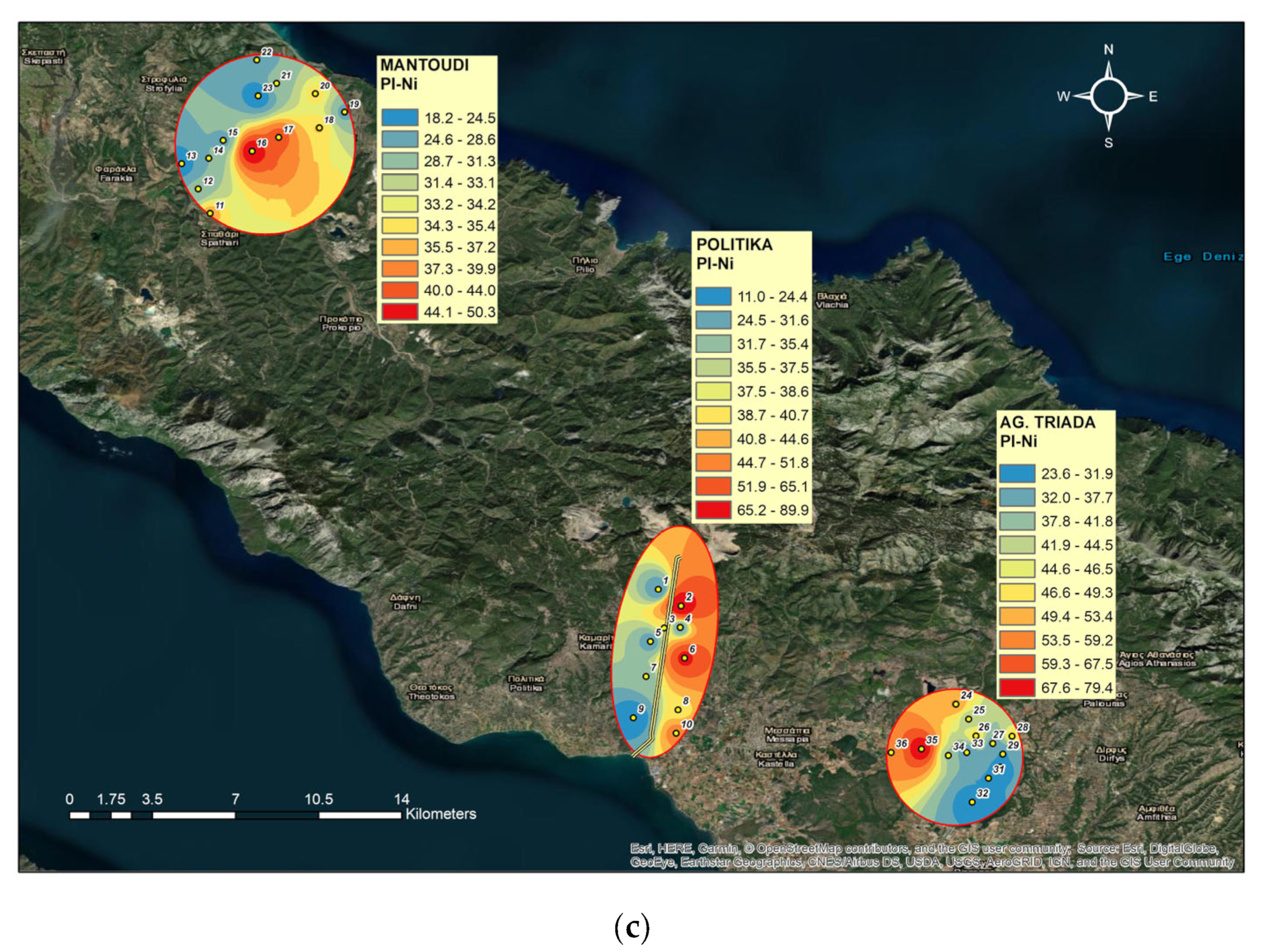
3.5. Single Pollution Index (PI)
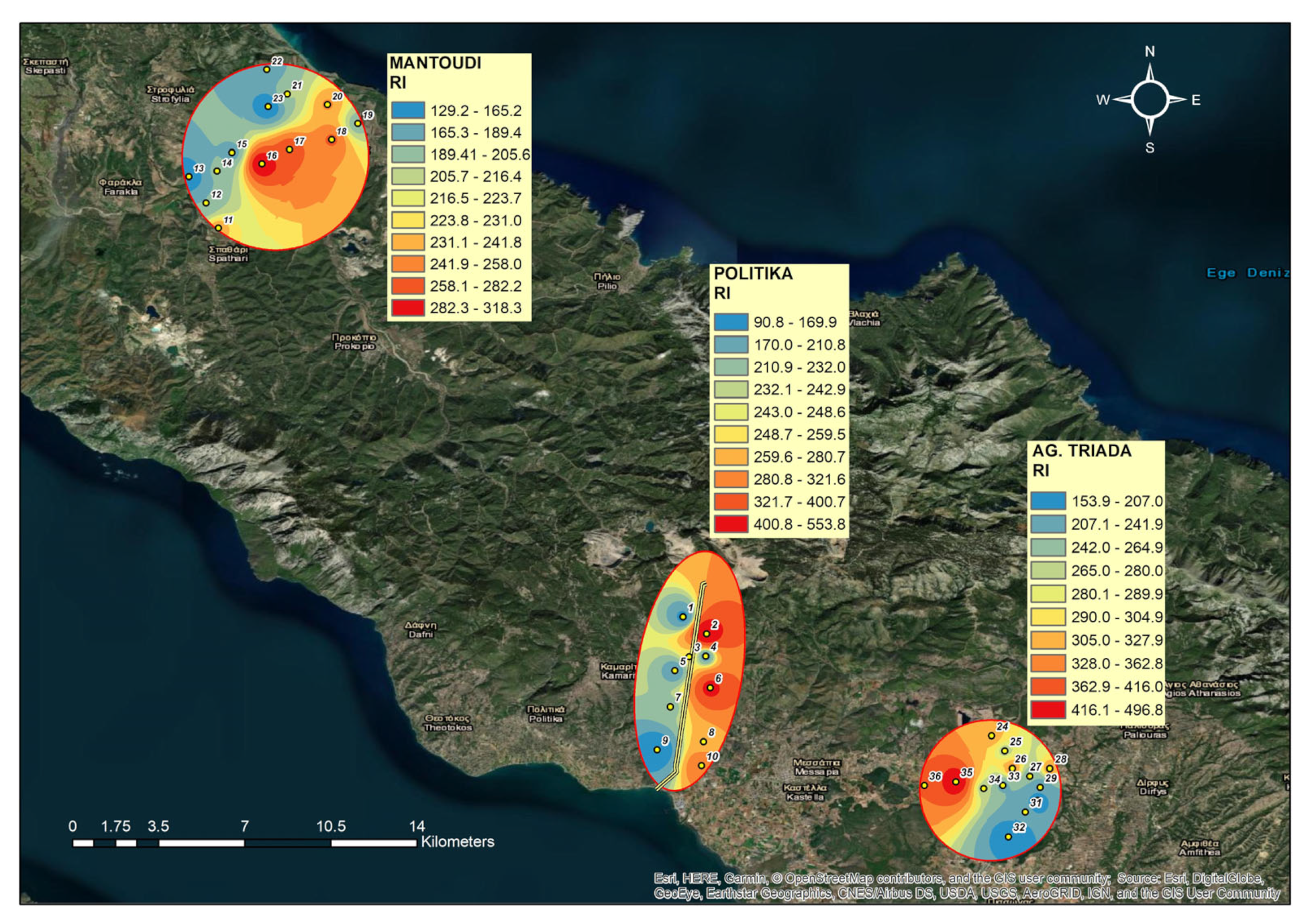
3.6. Potential Ecological Risk Index (RI)
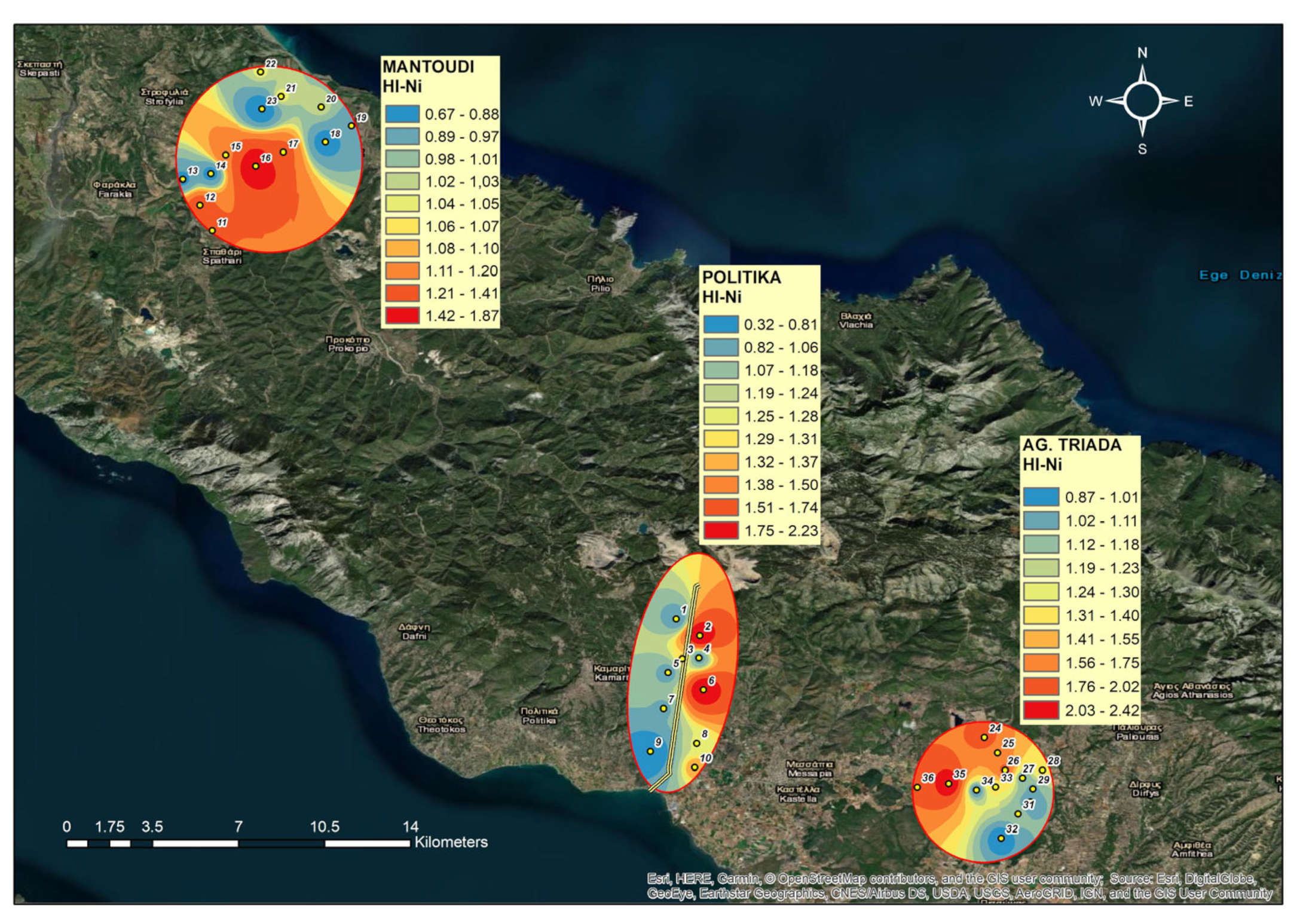
3.7. Hazard Index (HI)
3.8. Life Cancer Risk (LCR)
4. Discussion
4.1. Total and Bioavailable Metal Concentrations
4.2. Metal Fractionation in Soils
4.3. Single Pollution Indices for Ni, Cr, and Co
4.4. Potential Ecological Risk Index (RI)
4.5. Human Health Assessment
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Jayakumar, M.; Surendran, U.; Raja, P.; Kumar, A.; Senapathi, V. A Review of Heavy Metals Accumulation Pathways, Sources and Management in Soils. Arab. J. Geosci. 2021, 14, 2156. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Geng, R.; Ding, Z.; Luo, D.; Qiu, J.; Zheng, G.; Fan, Q. Exploring the Fate of Heavy Metals from Mining and Smelting Activities in Soil-Crop System in Baiyin, NW China. Ecotoxicol. Environ. Saf. 2021, 207, 111234. [Google Scholar] [CrossRef]
- Bilias, F.; Nikoli, T.; Kalderis, D.; Gasparatos, D. Towards a Soil Remediation Strategy Using Biochar: Effects on Soil Chemical Properties and Bioavailability of Potentially Toxic Elements. Toxics 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Asyakina, L.K.; Serazetdinova, Y.R.; Frolova, A.S.; Velichkovich, N.S.; Prosekov, A.Y. Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms 2023, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, Environmental Pollution; Alloway., B.J., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 22, pp. 11–50. [Google Scholar]
- Boskos, E.; Perraki, T.; Kolokotroni, K. The Ni and Cr distribution in the mineralogical phases of the nickeliferous laterites from Evia island. Min. Wealth 1996, 101, 9–26. [Google Scholar]
- Boskos, E.; Orphanoudaki, A.; Perraki, T.H. The Ni distribution in the mineral phases of Greek Fe–Ni laterite deposits. In Proceedings of the 3rd Congress of Mineral Wealth, Athens, Greece, 22–24 November 2000; Proceedings Vol. A; Technical Chamber of Greece: Athens, Greece, 2000; pp. 107–115. [Google Scholar]
- Eliopoulos, D.G.; Economou-Eliopoulos, M. Geochemical and Mineralogical Characteristics of Fe–Ni- and Bauxitic-Laterite Deposits of Greece. Ore Geol. Rev. 2000, 16, 41–58. [Google Scholar] [CrossRef]
- Montalván-Olivares, D.M.; Santana, C.S.; Velasco, F.G.; Luzardo, F.H.M.; Andrade, S.F.R.; Ticianelli, R.B.; Armelin, M.J.A.; Genezini, F.A. Multi-Element Contamination in Soils from Major Mining Areas in Northeastern of Brazil. Environ. Geochem. Health 2021, 43, 4553–4576. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Rinklebe, J.; Selim, H.M. (Eds.) Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Gasparatos, D.; Barbayiannis, N. The Origin of Nickel in Soils. In Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Alevizos, G. Mineralogy, Geochemistry and Origin of the Sedimentary Fe-Ni Ores of Lokris. Ph.D. Thesis, Technical University of Crete, Chania, Greece, 1997; p. 245. [Google Scholar]
- Economou-Eliopoulos, M. Apatite and Mn, Zn, Co-Enriched Chromite in Ni-Laterites of Northern Greece and Their Genetic Significance. J. Geochem. Explor. 2003, 80, 41–54. [Google Scholar] [CrossRef]
- Kumar, O.; Singh, S.K.; Singh, A.P.; Yadav, S.N.; Latare, A.M. Effect of Soil Application of Nickel on Growth, Micronutrient Concentration and Uptake in Barley (Hordeum vulgare L.) Grown in Inceptisols of Varanasi. J. Plant Nutr. 2017, 41, 50–66. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether Toxic or Essential for Plants and Environment—A Review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Lerdau, M. Mineral Nutrition of Plants: Principles and Perspectives. Second Edition. By Emanuel Epstein and, Arnold J Bloom. Sunderland (Massachusetts): Sinauer Associates. $79.95. Xiv + 400 p; Ill.; Author and Subject Indexes. ISBN: 0-87893-172-4. 2005. Q. Rev. Biol. 2005, 80, 359. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Economou-Eliopoulos, M.; Megremi, I.; Atsarou, C.; Theodoratou, C.; Vasilatos, C. Spatial Evolution of the Chromium Contamination in Soils from the Assopos to Thiva Basin and C. Evia (Greece) and Potential Source(s): Anthropogenic versus Natural Processes. Geosciences 2013, 3, 140–158. [Google Scholar] [CrossRef]
- OPEKEPE (in Greek). The Greek Payment Agency. Development of a Unified System for Geospatial Soil Data and the Delineation of the Rural Areas of the Country. Ministry of Rural Development and Food. 2014. Available online: https://iris.gov.gr/SoilServices (accessed on 10 February 2022).
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 25 January 2023).
- Bouyoucos, G.J. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils 1. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part. 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA-SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Roussos, P.A.; Gasparatos, D.; Kyriakou, C.; Tsichli, K.; Tsantili, E.; Haidouti, C. Growth, nutrient status, and biochemical changes of sour orange plants subjected to sodium chloride stress. Commun. Soil Sci. Plant Anal. 2013, 44, 805–816. [Google Scholar] [CrossRef]
- Mehra, O.P. Iron Oxide Removal from Soils and Clays by a Dithionite-Citrate System Buffered with Sodium Bicarbonate. Clays Clay Miner. 1958, 7, 317–327. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.M. Iron oxides. In Minerals in Soil Environments, 2nd ed.; Soil Science Society of America: Wisconsin, MA, USA, 1989; pp. 379–438. [Google Scholar]
- Gasparatos, D.; Haidouti, A. comparison of wet oxidation methods for determination of total phosphorus in soils. J. Plant Nutr. Soil Sci. 2001, 164, 435–439. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papaioannou, A.; Gasparatos, D.; Molassiotis, A. From Which Soil Metal Fractions Fe, Mn, Zn and Cu Are Taken up by Olive Trees (Olea Europaea L., Cv. ‘Chondrolia Chalkidikis’) in Organic Groves? J. Environ. Manag. 2017, 203, 489–499. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR Three Step Sequential Extraction Procedure Prior to the Certification of New Sediment and Soil Reference Materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Kalyvas, G.; Gasparatos, D.; Papassiopi, N.; Massas, I. Topsoil Pollution as Ecological Footprint of Historical Mining Activities in Greece. Land Degrad. Dev. 2018, 29, 2025–2035. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Megremi, I.; Ioannou, D.; Massas, I.; Economou-Eliopoulos, M. Assessment of Potentially Toxic Element Contamination in the Philippi Peatland, Eastern Macedonia, Greece. Minerals 2022, 12, 1475. [Google Scholar] [CrossRef]
- Webster, R.; Oliver, M.A. Geostatistics for Environmental Scientists; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Tobler, W.R. A Computer Movie Simulating Urban Growth in the Detroit Region. Econ. Geogr. 1970, 46, 234. [Google Scholar] [CrossRef]
- Netherlands Ministry of Housing, Physical Planning and Environment (Netherlands MHPPE). Annexes Circular on Target Values for Soil Remediation; MHPPE: The Hague, The Netherlands, 2000. [Google Scholar]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control. A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Aendo, P.; Netvichian, R.; Thiendedsakul, P.; Khaodhiar, S.; Tulayakul, P. Carcinogenic Risk of Pb, Cd, Ni, and Cr and Critical Ecological Risk of Cd and Cu in Soil and Groundwater around the Municipal Solid Waste Open Dump in Central Thailand. J. Environ. Public Health 2022, 2022, 3062215. [Google Scholar] [CrossRef] [PubMed]
- Kusin, F.M.; Azani, N.N.M.; Hasan, S.N.M.S.; Sulong, N.A. Distribution of Heavy Metals and Metalloid in Surface Sediments of Heavily-Mined Area for Bauxite Ore in Pengerang, Malaysia and Associated Risk Assessment. Catena 2018, 165, 454–464. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Okonkwo, S.I.; Idakwo, S.O.; Ameh, E.G. Heavy Metal Contamination and Ecological Risk Assessment of Soils around the Pegmatite Mining Sites at Olode Area, Ibadan Southwestern Nigeria. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100424. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites, OSWER 9355; Office of Emergency and Remedial Response: Washington, DC, USA, 2002. [Google Scholar]
- US Environmental Protection Agency (USEPA). Integrated Risk Information System of the US Environmental Protection Agency; Office of Emergency and Remedial Response: Washington, DC, USA, 2012. [Google Scholar]
- Johnson-Beebout, S.E.; Lauren, J.G.; Duxbury, J.M. Immobilization of Zinc Fertilizer in Flooded Soils Monitored by Adapted DTPA Soil Test. Commun. Soil Sci. Plant Anal. 2009, 40, 1842–1861. [Google Scholar] [CrossRef]
- Bhatt, M.K.; Raverkar, K.P.; Chandra, R.; Pareek, N.; Labanya, R.; Kumar, V.; Kaushik, S.; Singh, D.K. Effect of Long-term Balanced and Imbalanced Inorganic Fertilizer and FYM Application on Chemical Fraction of DTPA-extractable Micronutrients and Yields under Rice–Wheat Cropping System in Mollisols. Soil Use Manag. 2020, 36, 261–273. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Kaur, J.; Shukla, A.K.; Hossain, A.; Abdel-Hafez, S.H.; Gaber, A.; Sayed, S.; Singh, V.K. The Pedospheric Variation of DTPA-Extractable Zn, Fe, Mn, Cu and Other Physicochemical Characteristics in Major Soil Orders in Existing Land Use Systems of Punjab, India. Sustainability 2021, 14, 29. [Google Scholar] [CrossRef]
- Barman, M.; Datta, S.P.; Rattan, R.K.; Meena, M.C. Critical Limits of Deficiency of Nickel in Intensively Cultivated Alluvial Soils. J. Soil Sci. Plant Nutr. 2019, 20, 284–292. [Google Scholar] [CrossRef]
- Contin, M.; Miho, L.; Pellegrini, E.; Gjoka, F.; Shkurta, E. Effects of Natural Zeolites on Ryegrass Growth and Bioavailability of Cd, Ni, Pb, and Zn in an Albanian Contaminated Soil. J. Soils Sediments 2019, 19, 4052–4062. [Google Scholar] [CrossRef]
- Megremi, I. Distribution and bioavailability of Cr in central Euboea, Greece. Open Geosci. 2010, 2, 103–123. [Google Scholar] [CrossRef]
- Kumar, D.; Ramani, V.P.; Patel, K.C.; Shukla, A.K. Establishing Critical Limits for Nickel in Soil and Plant for Predicting the Response of Spinach (Spinacia Oleracea). J. Indian Soc. Soil Sci. 2021, 69, 105–110. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, R. Spatial Characterization and Prioritization of Heavy Metal Contaminated Soil-Water Resources in Peri-Urban Areas of National Capital Territory (NCT), Delhi. Environ. Monit. Assess. 2006, 123, 233–247. [Google Scholar] [CrossRef]
- Parvin, A.; Moniruzzaman, M.; Hossain, M.K.; Saha, B.; Parvin, A.; Suchi, P.D.; Hoque, S. Chemical Speciation and Potential Mobility of Heavy Metals in Organic Matter Amended Soil. Appl. Environ. Soil Sci. 2022, 2022, 2028860. [Google Scholar] [CrossRef]
- Mitrović, M.; Blanusa, T.; Pavlović, M.; Pavlović, D.; Kostić, O.; Perović, V.; Jarić, S.; Pavlović, P. Using Fractionation Profile of Potentially Toxic Elements in Soils to Investigate Their Accumulation in Tilia sp. Leaves Urban Areas Differ. Pollut. Levels. Sustain. 2021, 13, 9784. [Google Scholar] [CrossRef]
- Davidson, C.M.; Urquhart, G.J.; Ajmone-Marsan, F.; Biasioli, M.; da Costa Duarte, A.; Díaz-Barrientos, E.; Grčman, H.; Hossack, I.; Hursthouse, A.S.; Madrid, L.; et al. Fractionation of Potentially Toxic Elements in Urban Soils from Five European Cities by Means of a Harmonised Sequential Extraction Procedure. Anal. Chim. Acta 2006, 565, 63–72. [Google Scholar] [CrossRef]
- Lafuente, A.L.; González, C.; Quintana, J.R.; Vázquez, A.; Romero, A. Mobility of Heavy Metals in Poorly Developed Carbonate Soils in the Mediterranean Region. Geoderma 2008, 145, 238–244. [Google Scholar] [CrossRef]
- Ospina-Alvarez, N.; Głaz, Ł.; Dmowski, K.; Krasnodębska-Ostręga, B. Mobility of Toxic Elements in Carbonate Sediments from a Mining Area in Poland. Environ. Chem. Lett. 2014, 12, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Diami, S.M.; Kusin, F.M.; Madzin, Z. Potential Ecological and Human Health Risks of Heavy Metals in Surface Soils Associated with Iron Ore Mining in Pahang, Malaysia. Environ. Sci. Pollut. Res. 2016, 23, 21086–21097. [Google Scholar] [CrossRef]
- Chonokhuu, S.; Batbold, C.; Chuluunpurev, B.; Battsengel, E.; Dorjsuren, B.; Byambaa, B. Contamination and Health Risk Assessment of Heavy Metals in the Soil of Major Cities in Mongolia. Int. J. Environ. Res. Public Health 2019, 16, 2552. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and Heavy Metals Contamination Assessment in Soil and Water to Evaluate Human Health Risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef] [PubMed]
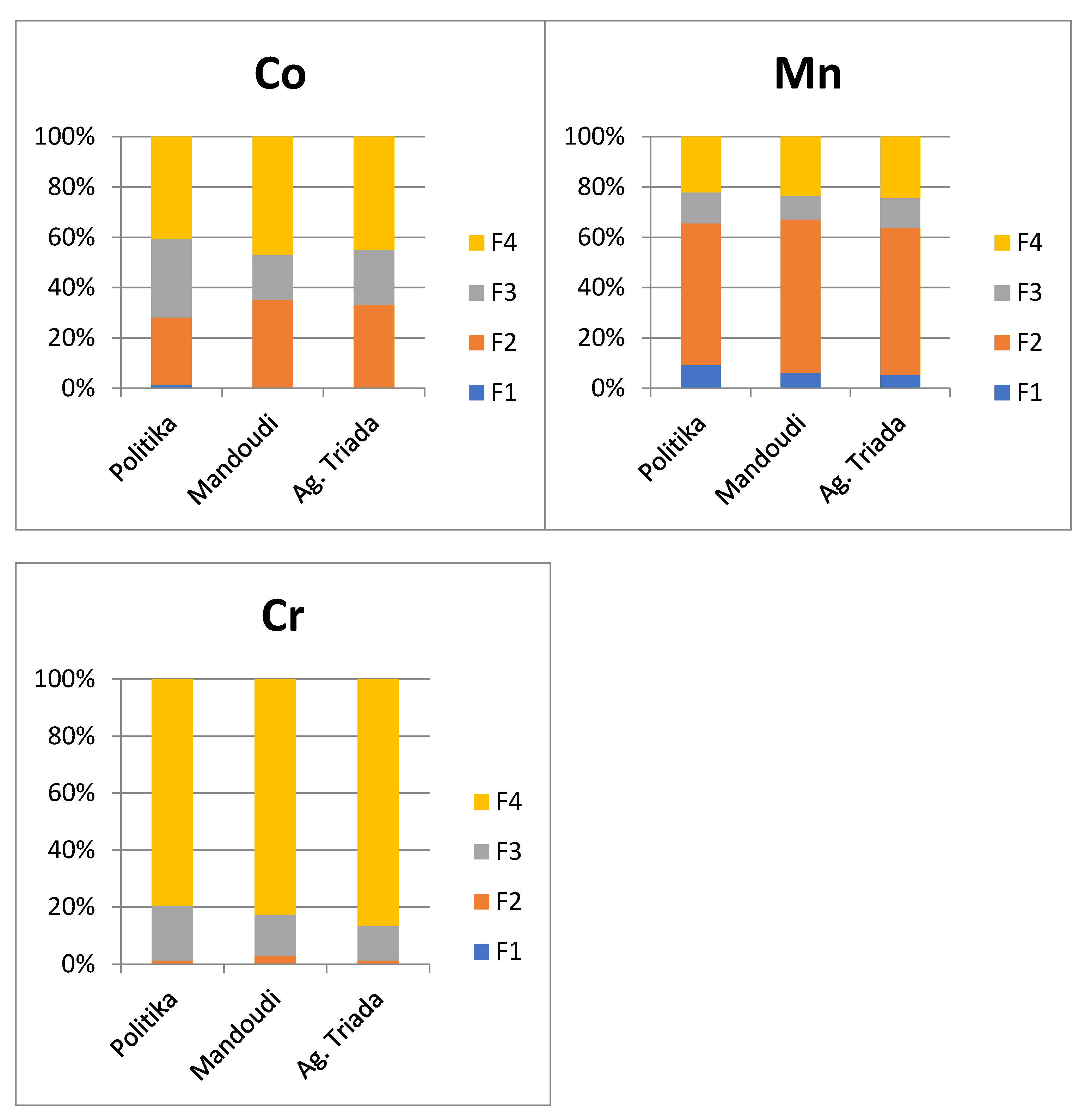
| Metal/Value | Ni | Cr | Co | Zn | Cu | Pb |
|---|---|---|---|---|---|---|
| a | 10 | 50 | 2 | 50 | 15 | 50 |
| b | 1 | 2 | 0.28 | 3 | 0.6 | 1 |
| c | 0 | 0 | 0 | 1.5 | 0.6 | 1 |
| Area | Sand (g kg−1) | Silt (g kg−1) | Clay (g kg−1) | pH (1:1) | E.C. (μS cm−1) | O.C. (g kg−1) | CaCO3eq (g kg−1) | Feo (mg kg−1) | Fed (mg kg−1) | Mno (mg kg−1) | Mnd (mg kg−1) | Alo (mg kg−1) | Ald (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 322 b | 286 b | 392 a | 7.24 a | 1002 ab | 26.77 b | 154.6 b | 1121.7 a | 16,864.50 a | 613.3 a | 851.75 a | 912.7 a | 640.15 a | |
| Politika (n = 10) | Median | 308 | 300 | 392 | 7.22 | 732 | 21.23 | 165.3 | 1071.0 | 15,420.00 | 629.1 | 793.75 | 841.5 | 489.25 |
| St.dev | 63 | 42 | 52 | 0.17 | 595 | 16.87 | 92.2 | 441.5 | 7274.14 | 138.5 | 217.66 | 374.4 | 342.40 | |
| Mean | 254 a | 297 b | 449 a | 7.34 a | 692 a | 10.95 a | 67.6 a | 1624.2 b | 12,465.77 a | 865.2 b | 877.50 a | 905.4 a | 538.73 a | |
| Mantoudi (n = 13) | Median | 247 | 308 | 435 | 7.35 | 671 | 9.75 | 69.7 | 1564.2 | 12.085 | 883.8 | 857.50 | 946.8 | 565.00 |
| St.dev | 84 | 75 | 107 | 0.19 | 211 | 5.51 | 54.5 | 531.1 | 2635.58 | 196.9 | 193.71 | 190.4 | 146.13 | |
| Mean | 334 b | 234 a | 432 a | 7.42 a | 1004 b | 12.67 a | 141.3 b | 1379.5 ab | 15,291.14 a | 707.2 ab | 743.46 a | 943.7 a | 643.54 a | |
| Ag. Triada (n = 13) | Median | 344 | 228 | 448 | 7.41 | 784 | 10.50 | 118.9 | 1123.2 | 12,690.00 | 606.6 | 727.50 | 910.8 | 702.00 |
| St.dev | 69 | 43 | 80 | 0.22 | 487 | 6.83 | 107.3 | 505.5 | 6571.93 | 385.9 | 248.88 | 315.5 | 249.25 |
| Fe (mg kg−1) | Mn (mg kg−1) | Ni (mg kg−1) | Cr (mg kg−1) | Cu (mg kg−1) | Zn (mg kg−1) | Pb (mg kg−1) | Co (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|
| Politika | ||||||||
| Mean | 51,800.00 a | 1360.38 a | 1946.88 ab | 711.43 a | 41.09 a | 51.68 a | 15.39 b | 122.02 a |
| Median | 48,390.00 | 1427.50 | 1774.06 | 636.07 | 39.66 | 38.18 | 13.50 | 124.37 |
| Std | 13,109.52 | 216.17 | 928.11 | 312.09 | 9.80 | 34.49 | 7.90 | 39.82 |
| Min | 37,890.00 | 971.88 | 510.00 | 327.86 | 30.56 | 31.29 | 5.00 | 57.42 |
| Max | 82,210.00 | 1627.50 | 3490.00 | 1173.57 | 62.25 | 145.25 | 32.25 | 182.25 |
| 25th | 45,630.00 | 1196.25 | 1449.38 | 472.14 | 32.63 | 33.83 | 10.63 | 98.50 |
| 75th | 53,980.00 | 1536.88 | 2132.50 | 1000.71 | 42.94 | 49.51 | 19.63 | 145.17 |
| Mantoudi | ||||||||
| Mean | 54,533.08 a | 1675.43 b | 1682.31 a | 445.88 b | 62.05 b | 38.52 a | 9.99 b | 121.76 a |
| Median | 56,880.00 | 1684.38 | 1630.63 | 460.00 | 59.94 | 39.03 | 8.88 | 123.75 |
| Std | 8167.26 | 279.48 | 485.71 | 90.50 | 18.24 | 4.82 | 6.84 | 23.95 |
| Min | 38,400.00 | 1242.50 | 1050.00 | 257.14 | 41.25 | 30.73 | 3.63 | 85.83 |
| Max | 68,730.00 | 2254.38 | 2926.25 | 580.71 | 108.19 | 45.63 | 31.75 | 181.75 |
| 25th | 54,530.00 | 1497.50 | 1316.88 | 390.00 | 49.44 | 33.10 | 5.83 | 108.00 |
| 75th | 57,900.00 | 1853.13 | 1907.50 | 505.71 | 68.69 | 44.59 | 10.98 | 131.42 |
| Ag. Triada | ||||||||
| Mean | 51,363.08 a | 1322.16 a | 2220.67 b | 1156.37 c | 40.60 a | 49.89 a | 3.63 a | 136.21 a |
| Median | 49,310.00 | 1256.88 | 2086.88 | 998.57 | 39.69 | 43.93 | 3.50 | 126.75 |
| Std | 12,548.97 | 471.38 | 731.40 | 569.97 | 9.89 | 19.76 | 2.56 | 34.23 |
| Min | 33,080.00 | 726.25 | 1358.13 | 475.00 | 22.06 | 27.73 | 0.25 | 90.33 |
| Max | 72,860.00 | 2202.13 | 3780.63 | 2444.29 | 59.94 | 92.53 | 8.75 | 184.58 |
| 25th | 41,080.00 | 1091.88 | 1667.50 | 675.71 | 34.81 | 32.86 | 1.30 | 114.75 |
| 75th | 60,630.00 | 1533.75 | 2736.87 | 1403.57 | 47.19 | 81.13 | 7.53 | 172.42 |
| Fe (mg kg−1) | Mn (mg kg−1) | Ni (mg kg−1) | Cr (mg kg−1) | Cu (mg kg−1) | Zn (mg kg−1) | Co (mg kg−1) | |
|---|---|---|---|---|---|---|---|
| Politika | |||||||
| Mean | 16.95 b | 34.79 b | 16.87 b | 0.08 a | 1.53 a | 4.45 b | 0.83 c |
| Median | 14.03 | 27.57 | 18.15 | 0.08 | 1.38 | 1.24 | 0.90 |
| Std | 8.25 | 22.27 | 4.51 | 0.02 | 0.54 | 9.48 | 0.28 |
| Min | 7.77 | 17.88 | 9.10 | 0.05 | 0.90 | 0.25 | 0.37 |
| Max | 36.50 | 93.22 | 22.98 | 0.10 | 2.37 | 31.25 | 1.18 |
| 25th | 13.34 | 20.92 | 14.96 | 0.06 | 1.13 | 0.67 | 0.58 |
| 75th | 20.57 | 38.14 | 19.81 | 0.09 | 2.01 | 3.06 | 0.95 |
| Mantoudi | |||||||
| Mean | 11.38 a | 19.08 a | 10.46 a | 0.12 a | 2.62 b | 0.95 a | 0.26 a |
| Median | 11.29 | 14.22 | 8.54 | 0.11 | 2.32 | 0.71 | 0.20 |
| Std | 3.52 | 12.06 | 8.40 | 0.02 | 1.70 | 0.87 | 0.16 |
| Min | 6.18 | 7.24 | 4.25 | 0.09 | 1.17 | 0.33 | 0.09 |
| Max | 17.23 | 43.52 | 37.48 | 0.15 | 7.68 | 3.44 | 0.62 |
| 25th | 8.34 | 10.84 | 7.70 | 0.10 | 1.51 | 0.43 | 0.16 |
| 75th | 12.94 | 24.32 | 10.26 | 0.14 | 2.91 | 1.00 | 0.31 |
| Ag. Triada | |||||||
| Mean | 11.50 a | 22.43 ab | 17.23 b | 0.12 a | 2.76 b | 2.88 b | 0.55 b |
| Median | 11.54 | 18.80 | 17.98 | 0.12 | 2.39 | 2.26 | 0.55 |
| Std | 3.03 | 7.97 | 6.66 | 0.02 | 1.21 | 1.64 | 0.20 |
| Min | 7.20 | 9.90 | 6.23 | 0.08 | 0.99 | 0.87 | 0.24 |
| Max | 16.89 | 36.04 | 27.62 | 0.15 | 5.12 | 5.56 | 0.90 |
| 25th | 9.56 | 17.86 | 14.46 | 0.11 | 2.08 | 1.66 | 0.42 |
| 75th | 12.98 | 28.44 | 19.32 | 0.13 | 3.08 | 4.12 | 0.69 |
| Politika (N = 10) | Mantoudi (N = 13) | Ag. Triada (N = 13) | |
|---|---|---|---|
| Mean | 266.5 ab | 210.2 b | 279.4 a |
| Median | 237.2 | 194.5 | 269.4 |
| Std | 140.8 | 55.4 | 92.4 |
| Min | 90.8 | 129.2 | 153.9 |
| Max | 553.8 | 318.3 | 497.0 |
| 25th | 173.2 | 180.0 | 210.9 |
| 75th | 322.5 | 244.0 | 329.6 |
| HI Index | LCR Index | |||
|---|---|---|---|---|
| Ni | Ni | Ni | Ni | |
| Child | Adult | Child | Adult | |
| Politika | ||||
| Mean | 1.248 ab | 0.135 ab | 0.0227 ab | 0.0024 ab |
| Median | 1.137 | 0.123 | 0.0206 | 0.0022 |
| Std | 0.595 | 0.064 | 0.0108 | 0.0012 |
| Min | 0.327 | 0.035 | 0.0059 | 0.0006 |
| Max | 2.237 | 0.242 | 0.0406 | 0.0044 |
| 25th | 0.929 | 0.104 | 0.0169 | 0.0018 |
| 75th | 1.367 | 0.148 | 0.0248 | 0.0027 |
| Mantoudi | ||||
| Mean | 1.078 a | 0.117 a | 0.0196 a | 0.0021 a |
| Median | 1.045 | 0.113 | 0.0190 | 0.0020 |
| Std | 0.311 | 0.033 | 0.0057 | 0.0006 |
| Min | 0.673 | 0.073 | 0.0122 | 0.0013 |
| Max | 1.876 | 0.203 | 0.0340 | 0.0036 |
| 25th | 0.844 | 0.092 | 0.0153 | 0.0016 |
| 75th | 1.223 | 0.133 | 0.0222 | 0.0024 |
| Ag. Triada | ||||
| Mean | 1.423 b | 0.154 b | 0.0258 b | 0.0028 b |
| Median | 1.338 | 0.145 | 0.0243 | 0.0026 |
| Std | 0.469 | 0.051 | 0.0085 | 0.0009 |
| Min | 0.871 | 0.094 | 0.0158 | 0.0017 |
| Max | 2.423 | 0.263 | 0.0430 | 0.0047 |
| 25th | 1.069 | 0.116 | 0.0194 | 0.0021 |
| 75th | 1.754 | 0.190 | 0.0318 | 0.0034 |
| Clay | O.C. | CaCO3 | Fet1 | Mnt | Nit | Crt | Cut | Znt | Pbt | Cot | Feav2 | Mnav | Niav | Crav | Znav | Fed3 | Feo4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay | ||||||||||||||||||
| O.C. | ||||||||||||||||||
| CaCO3 | ||||||||||||||||||
| Fet | **/− | |||||||||||||||||
| Mnt | ***/− | ***/+ | ||||||||||||||||
| Nit | ***/+ | |||||||||||||||||
| Crt | ***/+ | |||||||||||||||||
| Cut | ***/- | */+ | ***/+ | |||||||||||||||
| Znt | ***/+ | */+ | ||||||||||||||||
| Pbt | ***/+ | **/+ | ||||||||||||||||
| Cot | ***/+ | **/+ | ***/+ | ***/+ | ||||||||||||||
| Feav | ***/+ | ***/+ | ***/+ | |||||||||||||||
| Mnav | ***/+ | ***/+ | ***/+ | ***/+ | ||||||||||||||
| Niav | */+ | ***/+ | ***/+ | ***/+ | ||||||||||||||
| Crav | ||||||||||||||||||
| Cuav | ***/+ | |||||||||||||||||
| Znav | ***/+ | ***/+ | ***/+ | ***/+ | ***/+ | |||||||||||||
| Pbav | ||||||||||||||||||
| Coav | */- | **/+ | */+ | ***/+ | **/+ | */- | ||||||||||||
| Fed | */+ | */+ | */+ | |||||||||||||||
| Feo | ***/− | ***/+ | ***/+ | */+ | ***/+ | **/+ | ||||||||||||
| Mnd | */− | */+ | ***/+ | */+ | */+ | */+ | */+ | |||||||||||
| Mno | */+ | ***/− | ***/+ | ***/+ | ***/+ | **/+ | ***/+ | |||||||||||
| Ald | **/+ | */+ | **/+ | **/+ | ***/+ | ***/+ | **/+ | |||||||||||
| Alo | */+ | */− | ***/+ | */+ | **/+ | **/+ | ***/+ | */+ | **/+ |
| Mn | Ni | Cr | Co | |
|---|---|---|---|---|
| Politika (N = 10) | ||||
| Mean | 877.83 a | 150.84 a | 7.47 b | 32.27 a |
| Median | 881.74 | 107.34 | 6.40 | 26.46 |
| Std | 165.46 | 113.28 | 5.42 | 23.07 |
| Mantoudi (N = 13) | ||||
| Mean | 1081.06 b | 205.38 a | 11.71 a | 39.05 a |
| Median | 1048.5 | 218.92 | 11.84 | 40.16 |
| Std | 244.9 | 69.50 | 3.19 | 10.90 |
| Ag. Triada (N = 13) | ||||
| Mean | 823.13 a | 220.41 a | 13.27 a | 43.01 a |
| Median | 818.56 | 221.52 | 13.28 | 40.20 |
| Std | 347.18 | 118.76 | 8.33 | 20.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massas, I.; Kairis, O.; Gasparatos, D.; Ioannou, D.; Vatougios, D.; Zafeiriou, I. Impaired Soil Health in Agricultural Areas Close to Fe-Ni Mines on Euboea Island, Greece, Caused by Increased Concentrations of Potentially Toxic Elements, and the Associated Impacts on Human Health. Environments 2023, 10, 150. https://doi.org/10.3390/environments10090150
Massas I, Kairis O, Gasparatos D, Ioannou D, Vatougios D, Zafeiriou I. Impaired Soil Health in Agricultural Areas Close to Fe-Ni Mines on Euboea Island, Greece, Caused by Increased Concentrations of Potentially Toxic Elements, and the Associated Impacts on Human Health. Environments. 2023; 10(9):150. https://doi.org/10.3390/environments10090150
Chicago/Turabian StyleMassas, Ioannis, Orestis Kairis, Dionisios Gasparatos, Dafni Ioannou, Dimitrios Vatougios, and Ioannis Zafeiriou. 2023. "Impaired Soil Health in Agricultural Areas Close to Fe-Ni Mines on Euboea Island, Greece, Caused by Increased Concentrations of Potentially Toxic Elements, and the Associated Impacts on Human Health" Environments 10, no. 9: 150. https://doi.org/10.3390/environments10090150
APA StyleMassas, I., Kairis, O., Gasparatos, D., Ioannou, D., Vatougios, D., & Zafeiriou, I. (2023). Impaired Soil Health in Agricultural Areas Close to Fe-Ni Mines on Euboea Island, Greece, Caused by Increased Concentrations of Potentially Toxic Elements, and the Associated Impacts on Human Health. Environments, 10(9), 150. https://doi.org/10.3390/environments10090150






