Contaminant Risk and Social Vulnerability Associated with Crustacean Shellfish Harvest in the Highly Urbanized San Diego Bay, USA
Abstract
1. Introduction
1.1. The Risks
1.2. Those Who Harvest Shellfish
2. Materials and Methods
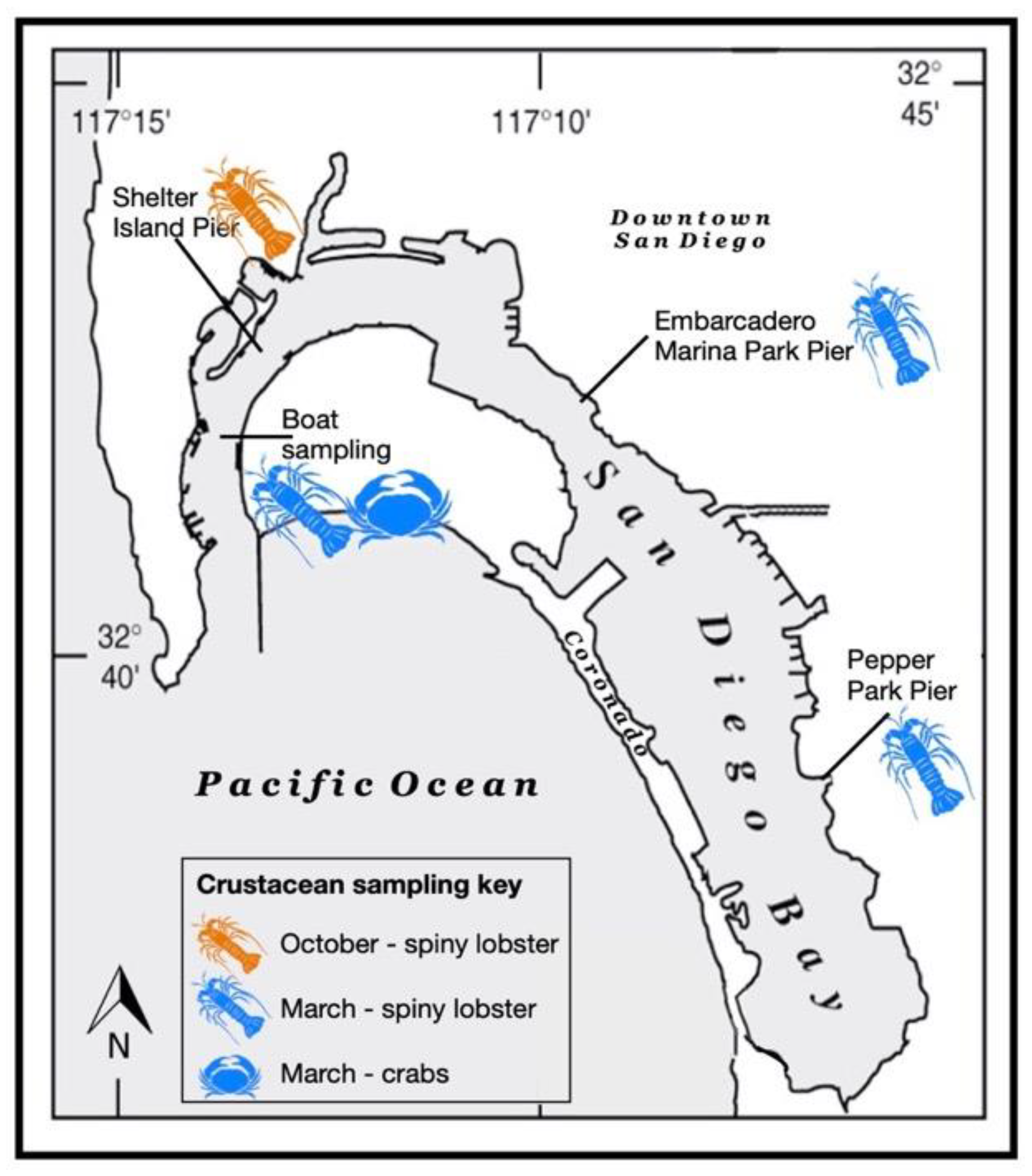
2.1. Field Sampling
2.2. Demographic Analysis
2.3. Contaminant Analysis
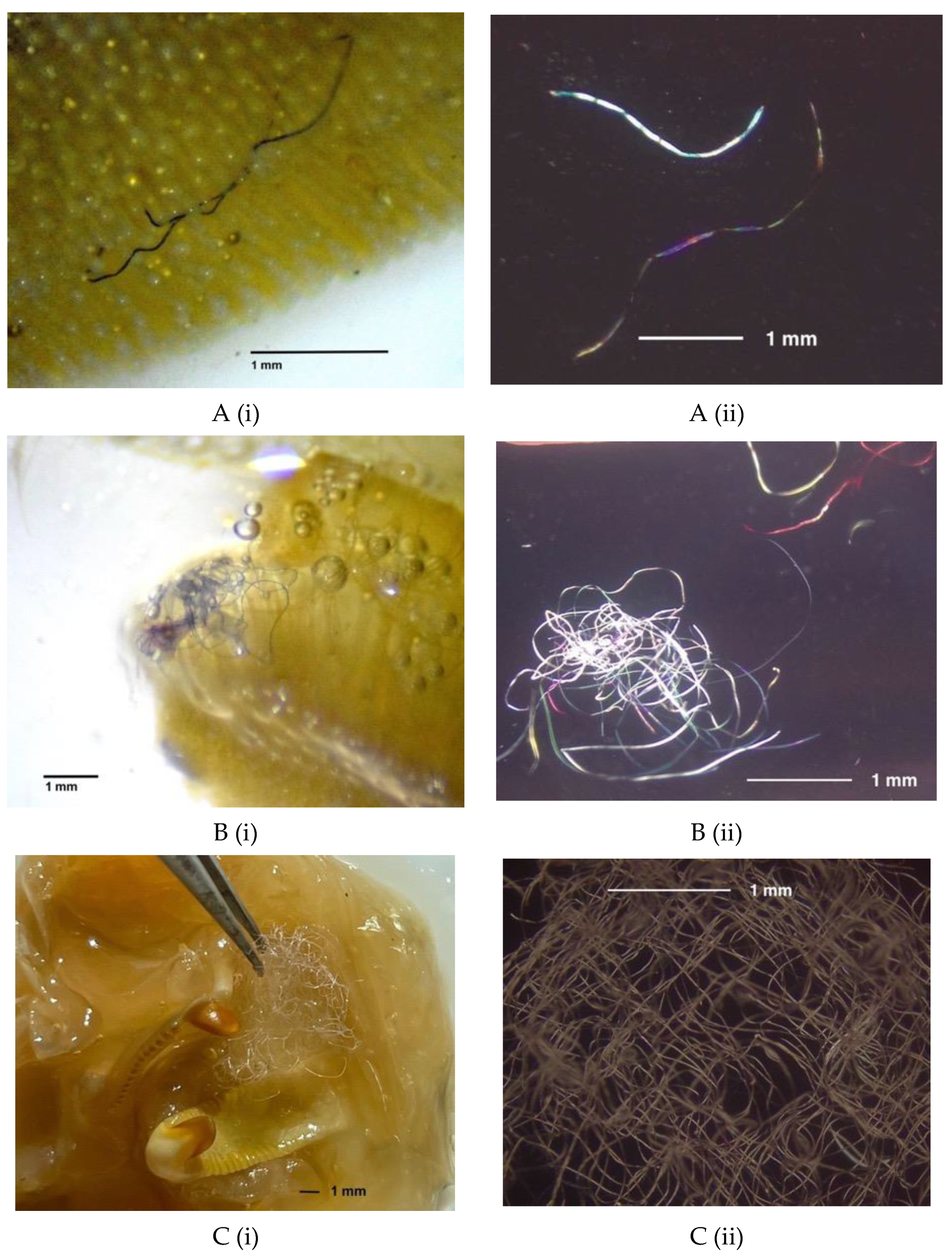
2.4. Anthropogenic Debris Analysis
3. Results
3.1. Shellfish Contamination
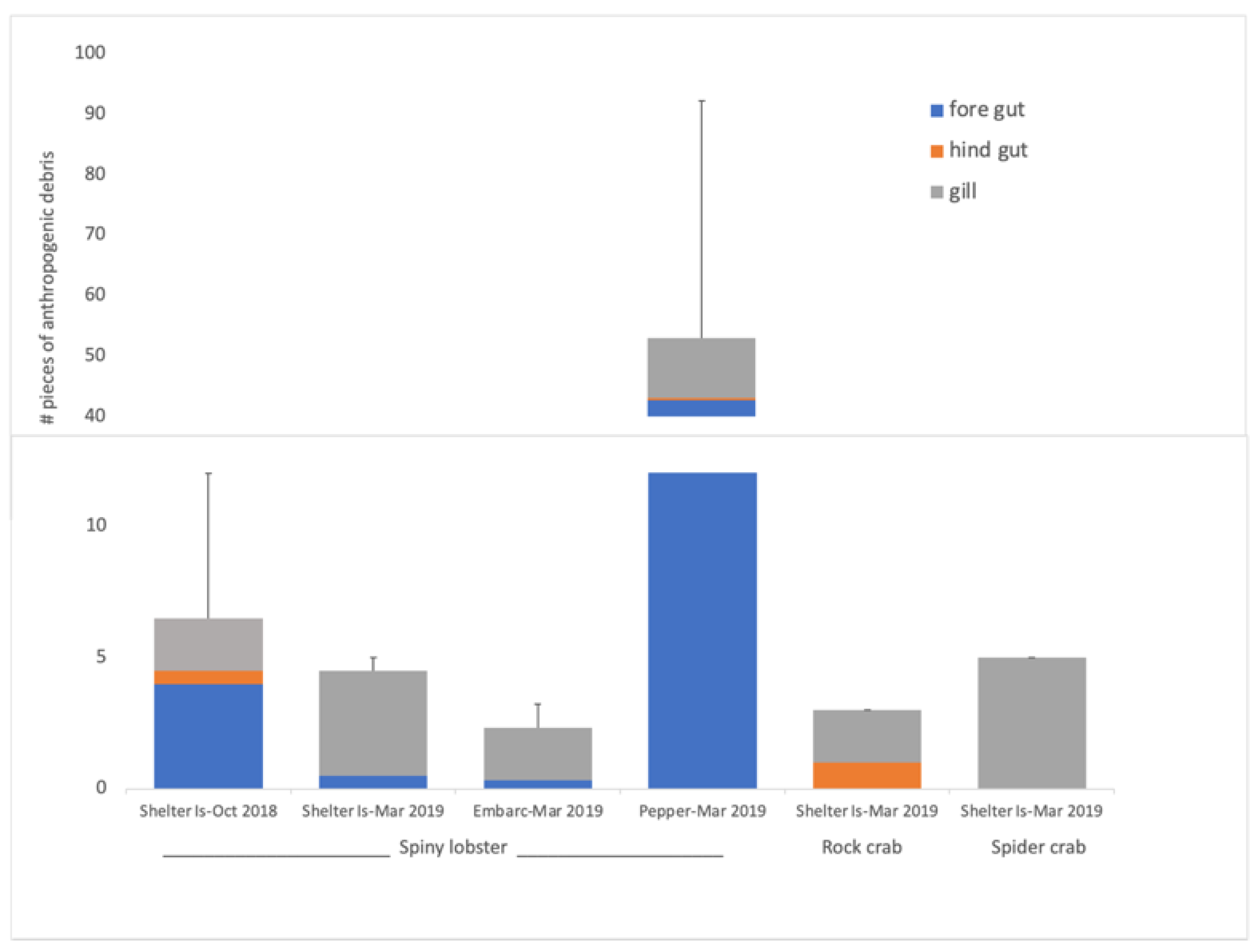
3.2. Shellfish Anthropogenic Debris
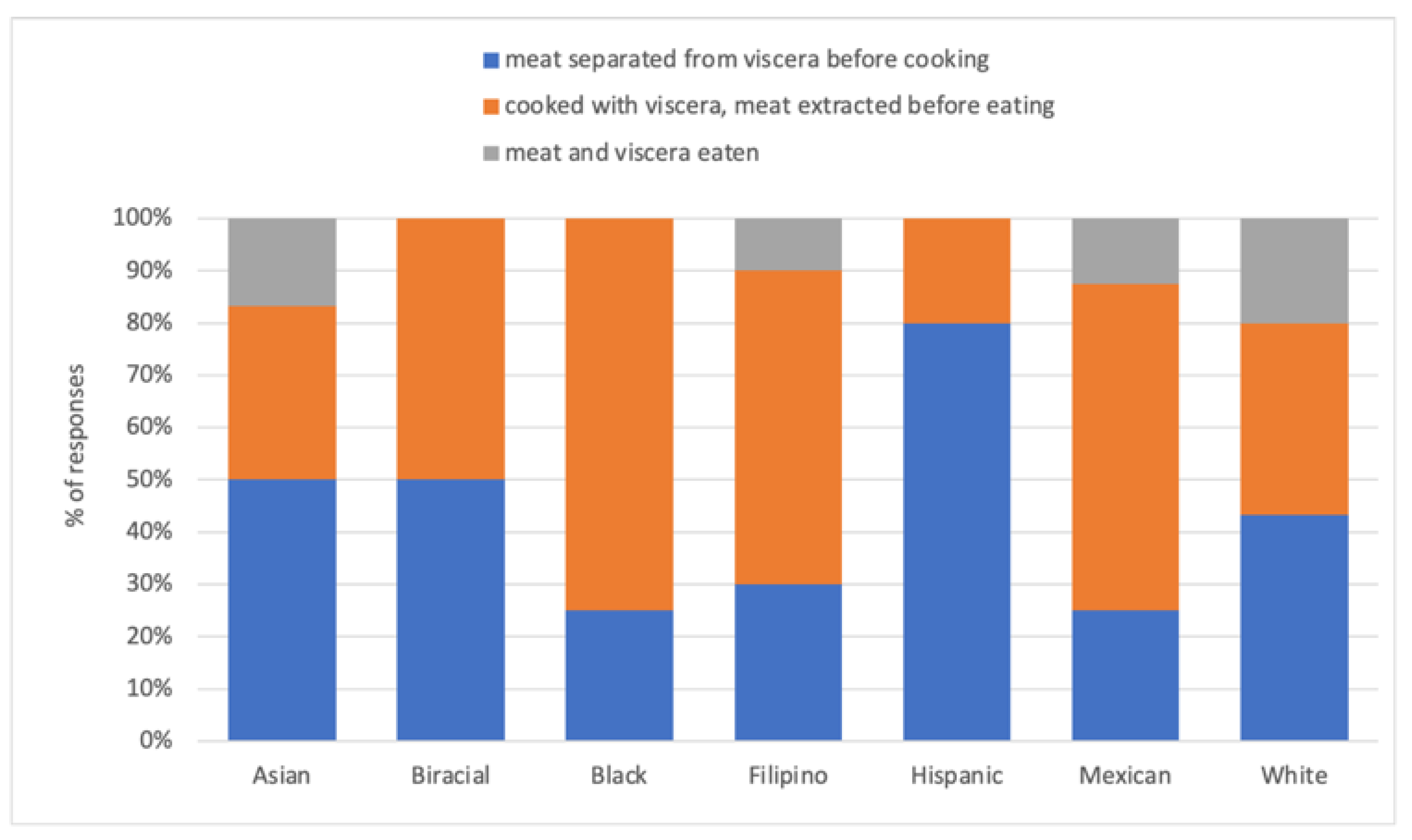
3.3. Shellfish Harvesters
4. Discussion
4.1. Contaminant Exposure Risks Associated with Eating Whole Lobster and Crab
4.2. Who Is Shellfishing?
4.3. Patterns of Shellfish Harvest and Potential Exposure
4.4. Monitoring Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPA (Environmental Protection Agency). Exposure Factors Handbook (Final Report). EPA/600/R-09/052F. 2011. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 27 January 2023).
- Guéguen, M.; Amiard, J.C.; Arnich, N.; Badot, P.M.; Claisse, D.; Guérin, T.; Vernoux, J.P. Shellfish and Residual Chemical Contaminants: Hazards, Monitoring, and Health Risk Assessment Along French Coasts. Rev. Environ. Contam. Toxicol. 2011, 213, 55–111. [Google Scholar] [CrossRef] [PubMed]
- Tatters, A.; Howard, M.; Nagoda, C.; Busse, L.; Gellene, A.; Caron, D. Multiple Stressors at the Land-Sea Interface: Cyanotoxins at the Land-Sea Interface in the Southern California Bight. Toxins 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Abollo, E.; Marrone, R.; Bernardi, C.E.M.; Chirollo, C.; Anastasio, A.; Del Hierro, S.P. Risk-Based Scoring and Genetic Identification for Anisakids in Frozen Fish Products from Atlantic FAO Areas. BMC Vet. Res. 2020, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Urton, A.; Turf, E.; Monti, M.M. Fish and Shellfish Consumption Estimates and Perceptions of Risk in a Cohort of Occupational and Recreational Fishers of the Chesapeake Bay. Environ. Res. 2009, 109, 108–115. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). Fish Consumption in Connecticut, Florida, Minnesota, and North Dakota (Final Report; EPA/600/R-13/098F). 2013. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=258242 (accessed on 11 April 2020).
- Pitchon, A.; Norman, K. Fishing off the Dock and Under the Radar in Los Angeles County: Demographics and Risks. Bull. South. Calif. Acad. Sci. 2012, 111, 141–152. [Google Scholar] [CrossRef]
- Steinberg, S.J.; Moore, S.L. San Diego Bay Fish Consumption Study. SCCWRP (Southern California Coastal Water Research Project) Technical Report 976. 2017. Available online: https://ftp.sccwrp.org/pub/download/DOCUMENTS/TechnicalReports/976_SanDiegoFishConsumptionStudy.pdf (accessed on 3 December 2017).
- Bay, S.; Greenstein, D.J.; Parks, A.N.; Zeeman, C.Q.T. Assessment of Bioaccumulation in San Diego Bay. SCCWRP (Southern California Coastal Water Research Project) Technical Report 953. 2016. Available online: http://ftp.sccwrp.org/pub/download/DOCUMENTS/TechnicalReports/953_SDBay_Bioaccum.pdf (accessed on 19 April 2019).
- Hunter, L.; Rodriguez, S.; Williams, J. Surveys of Fishers on Piers in San Diego Bay; Environmental Health Coalition: San Diego, CA, USA, 2005. Available online: https://www.waterboards.ca.gov/sandiego/water_issues/programs/npdes/southbay_power_plant/docs/updates_022410/2005_EHC_Pier_StudyFINALMarch.30.05.pdf (accessed on 12 February 2016).
- Pedersen, D.; Talley, T.S. Cultural, Economic, and Public Health Determinants of Social Vulnerability to Seafood Contaminants in an Urban Embayment in Southern California; Final Report Submitted to California Sea Grant, Project no. R/SFA-04A; California Sea Grant: San Diego, CA, USA, 2021. [Google Scholar]
- Stransky, C.; Tait, K.; Sheredy, C.; Schottle, R.; Kolb, R.; Bernstein, B. Aquatic Food Web Bioaccumulation Study of San Diego Bay: Final Report; Prepared by Amec Foster Wheeler Environmental, Inc for the City of San Diego: San Diego, CA, USA. 2016. Available online: https://www.waterboards.ca.gov/sandiego/water_issues/programs/sdbay_strategy/doc/R0516-074_Food_Web_Bioaccumulation_Study_Report_for_SD_Bay_FINAL_120716_update.pdf (accessed on 30 November 2018).
- Loflen, C.L.; Buck, T.; Bonnema, A.; Heim, W.A. Pollutant Bioaccumulation in the California Spiny Lobster (Panulirus Interruptus) in San Diego Bay, California, and Potential Human Health Implications. Mar. Pollut. Bull. 2018, 128, 585–592. [Google Scholar] [CrossRef]
- OEHHA (Office of Environmental Health Hazard Assessment, California Environmental Protection Agency). Health Advisory and Guidelines For Eating Fish From San Diego Bay (San Diego County). Available online: https://oehha.ca.gov/advisories/san-diego-bay (accessed on 27 January 2023).
- Busse, L. Surface Water Ambient Monitoring Program (SWAMP) Monitoring Plan for Region 9: Pilot Study on Pharmaceutical and Personal Care Products in the San Diego Region. California Regional Water Quality Control Board. 2010. Available online: https://www.waterboards.ca.gov/rwqcb9/water_issues/programs/swamp/docs/regional/SWAMP_PPCP_Workplan_2010_11.pdf (accessed on 10 May 2016).
- Busse, L.; Nagoda, C. Detection of Caffeine in the Streams and Rivers within the San Diego Region: Pilot Study. California Regional Water Quality Control Board. 2015. Available online: www.waterboards.ca.gov/sandiego/water_issues/programs/swamp/docs/Caffeine_FINAL_22Dec2015.pdf (accessed on 12 February 2016).
- Buzby, N.; Lin, D.; Sutton, R. Neonicotinoids and Their Degradates in San Francisco Bay Water. SFEI Contribution #1002. Regional Monitoring Program for Water Quality in San Francisco Bay. 2020. Available online: https://www.sfei.org/sites/default/files/biblio_files/Neonic%20Report%20-%20FINAL.pdf (accessed on 27 January 2021).
- Talley, T.S.; Venuti, N.; Whelan, R. Natural History Matters: Plastics in Estuarine Fish and Sediments at the Mouth of an Urban Watershed. PLoS ONE 2020, 15, e0229777. [Google Scholar] [CrossRef]
- Talley, T.S.; Loflen, C.; Gossett, R.; Pedersen, D.; Venuti, N.; Nguyen, J.; Gersberg, R. Contaminant Concentrations and Risks Associated with the Pacific Oyster in the Highly Urbanized San Diego Bay. Mar. Pollut. Bull. 2022, 174, 113132. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in Oceanic Surface Waters: A Global Characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic Contamination in the Decapod Crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Zabik, M.E.; Harte, J.B.; Zabik, M.J.; Dickmann, G. Effect of Preparation and Cooking on Contaminant Distributions in Crustaceans: PCBs in Blue Crab. J. Agric. Food Chem. 1992, 40, 1197–1203. [Google Scholar] [CrossRef]
- Adams, D.H.; Engel, M.E. Mercury, Lead, and Cadmium in Blue Crabs, Callinectes sapidus, from the Atlantic Coast of Florida, USA: A Multipredator Approach. Ecotoxicol. Environ. Saf. 2014, 102, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Culver, C.; Pomeroy, C.; Tyburczy, J. Natural Biotoxins in California Crabs: Domoic Acid. California Sea Grant. Available online: https://caseagrant.ucsd.edu/sites/default/files/Biotoxins-SU16-FAQ-v2.pdf (accessed on 20 November 2016).
- CDFW (California Department of Fish and Wildlife). Health Advisories and Closures for California Finfish, Shellfish and Crustaceans. 2022. Available online: https://wildlife.ca.gov/Fishing/Ocean/Health-Advisories (accessed on 12 April 2023).
- FDA (Food and Drug Administration). Technical Information on Development of FDA/EPA Advice about Eating Fish for Those Who Might Become or Are Pregnant or Breastfeeding and Children Ages 1–11 Years. FDA. 2021. Available online: https://www.fda.gov/food/environmental-contaminants-food/technical-information-development-fdaepa-advice-about-eating-fish-those-who-might-become-or-are (accessed on 27 January 2023).
- CDPH (California Department of Public Health). Shellfish Advisories. 2022. Available online: https://www.cdph.ca.gov/Programs/OPA/Pages/Shellfish-Advisories.aspx# (accessed on 12 April 2023).
- CDFW (California Department of Fish and Wildlife). California Ocean Sportfishing Regulations. 2019. Available online: https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=165608&inline (accessed on 29 June 2019).
- City-Data.com San Diego, California (CA) Profile. Available online: https://www.city-data.com/city/San-Diego-California.html (accessed on 11 April 2021).
- JMP® 15 Pro SAS Institute, Inc. Cary, NC 1989–2022. Available online: https://www.jmp.com/en_us/home.html (accessed on 11 March 2021).
- Krone, C.A.; Brown, D.W.; Burrows, D.G.; Bogar, R.G.; Chan, S.L.; Varanasi, U. A Method for Analysis of Butyltin Species and Measurement of Butyltins in Sediment and English Sole Livers from Puget Sound. Mar. Enviro. Res. 1989, 27, 1–18. [Google Scholar] [CrossRef]
- MMC1800 Sheep Crab AKA California King Crab-Cleaning and Meat Yield. Spearboard.Com-The World’s Largest Spearfishing Diving Boating Social Media Forum. Available online: http://www.spearboard.com/showthread.php?t=180705 (accessed on 12 March 2022).
- Grygus, A. Crabs: Pacific Rock Crab. Available online: https://clovegarden.com/ingred/seacrab.html (accessed on 12 April 2023).
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Labbe, A.B.; Bagshaw, C.R.; Uttal, L. Inexpensive Adaptations of Basic Microscopes for the Identification of Microplastic Contamination Using Polarization and Nile Red Fluorescence Detection. J. Chem. Educ. 2020, 97, 4026–4032. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic Debris in Seafood: Plastic Debris and Fibers from Textiles in Fish and Bivalves Sold for Human Consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- SCCWRP Microplastics Health Effects Webinar Series. Available online: https://www.sccwrp.org/about/research-areas/additional-research-areas/trash-pollution/microplastics-health-effects-webinar-series/ (accessed on 12 April 2023).
- ToxServices 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-Hexamethylcyclopenta-γ-2-Benzopyran (HHCB) (CAS# 1222-05-5) GreenScreen® for Safer Chemicals Assessment. Prepared for Women’s Voices for the Earth. 2015. Available online: https://www.womensvoices.org/wp-content/uploads/2016/04/1222-05-5-HHCB-aka-Galaxolide-GS-546-v-1-2-Certified-April-2015-3.pdf (accessed on 4 October 2022).
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Clark, J.R.; Goodman, L.R.; Borthwick, P.W.; Patrick, J.M.; Cripe, G.M.; Moody, P.M.; Moore, J.C.; Lores, E.M. Toxicity of Pyrethroids to Marine Invertebrates and Fish: A Literature Review and Test Results with Sediment-Sorbed Chemicals. Environ. Toxicol. Chem. 1989, 8, 393–401. [Google Scholar] [CrossRef]
- Jackson, D.; Luukinen, B.; Buhl, K.; Stone, D. DEET Technical Fact Sheet. National Pesticide Information Center, US Environmental Protection Agency and Oregon State University. Available online: http://npic.orst.edu/factsheets/archive/DEETtech.html (accessed on 12 April 2023).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for DEET (N,N-Diethyl-Meta-Toluamide); Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2017. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp185.pdf (accessed on 14 February 2023).
- EFSA (Panel on Contaminants in the Food Chain); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.; et al. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef]
- New Hampshire DES. Fish, Shellfish, Recreational Swimming and Wading Screening Levels (SLs) for Five Perfluoroalkyl Substances Including: PFOA, PFOS, PFHxS, PFNA and PFBS; New Hampshire Department of Environmental Services: Concord, NH, USA, 2019. Available online: https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/2019-pease-screening-levels.pdf (accessed on 14 February 2023).
- Maine CDC. Maine CDC Scientific Brief: PFOS Fish Consumption Advisory; Maine Center for Disease Control and Prevention: Augusta, ME, USA, 2022. Available online: www.maine.gov/dhhs/mecdc/environmental-health/eohp/fish/documents/pfas-fish-science-brief-05052022.pdf (accessed on 5 June 2022).
- Goodrow, S.M. Investigation of Levels of Perfluorinated Compounds in New Jersey Fish, Sediment and Surface Water; New Jersey Department of Environmental Protection: Trenton, NJ, USA, 2019; Available online: https://www.state.nj.us/drbc/library/documents/TAC/06182019/PFAS_NJsediment-fish-water_Goodrow_NJDEpdf (accessed on 19 April 2020).
- Klasing, S.; Brodberg, R. Development of Fish Contaminant Goals and Advisory Tissue Levels for Common Contaminants in California Sport Fish: Chlordane, DDTs, Dieldrin, Methylmercury, PCBs, Selenium, and Toxaphene; Office of Environmental Health Hazard Assessment, California Environmental Protection Agency: Sacramento, CA, USA, 2008. Available online: https://.ca.gov/media/downloads/fish/report/atlmhgandothers2008c.pdf (accessed on 27 January 2023).
- Krishnan, K.; Brodeur, J. Toxic Interactions among Environmental Pollutants: Corroborating Laboratory Observations with Human Experience. Environ. Health Perspect. 1994, 102, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Lin, C.-S.; Wang, W.-H. Metal Accumulation in Marine Bivalves under Various Tributyltin Burdens. Environ. Toxicol. Chem. 2009, 28, 2333. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Schiff, K.C.; James Allen, M.; Zeng, E.Y.; Bay, S.M. Southern California. Mar. Pollut. Bull. 2000, 41, 76–93. [Google Scholar] [CrossRef]
- Rowe, C.L. “The Calamity of So Long Life”: Life Histories, Contaminants, and Potential Emerging Threats to Long-Lived Vertebrates. BioScience 2008, 58, 623–631. [Google Scholar] [CrossRef]
- Katagi, T. Bioconcentration, Bioaccumulation, and Metabolism of Pesticides in Aquatic Organisms. In Review of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2010; Volume 204, pp. 1–132. ISBN 978-1-4419-1445-3. [Google Scholar]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and Seafood_ Lower Trophic Organisms at Highest Risk of Contamination | Elsevier Enhanced Reader. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef]
- Inoue, S.; Abe, S.; Oshima, Y.; Kai, N.; Honjo, T. Tributyltin Contamination of Bivalves in Coastal Areas around Northern Kyushu, Japan. Environ. Toxicol. 2006, 21, 244–249. [Google Scholar] [CrossRef]
- EPA (U.S. Environmental Protection Agency). Draft Scope of the Risk Evaluation for Butyl Benzyl Phthalate (1,2 Benzenedicarboxylic Acid, 1-Butyl 2-(Phenylmethyl) Ester) CASRN 85-68-7; EPA-740-D-20-015: Washington D.C., 2020. Available online: https://www.epa.gov/sites/default/files/2020-04/documents/casrn-84-74-2_butyl_benzyl_phthalate_draft_scope_4-15-2020.pdf (accessed on 10 May 2019).
- OEHHA (Office of Environmental Health Hazard Assessment). Proposition 65 Maximum Allowable Dose Level for Butyl Benzyl Phthalate; California Environmental Protection Agency: Sacramento, CA, USA, 2007. Available online: https://oehha.ca.gov/media/downloads/proposition-65/chemicals/abpkg6a.pdf (accessed on 22 January 2022).
- Ali, N.; Dirtu, A.C.; den Eede, N.V.; Goosey, E.; Harrad, S.; Neels, H.; ’t Mannetje, A.; Coakley, J.; Douwes, J.; Covaci, A. Occurrence of Alternative Flame Retardants in Indoor Dust from New Zealand: Indoor Sources and Human Exposure Assessment. Chemosphere 2012, 88, 1276–1282. [Google Scholar] [CrossRef]
- Ruxton, C. Health Aspects of Caffeine: Benefits and Risks. Nurs. Stand. 2009, 24, 41–48. [Google Scholar] [CrossRef]
- Town & Tourist Top 12, U. S States to Go Crab Fishing!-Licenses, Locations, Crabs with the Most Meat! Available online: https://www.townandtourist.com/top-12-u-s-states-to-go-crab-fishing-do-you-need-a-license/ (accessed on 12 April 2023).
- Crabbing HQ 5 Best Places to Crab. Available online: https://crabbinghq.com/tips/5-best-places-to-crab/ (accessed on 4 October 2022).
- Lipton, D.W.; Strand, I.E. Economic Effects of Pollution in Fish Habitats. Trans. Am. Fish. Soc. 1997, 126, 514–518. [Google Scholar] [CrossRef]
- Robles, C. Predator Foraging Characteristics and Prey Population Structure on a Sheltered Shore. Ecology 1987, 68, 1502–1514. [Google Scholar] [CrossRef]
- Carter, M.; Hilbern, M.; Mazzillo, F.; Culver, C.; Langlois, G. A Southern California Perspective on Harmful Algal Blooms. CalCOFI Rep. 2013, 54, 1–4. [Google Scholar]
- Tyson, R. Contaminated Fish Warnings Fail to Reach People Most at Risk. Scientific American. 2012. Available online: https://www.scientificamerican.com/article/contaminated-fish-warning-fail-to-reach-people-most-at-risk/ (accessed on 12 April 2022).
- Neira, C.; Cossaboon, J.; Mendoza, G.; Hoh, E.; Levin, L.A. Occurrence and Distribution of Polycyclic Aromatic Hydrocarbons in Surface Sediments of San Diego Bay Marinas. Mar. Pollut. Bull. 2017, 114, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Dewees, C.M.; Strange, E.M.; Guagnano, G. Competing for the Recreational Dollar: An Analysis of the California Commercial Passenger-Carrying Fishing Vessel Industry. 1990. Available online: https://aquadocs.org/handle/1834/26554 (accessed on 12 December 2021).
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Patricio Ojeda, F.; Duarte, C.; Galbán-Malagón, C. Is the Feeding Type Related with the Content of Microplastics in Intertidal Fish Gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef]
- Parker, D.O. Rock Crabs; California’s Living Marine Resources: A Status Report; California Department of Fish and Wildlife: Sacremento, CA, USA, 2001. Available online: https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=34335&inline (accessed on 14 February 2016).
- Culver, C.; Kuris, A. Sheep Crab; Status of the Fisheries Reports. 2003. Available online: https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=34389 (accessed on 12 February 2016).
- Neilson, D.J.; Barsky, K.C. California Spiny Lobster, Panuliris Interruptus; Status of the Fisheries Report; California Department of Fish and Wildlife: Sacremento, CA, USA, 2011. Available online: nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=65491&inline (accessed on 12 February 2016).




| Date Collected | Site | Sex | Avg ± 1SE Length (mm) | Avg ± 1SE Weight (g) | Viscera or Tail Muscle | % Moisture | % Lipids |
|---|---|---|---|---|---|---|---|
| California spiny lobster (Panulirus interruptus) | |||||||
| October-18 | Shelter Island | F,M | 79±0 | 558±2 | viscera | 64 | 58.50 |
| muscle | 71 | 0.75 | |||||
| March-19 | Shelter Island | F,F | 78 ± 2 | 549 ± 11 | viscera | 70 | 36.20 |
| muscle | 72 | 0.82 | |||||
| March-19 | Embarcadero | F,F,M | 86 ± 2 | 624 ± 23 | viscera | 70 | 13.10 |
| muscle | 76 | 1.01 | |||||
| March-19 | Pepper Park | F,F,M | 77 ± 4 | 526 ± 53 | viscera | 67 | 20.30 |
| muscle | 77 | 1.01 | |||||
| Spider crab (Loxorhynchus grandis) | |||||||
| March-19 | Shelter Island | F | 124 | 2721 | viscera | 69 | 26.60 |
| muscle | 79 | 3.94 | |||||
| Yellow rock crab (Metacarcinus anthonyii) | |||||||
| March-19 | Shelter Island | M | 108 | 411 | viscera | 86 | 9.81 |
| muscle | 75 | 0.63 | |||||
| Contaminant Class | Analytical Method |
|---|---|
| Acid Extractable Compounds (phenols) | EPA 8270D |
| Base/Neutral Extractable Compounds (phthalates, caffeine) | EPA 8270D |
| Fipronil and Degradates | EPA 8270D-NCI |
| Neonicotinoid Compounds | EPA 8270D-NCI |
| Organophosphorus Pesticides (chlorpyrifos, DEET) | EPA 8270D |
| Organotins | [32] |
| Phosphate Flame Retardants | EPA 8270D |
| PolyBrominated Diphenyl Ethers (PBDEs) | EPA 8270D-NCI |
| Pyrethroid Pesticides | EPA 8270D-NCI |
| Perfluorooctane sulfonic acid (PFOS) | PFAS Isotope Dilution Method |
| Pharmaceuticals and Personal Care Products | Modified EPA 1694 |
| California spiny lobster | yellow rock crab | spider crab | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| viscera + muscle | muscle only | viscera + muscle | muscle only | viscera + muscle | muscle only | ||||||||||||||
| avg | ± 1SD | max | avg | ± 1SD | max | ||||||||||||||
| 2018–2019 | |||||||||||||||||||
| Anthropogenic debris † | 19 | 40 | 130 | -- | 3 | -- | 5 | -- | |||||||||||
| Benzylbutyl phthalate †* | 10958 | 8899 | 19500 | 9791 | 7964 | 19500 | 9304 | 9304 | ND | ND | |||||||||
| Caffeine † | 138 | 276 | 553 | 138 | 276 | 553 | ND | ND | ND | ND | |||||||||
| DEET †* | ND | ND | ND | ND | ND | 1106 | |||||||||||||
| Galaxolide † | 24 | 47 | 94 | 24 | 47 | 94 | 155 | 155 | ND | ND | |||||||||
| Organotins †* | 149 | 138 | 334 | 57 | 77 | 164 | ND | ND | ND | ND | |||||||||
| Pyrethroids † | 316 | 350 | 809 | 162 | 189 | 354 | ND | ND | ND | ND | |||||||||
| PFOs †* | 134 | 131 | 264 | 61 | 72 | 140 | ND | ND | ND | ND | |||||||||
| Phosphate Flame Retardants †* | 38 | 77 | 153 | 38 | 77 | 153 | 210 | 210 | ND | ND | |||||||||
| PBDEs | 7 | 6 | 12 | 7 | 6 | 12 | 92 3 | ND | 1389 0 | 388 1 | |||||||||
| 2014–2015 | whole individual | muscle (tail) only | |||||||||||||||||
| PBDEs | 69277 1 | 69277 1 | 0 | ||||||||||||||||
| PCBs | 3970 3 | 18.0 | 7543 2 | 1590 | 45 | 1679 | -- | -- | -- | -- | |||||||||
| Chlordanes | 349.00 | 349.36 | 0 | -- | -- | -- | -- | ||||||||||||
| Silver | 180635 | 180635 | -- | -- | -- | -- | -- | ||||||||||||
| Arsenic | 10222750 | 10222750 | -- | -- | -- | -- | -- | ||||||||||||
| Cadmium | 206440 0 | 206440 0 | 553 | 14 | -- | -- | -- | -- | |||||||||||
| Chromium | 260035 | 260035 | -- | -- | -- | -- | -- | ||||||||||||
| Copper | 41883500 | 41883500 | -- | -- | -- | -- | -- | ||||||||||||
| Manganese | 369210 | 369210 | -- | -- | -- | -- | -- | ||||||||||||
| Nickel | 123070 | 123070 | -- | -- | -- | -- | -- | ||||||||||||
| Lead | 23820 | 23820 | 142 | 4 | -- | -- | -- | -- | |||||||||||
| Selenium | 837670 0 | 837670 0 | 187176 | 110179 | 375770 2 | -- | -- | -- | -- | ||||||||||
| Zinc | 23423000 | 23423000 | -- | -- | -- | -- | -- | ||||||||||||
| Mercury | -- | 30771 2 | 12478 | 56862 1 | -- | -- | -- | -- | |||||||||||
| OEHHA advisory (based on concentrations (ng) per g wet weight) | |||||||||||||||||||
| 0 = no consumption advised | † = no OEHHA seafood consumption guidelines available | ||||||||||||||||||
| 1 = 1 serving/week limit | * = other dose guidelines exist | ||||||||||||||||||
| 2 = 2 servings/week limit | -- = no data | ||||||||||||||||||
| 3 = 3 servings/week limit | ND = not detected | ||||||||||||||||||
| no notation= no serving limit | |||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talley, T.S.; Loflen, C.; Venuti, N.; Pedersen, D.; Gossett, R.; Baker, M.D. Contaminant Risk and Social Vulnerability Associated with Crustacean Shellfish Harvest in the Highly Urbanized San Diego Bay, USA. Environments 2023, 10, 91. https://doi.org/10.3390/environments10060091
Talley TS, Loflen C, Venuti N, Pedersen D, Gossett R, Baker MD. Contaminant Risk and Social Vulnerability Associated with Crustacean Shellfish Harvest in the Highly Urbanized San Diego Bay, USA. Environments. 2023; 10(6):91. https://doi.org/10.3390/environments10060091
Chicago/Turabian StyleTalley, Theresa Sinicrope, Chad Loflen, Nina Venuti, David Pedersen, Richard Gossett, and Mark D. Baker. 2023. "Contaminant Risk and Social Vulnerability Associated with Crustacean Shellfish Harvest in the Highly Urbanized San Diego Bay, USA" Environments 10, no. 6: 91. https://doi.org/10.3390/environments10060091
APA StyleTalley, T. S., Loflen, C., Venuti, N., Pedersen, D., Gossett, R., & Baker, M. D. (2023). Contaminant Risk and Social Vulnerability Associated with Crustacean Shellfish Harvest in the Highly Urbanized San Diego Bay, USA. Environments, 10(6), 91. https://doi.org/10.3390/environments10060091






