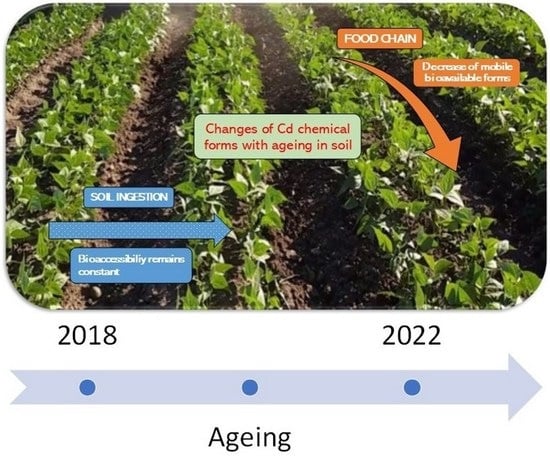
Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Soil Characterization and Cd Bioavailability and Bioaccessibility Evaluation
2.3. Microcosm Bioassay Experimental Design
2.4. Cadmium Analysis
2.5. Quality Assurance and Quality Control
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Analysis
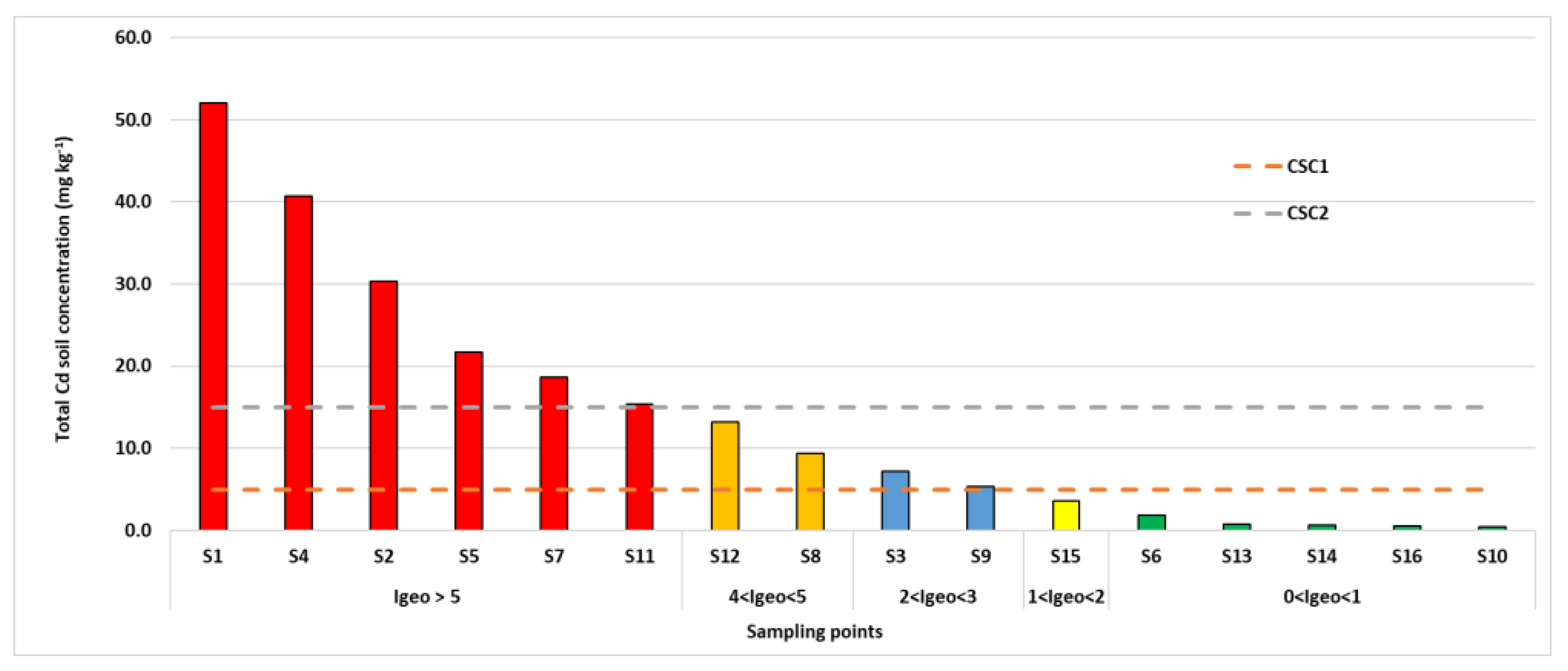
3.2. Soil Contamination
3.3. Changes in Cd Extractability with Aging
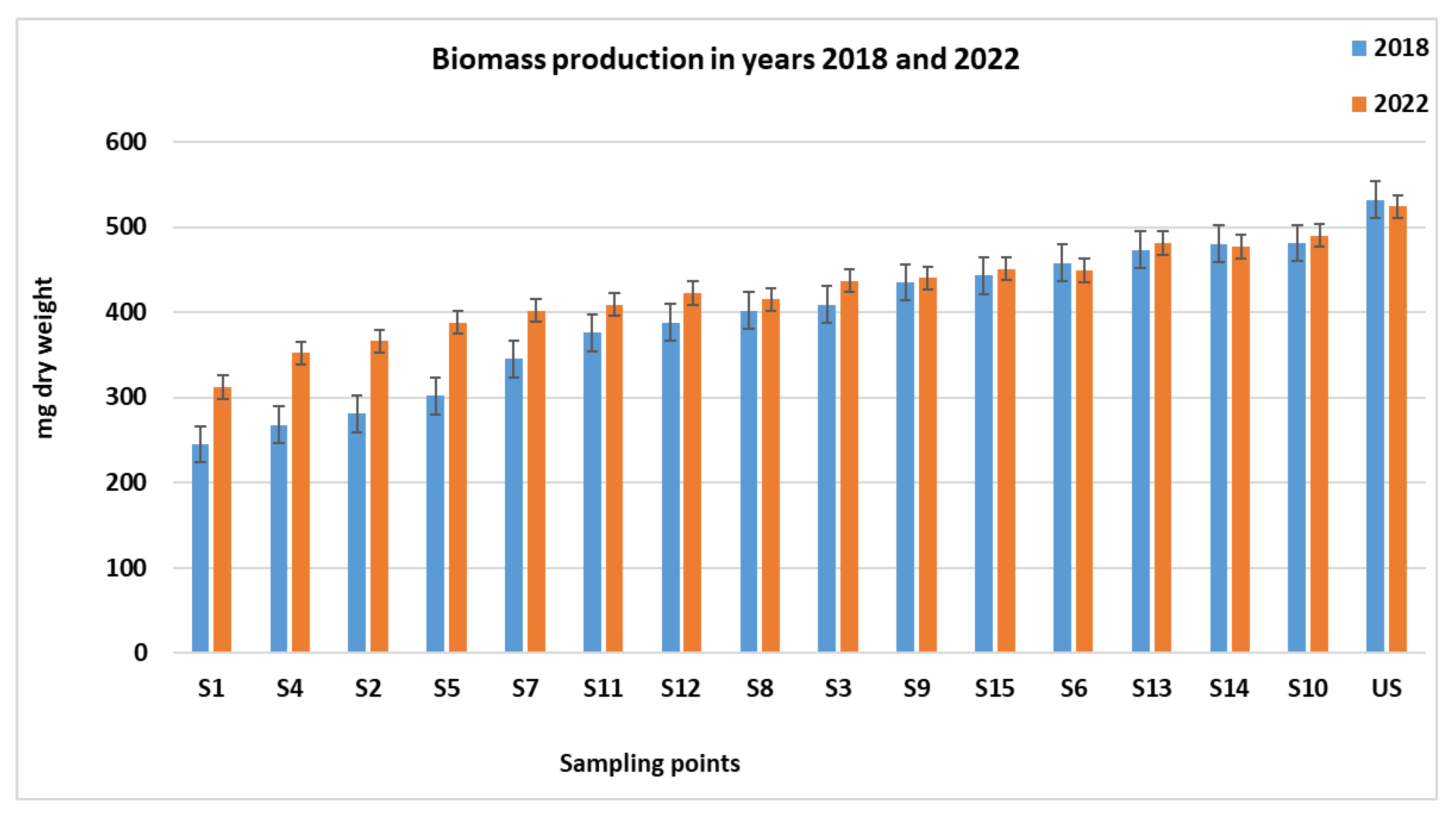
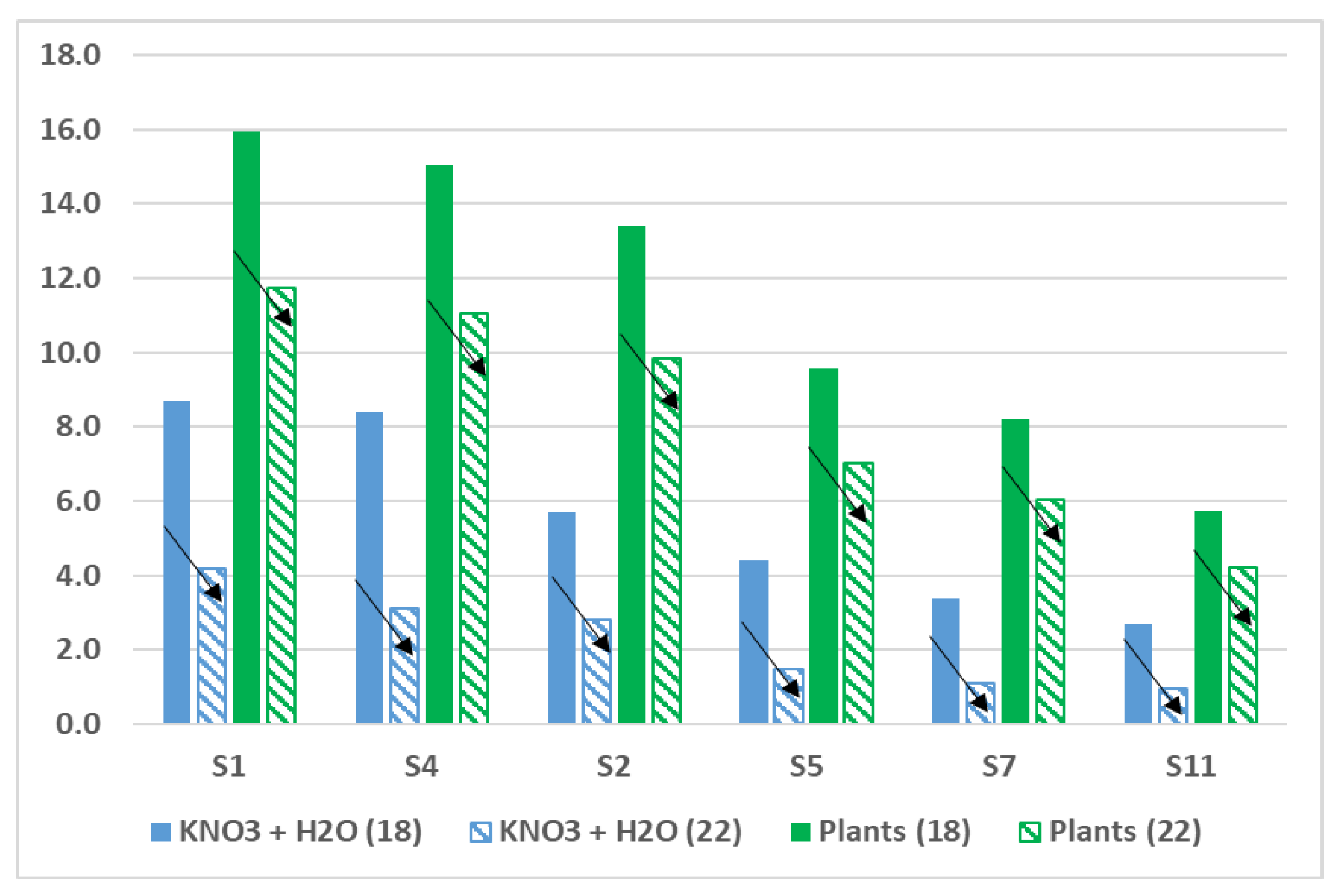
3.4. Effects of Aging on Cd Plant Uptake
3.5. Effect of Ageing on Cd Bioaccessibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyrogasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Capitano, C.; Cirrincione, L.; Peri, G.; Rizzo, G.; Scaccianoce, G. A simplified method for the indirect evaluation of the “embodied pollution” of natural stones (marble) working chain to be applied for achieving the Ecolabel brand of the product. J. Clean. Prod. 2022, 362, 132576. [Google Scholar] [CrossRef]
- Peri, G.; Licciardi, G.R.; Matera, N.; Mazzeo, D.; Cirrincione, L.; Scaccianoce, G. Disposal of green roofs: A contribution to identifying an “Allowed by legislation” end–of–life scenario and facilitating their environmental analysis. Build. Environ. 2022, 226, 109739. [Google Scholar] [CrossRef]
- Vocciante, M.; Meshalkin, V. An accurate inverse model for the detection of leaks in sealed landfills. Sustainability 2020, 12, 5598. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Rizzo, G.; Scaccianoce, G. The landfilling of municipal solid waste and the sustainability of the related transportation activities. Sustainability 2022, 14, 5272. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.A.P.; Duarte, A.C. Soil and Pollution: An Introduction to the Main Issues. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T.A.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Biores. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef]
- Trofa, M.; D’Avino, G.; Fabiano, B.; Vocciante, M. Nanoparticles synthesis in wet-operating stirred media: Investigation on the grinding efficiency. Materials 2020, 13, 4281. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Reverberi, A.P. A novel graphene-based sorbent for oil spill cleanup. Materials 2022, 5, 609. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Franchi, E.; Cardaci, A.; Pietrini, I.; Fusini, D.; Conte, A.; De Folly D’Auris, A.; Grifoni, M.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; et al. Nature-based solutions for restoring an agricultural area contaminated by an oil spill. Plants 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Sauer, T.J.; Cruse, R.M. Soil: The Forgotten Piece of the Water, Food, Energy Nexus. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 143, pp. 1–46. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 297, 126723. [Google Scholar] [CrossRef]
- Grifoni, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Barbafieri, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. Soil Remediation: Towards a Resilient and Adaptive Approach to Deal with the Ever-Changing Environmental Challenges. Environments 2022, 9, 18. [Google Scholar] [CrossRef]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; Reverberi, A.P.; Dovì, V.G. Approximate solution of the inverse Richards’ problem. Appl. Math. Model. 2016, 40, 5364–5376. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Gorini, F.; Pezzarossa, B.; Pedron, F. The fate of pollutants in soil. In CNR Environment and Health Inter-Departmental Project: Present Knowledge and Prospects for Future Research; CNR: Roma, Italy, 2010; pp. 1–38. ISBN 978-88-8080-113-9. [Google Scholar]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Monographs on the Identification of Carcinogenic Hazards to Humans; IARC: Lyon, France, 2012. [Google Scholar]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Akesson, A.; Julin, B.; Wolk, A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study. Cancer Res. 2008, 68, 6435–6441. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Cien. Saude Colet. 2011, 16, 2587–2602. [Google Scholar] [CrossRef]
- Grioni, S.; Agnoli, C.; Krogh, V.; Pala, V.; Rinaldi, S.; Vinceti, M.; Contiero, P.; Vescovi, L.; Malavolti, M.; Sieri, S. Dietary cadmium and risk of breast cancer subtypes defined by hormone receptor status: A prospective cohort study. Int. J. Cancer. 2019, 144, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef] [PubMed]
- UNEP (United Nations Environment Programme). Final Review of Scientific Information on Cadmium; UNEP: Nairobi, Kenya, 2010; pp. 1–201. [Google Scholar]
- EFSA (European Food Safety Authority). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liao, X.; Tao, H.; Li, Y. Chain modeling for the biogeochemical nexus of cadmium in soil–rice–human health system. Environ. Int. 2022, 167, 107424. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.; Mosby, D.E.; Casteel, S.W.; Berti, W.; Carpenter, M.; et al. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Luo, X.S.; Ding, J.; Xu, B.; Wang, Y.J.; Li, H.B.; Yu, S. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F.; Rosellini, I. Bioavailability and bioaccessibility in soil: A short review and a case study. AIMS Environ. Sci. 2020, 7, 208–225. [Google Scholar] [CrossRef]
- Alexander, M. Ageing, bioavailability and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 2000, 34, 4259–4265. [Google Scholar] [CrossRef]
- Ma, Y.B.; Lombi, E.; Oliver, I.W.; Nolan, A.L.; McLaughlin, M.J. Long-term aging of copper added to soils. Environ. Sci. Technol. 2006, 40, 6310–6317. [Google Scholar] [CrossRef]
- Li, J.; Peng, Q.; Liang, D.L.; Liang, S.J.; Chen, J.; Sun, H.; Li, S.Q.; Lei, P.H. Effects of aging on the fraction distribution and bioavailability of selenium in three different soils. Chemosphere 2016, 144, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.K.; McLaughlin, M.J.; Ma, Y.B.; Ajiboye, B. Aging effects on molybdate lability in soils. Chemosphere 2012, 89, 876–883. [Google Scholar] [CrossRef]
- Settimio, L.; McLaughlin, M.J.; Kirby, J.K.; Langdon, K.A.; Lombi, E.; Donner, E.; Scheckel, K.G. Fate and lability of silver in soils: Effect of ageing. Environ. Pollut. 2014, 191, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Sun, J.Y.; Xu, X.J.; Yu, H.W. Distribution and availability of fungicide-derived copper in soil aggregates. J. Soils Sediments 2020, 20, 816–823. [Google Scholar] [CrossRef]
- Abbasi, S.; Lamb, D.T.; Kader, M.; Naidu, R.; Megharaj, M. The influence of long-term ageing on arsenic ecotoxicity in soil. J. Haz. Mat. 2021, 407, 124819. [Google Scholar] [CrossRef]
- Han, Y.S.; Park, J.H.; Ahn, J.S. Aging effects on fractionation and speciation of redox sensitive metals in artificially contaminated soil. Chemosphere 2021, 263, 127931. [Google Scholar] [CrossRef]
- Smolders, E.; Oorts, K.; Peeters, S.; Lanno, R.; Cheyns, K. Toxicity in lead salt spiked soils to plants, invertebrates and microbial processes: Unraveling effects of acidification, salt stress and ageing reactions. Sci. Total Environ. 2015, 536, 223–231. [Google Scholar] [CrossRef]
- Tang, X.Y.; McBride, M.B. Phytotoxicity and microbial respiration of Ni-spiked soils after field aging for 12 Yr. Environ. Toxicol. Chem. 2018, 37, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Sun, J.Y.; Yu, H.W.; Yang, X.T.; Yue, J.; Hu, N.W. Laboratory versus field soil aging: Impacts on cadmium distribution, release, and bioavailability. Sci. Total Environ. 2021, 779, 146442. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Chen, Z.Y.; Wang, J.; Hou, Q.X.; Zhang, Y. Impact of temperature on the aging mechanisms of arsenic in soils: Fractionation and bioaccessibility. Environ. Sci. Pollut. Res. 2016, 23, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, Z.; Liu, X.; Xue, W.; Dong, L.; Liu, Y. Aging Process of Cadmium, Copper, and Lead under Different Temperatures and Water Contents in Two Typical Soils of China. J. Chem. 2020, 2583819. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, H.; Luo, Y.; Christie, P. Effects of soil drying and wetting-drying cycles on the availability of heavy metals and their relationship to dissolved organic matter. J. Soils Sediments 2015, 15, 1510–1519. [Google Scholar] [CrossRef]
- Italian Government, D. Lgs 152. Official Gazette No. 88 of the Italian Republic of 14-04-2006; Ordinary Supplement No. 96 (in Italian). Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2006. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis, Part 3—Chemical Method; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Petruzzelli, G.; Barbafieri, M.; Bonomo, L.; Saponaro, S.; Milani, A.; Pedron, F. Bench Scale Evaluation of Soil Washing for Heavy Metal Contaminated Soil at a Former Manufactured Gas Plant Site. Bull. Environ. Contam. Toxicol. 2004, 73, 38–44. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E. Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 2009, 75, 808–814. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Franchi, E.; Vocciante, M.; Petruzzelli, G. Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies. Environment 2022, 9, 139. [Google Scholar] [CrossRef]
- Grifoni, M.; Petruzzelli, G.; Barbafieri, M.; Rosellini, I.; Pedron, F. Soil quality protection at heavy metal-contaminated manufactured gas plant sites: Role of biological remediation. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 11, pp. 231–260. [Google Scholar]
- Pietrini, I.; Grifoni, M.; Franchi, E.; Cardaci, A.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants. Soil Syst. 2021, 5, 55. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). In Vitro Bioaccessibility Assay for Lead in Soil. Validated Test Method 1340; Washington, DC, USA. 2017. Available online: https://www.epa.gov/sites/production/files/2017-03/documents/method_1340_update_vi_final_3-22-17.pdf (accessed on 18 May 2023).
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J. Haz. Mater. 2009, 171, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Padoan, E.; Romè, C.; Ajmone-Marsan, F. Bioaccessibility and size distribution of metals in road dust and roadside soils along a peri-urban transect. Sci. Total Environ. 2017, 601–602, 89–98. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, T.; Shao, Y.; Tian, C.; Cattle, S.R.; Zhu, Y.; Song, J. Fractionation, Bioaccessibility, and Risk Assessment of Heavy Metals in the Soil of an Urban Recreational Area Amended with Composted Sewage Sludge. Int. J. Environ. Res. Public Health 2018, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- Müller, G. Schwermetalle in den sedimenten des Rheins-Veräderungen seit. Umschau 1979, 79, 778–783. [Google Scholar]
- Barbieri, M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J. Geol. Geophys. 2016, 5, 237–240. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; Method 3051A (Rev.1); USEPA: Washington, DC, USA, 2007.
- Wang, Q.Y.; Sun, J.J.; Hu, N.W.; Wang, T.Y.; Yue, J.; Hu, B.; Yu, H.W. Effects of soil aging conditions on distributions of cadmium distribution and phosphatase activity in different soil aggregates. Sci. Tot. Environ. 2022, 834, 155440. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Luo, J. Characterizing pollution and source identification of heavy metals in soils using geochemical baseline and PMF approach. Sci. Rep. 2020, 10, 6460. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, S.; Hashmi, M.Z.; Malik, R.N. Heavy metals distribution, risk assessment and water quality characterization by water quality index of the River Soan, Pakistan. Ecol. Indic. 2014, 43, 262–270. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Han, R.; Xu, Z. Spatial distribution and ecological risk assessment of heavy metals in karst soils from the Yinjiang County, Southwest China. PeerJ 2022, 10, e12716. [Google Scholar] [CrossRef]
- Schreck, E.; Foucault, Y.; Geret, F.; Pradère, P.; Dumat, C. Influence of soil ageing on bioavailability and ecotoxicity of lead carried by process waste metallic ultrafine particles. Chemosphere 2011, 85, 1555–1562. [Google Scholar] [CrossRef]
- Bloom, N.S.; Preus, E.; Katon, J.; Hiltner, M. Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediments and soils. Anal. Chim. Acta 2003, 479, 233–248. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC, Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Matong, J.M.; Nyaba, L.; Nomngongo, P.N. Fractionation of trace elements in agricultural soils using ultrasound assisted sequential extraction prior to inductively coupled plasma mass spectrometric determination. Chemosphere 2016, 154, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Kari, F.G.; Krüger, H.G. The remobilization of metals from iron oxides and sediments by metal-edta complexes. Water Air Soil Pollut. 2001, 125, 243–257. [Google Scholar] [CrossRef]
- Jalali, M.; Khanlari, Z.V. Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 2008, 143, 26–40. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, S.; Shan, X.Q. Time effect on the fractionation of heavy metals in soils. Geoderma 2005, 125, 225–234. [Google Scholar] [CrossRef]
- Naidu, R.; Juhasz, A.; Mallavarapu, M.; Smith, E.; Lombi, E.; Bolan, N.S.; Wong, M.H.; Harmsen, J. Chemical Bioavailability in the Terrestrial Environment—Recent advances. J. Haz. Mater. 2013, 261, 685–686. [Google Scholar] [CrossRef]
- Harmsen, J.; Naidu, R. Bioavailability as a tool in site management. J. Haz. Mater. 2013, 261, 840–846. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Rasafi, T.E.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Abin Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E.; Ambrosini, P.; Patata, L. Mercury Mobilization in a Contaminated Industrial Soil for Phytoremediation. Commun. Soil Sci. Plant Anal. 2011, 42, 2767–2777. [Google Scholar] [CrossRef]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted phytoremediation of a multi-contaminated soil: Investigation on arsenic and lead combined mobilization and removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef]
- Menzies, N.W.; Donn, M.J.; Kopittke, P.M. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ. Pollut. 2007, 145, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace Element Chemistry in Residual-Treated Soil: Key Concepts and Metal Bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Long, X.H.; Zhang, Z.H.; Zheng, X.T.; Rengel, Z.; Liu, Z.P. Cadmium Accumulation and Translocation in Two Jerusalem Artichoke (Helianthus tuberosus L.) Cultivars. Pedosphere 2011, 21, 573–580. [Google Scholar] [CrossRef]
- Rezapour, S.; Atashpaz, B.; Moghaddam, S.S.; Kalavrouziotis, I.K.; Damalas, C.A. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 2019, 231, 579–587. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wu, Y.J.; Wang, Q.; Feng, Y. The endophytic bacterium relieved healthy risk of pakchoi intercropped with hyperaccumulator in the cadmium polluted greenhouse vegetable field. Environ. Pollut. 2020, 264, 114796. [Google Scholar] [CrossRef]
- Ma, L.Y.; Liu, Y.R.; Wu, Y.J.; Wang, Q.; Sahito, Z.A.; Zhou, Q.Y.; Huang, L.; Li, T.; Feng, Y. The effects and health risk assessment of cauliflower co-cropping with Sedum alfredii in cadmium contaminated vegetable field. Environ. Pollut. 2021, 268, 115869. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E. Remediation of a Mercury-Contaminated Industrial Soil Using Bioavailable Contaminant Stripping. Pedosphere 2013, 23, 104–110. [Google Scholar] [CrossRef]
- Santa-Cruz, J.; Robinson, B.; Krutyakov, Y.A.; Shapoval, O.A.; Peñaloza, P.; Yáñez, C.; Neaman, A. An Assessment of the Feasibility of Phytoextraction for the Stripping of Bioavailable Metals from Contaminated Soils. Environ. Toxicol. Chem. 2023, 42, 558–565. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Xu, W.; Chi, S.; Li, T.; Li, Y.; He, Z.; Yang, M.; Feng, D. Resistance of alfalfa and Indian mustard to Cd and the correlation of plant Cd uptake and soil Cd form. Environ. Sci. Pollut. Res. Int. 2019, 26, 13804–13811. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Phytoremediation of cadmium contaminated soil using Brassica juncea: Influence on PSII activity, leaf gaseous exchange, carbohydrate metabolism, redox and elemental status. Bull. Environ. Contam. Toxicol. 2020, 105, 411–421. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Zeng, C.; Dong, X.; Li, M.; Liu, Z.; Yan, M. The elemental defense effect of cadmium on Alternaria brassicicola in Brassica juncea. BMC Plant Biol. 2022, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Xia, B.; Zeng, X.; Qiu, R.; Tang, Y.; Hu, Z. Adsorption of Cadmium by Brassica juncea (L.) Czern. and Brassica pekinensis (Lour.) Rupr in Pot Experiment. Sustainability 2022, 14, 429. [Google Scholar] [CrossRef]
- Sun, Y.B.; Zhou, Q.X.; An, J.; Liu, W.T.; Liu, R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 2009, 150, 106–112. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Oomen, A.; Duits, M.; Selinus, O.; Berglund, M. Bioaccessibility of metals in urban playground soils. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2007, 42, 1241–1250. [Google Scholar] [CrossRef]
- Ruby, M.V.; Lowney, Y.W. Selective soil particle adherence to hands: Implications for understanding oral exposure to soil contaminants. Environ. Sci. Technol. 2012, 46, 12759–12771. [Google Scholar] [CrossRef]
- Ruby, M.V.; Lowney, Y.W.; Bunge, A.L.; Roberts, S.M.; Gomez-Eyles, J.L.; Ghosh, U.; Kissel, J.C.; Tomlinson, P.; Menzie, C. Oral Bioavailability, Bioaccessibility, and Dermal Absorption of PAHs from Soil—State of the Science. Environ. Sci. Technol. 2016, 50, 2151–2164. [Google Scholar] [CrossRef]
- Morman, S.A.; Plumlee, G.S.; Smith, D.B. Application of in vitro extraction studies to evaluate element bioaccessibility in soils from a transect across the United States and Canada. Appl. Geochem. 2009, 24, 1454–1463. [Google Scholar] [CrossRef]
- Pedron, F.; Rosellini, I.; Pezzarossa, B.; Bianchi, L.; Petruzzelli, G. Assessment of heavy metal bioavailability and human bioaccessibility in soils in the vicinity of a cement plant. Agrochimica 2014, LVIII, 178–192. [Google Scholar]
- Li, S.W.; Chang, M.; Huang, X.; Li, H.; Li, H.B.; Ma, L.Q. Coupling in vitro assays with sequential extraction to investigate cadmium bioaccessibility in contaminated soils. Chemosphere 2022, 288, 132655. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.J.; Garrido, R.T.; Quilodrán, R.C.; Segovia, C.M.; Parada, A.J. Evaluation of the bioaccessible gastric and intestinal fractions of heavy metals in contaminated soils by means of a simple bioaccessibility extraction test. Chemosphere 2017, 176, 81–88. [Google Scholar] [CrossRef]
- Riding, M.J.; Doick, K.J.; Martin, F.L.; Jones, K.C.; Semple, K.T. Chemical measures of bioavailability/bioaccessibility of PAHs in soil: Fundamentals to application. J. Haz. Mater. 2013, 261, 687–700. [Google Scholar] [CrossRef]
- Yan, K.; Dong, Z.; Wijayawardena, M.A.A.; Liu, Y.; Naidu, R.; Semple, K.T. Measurement of soil lead bioavailability and influence of soil types and properties: A review. Chemosphere 2017, 184, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ti, Q.; Gu, C.; Liu, C.; Cai, J.; Bian, Y.; Yang, X.; Song, Y.; Wang, F.; Sun, C.; Jiang, X. Comparative evaluation of influence of aging, soil properties and structural characteristics on bioaccessibility of polychlorinated biphenyls in soil. Chemosphere 2018, 210, 941–948. [Google Scholar] [CrossRef]
- Kastury, F.; Smith, E.; Doelsch, E.; Lombi, E.; Donnelley, M.; Cmielewski, P.L.; Parsons, D.W.; Scheckel, K.G.; Paterson, D.; de Jonge, M.D.; et al. In vitro, in vivo and spectroscopic assessment of lead exposure reduction via ingestion and inhalation pathways using phosphate. Environ. Sci. Technol. 2019, 53, 10329–10341. [Google Scholar] [CrossRef] [PubMed]
- Villen-Guzman, M.; Garcia-Rubio, A.; Paz-Garcia, J.M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M. Aging effects on the mobility of Pb in soil: Influence on the energy requirements in electroremediation. Chemosphere 2018, 213, 351–357. [Google Scholar] [CrossRef]
| Soil Characteristics | Mean Value ± SD |
|---|---|
| pH | 6.3 ± 0.4 |
| EC (mS cm−1) | 14.4 ± 1.3 |
| Clay (%) | 13.8 ± 1.6 |
| Silt (%) | 29.2 ± 2.1 |
| Sand (%) | 55.5 ± 3.5 |
| CEC (cmol(+)kg−1) | 14.4 ± 1.3 |
| Organic matter (%) | 0.9 ± 0.05 |
| Sampling Point | Total Cd (2018) | Total Cd (2022) |
|---|---|---|
| S1 | 52.1 ± 3.4 a | 50.6 ± 4.2 a |
| S4 | 40.7 ± 3.0 a | 41.8 ± 3.7 a |
| S2 | 30.3 ± 2.5 a | 31.6 ± 2.9 a |
| S5 | 21.7 ± 2.1 a | 22.1 ± 2.5 a |
| S7 | 18.6 ± 1.8 a | 17.7 ± 1.9 a |
| S11 | 15.4 ± 1.2 a | 17.1 ± 2.3 a |
| S12 | 13.2 ± 1.1 a | 11.9 ± 2.7 a |
| S8 | 9.4 ± 1.0 a | 10.2 ± 1.7 a |
| S3 | 7.2 ± 0.7 a | 6.9 ± 0.82 a |
| S9 | 5.3 ± 0.5 a | 5.9 ± 0.9 a |
| S15 | 3.6 ± 0.3 a | 4.1 ± 0.4 a |
| S6 | 1.81 ± 0.2 a | 1.63 ± 0.2 a |
| S13 | 0.66 ± 0.04 a | 0.60 ± 0.07 a |
| S14 | 0.54 ± 0.04 a | 0.56 ± 0.05 a |
| S10 | 0.38 ± 0.03 a | 0.40 ± 0.06 a |
| US | 0.5 ± 0.001 a | 0.5 ± 0.003 a |
| Sample | H2O | KNO3 | EDTA | |||
|---|---|---|---|---|---|---|
| 2018 | 2022 | 2018 | 2022 | 2018 | 2022 | |
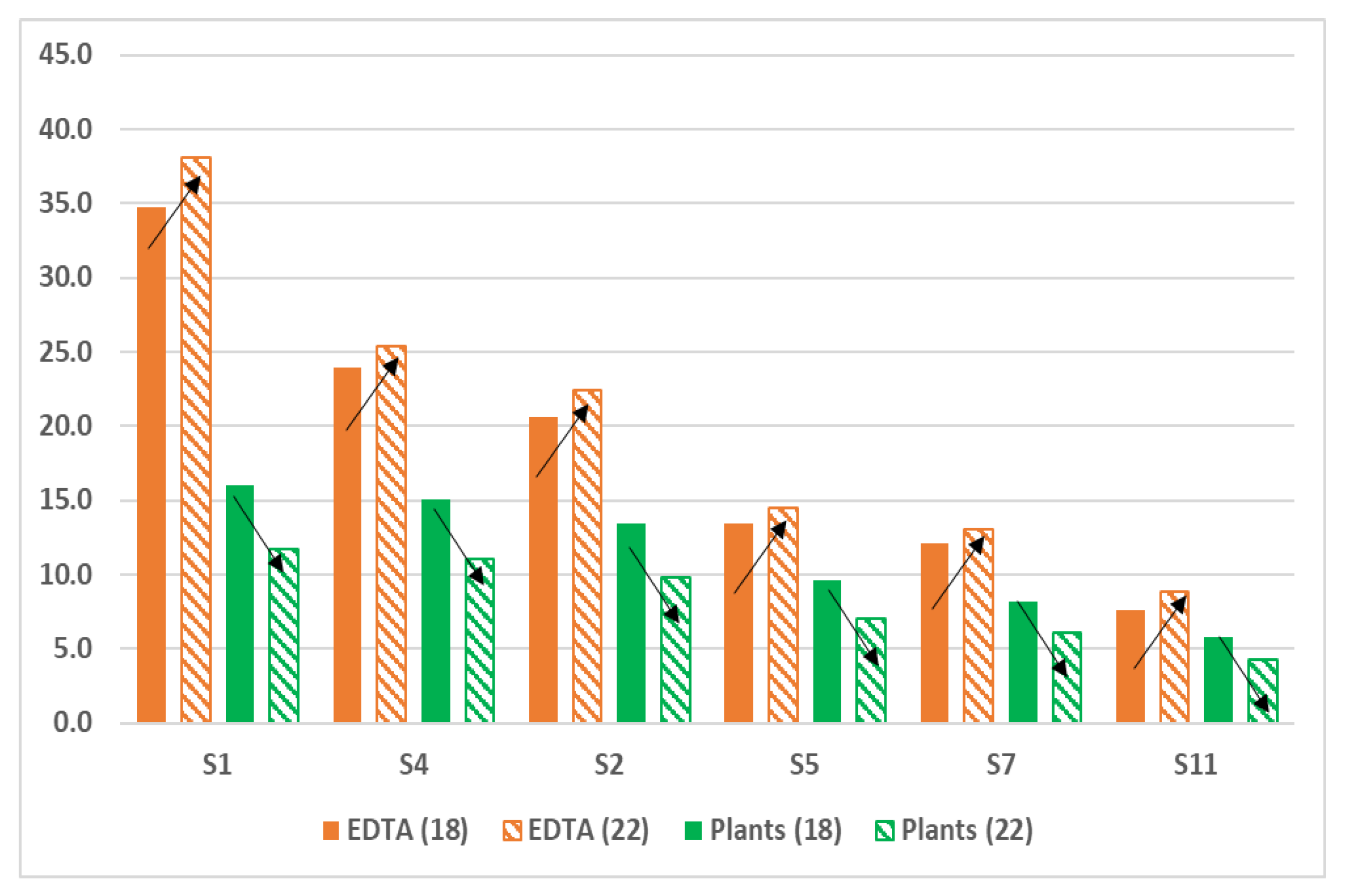
| S1 | 1.5 ± 0.02 | nd | 7.2 ± 0.11 b | 4.2 ± 0.03 a | 34.7 ± 1.48 a | 38.1 ± 1.61 b |
| S4 | 1.8 ± 0.03 | nd | 6.6 ± 0.09 b | 3.1 ± 0.02 a | 23.9 ± 1.30 a | 25.4 ± 1.23 b |
| S2 | 1.5 ± 0.03 | nd | 4.2 ± 0.07 b | 2.8 ± 0.03 a | 20.6 ± 1.15 a | 22.4 ± 1.11 b |
| S5 | 1.3 ± 0.02 | nd | 3.1 ± 0.03 b | 1.5 ± 0.01 a | 13.4 ± 0.72 a | 14.5 ± 0.09 b |
| S7 | 0.80 ± 0.01 | nd | 2.6 ± 0.03 b | 1.1 ± 0.01 a | 12.1 ± 0.68 a | 13.0 ± 0.08 b |
| S11 | 0.60 ± 0.01 | nd | 2.1 ± 0.02 b | 0.96 ± 0.01 a | 7.6 ± 0.10 a | 8.8 ± 0.09 b |
| S12 | 0.65 ± 0.02 | nd | 1.5 ± 0.02 b | 0.90 ± 0.02 a | 6.2 ± 0.09 a | 6.9 ± 0.07 b |
| S8 | nd | nd | 1.2 ± 0.01 b | 0.85 ± 0.01 a | 5.2 ± 0.10 a | 5.7 ± 0.05 b |
| S3 | nd | nd | 1.1 ± 0.02 b | 0.80 ± 0.01 a | 3.9 ± 0.06 a | 4.0 ± 0.05 b |
| S9 | nd | nd | 0.82 ± 0.01 b | 0.76 ± 0.01 a | 3.1 ± 0.06 a | 3.2 ± 0.03 b |
| S15 | nd | nd | 0.68 ± 0.01 b | 0.63 ± 0.02 a | 1.8 ± 0.05 a | 2.0 ± 0.03 b |
| S6 | nd | nd | 0.55 ± 0.02 b | 0.28 ± 0.02 a | 0.92 ± 0.01 a | 1.1 ± 0.01 b |
| S13 | nd | nd | 0.14 ± 0.01 b | 0.10 ± 0.01 a | 0.24 ± 0.01 a | 0.26 ± 0.02 b |
| S14 | nd | nd | 0.11 ± 0.01 b | 0.060 ± 0.002 a | 0.28 ± 0.02 a | 0.29 ± 0.02 b |
| S10 | nd | nd | 0.025 ± 0.02 b | 0.010 ± 0.001 a | 0.18 ± 0.02 a | 0.18 ± 0.01 b |
| US | nd | nd | 0.010 ± 0.01 a | 0.010 ± 0.001 a | 0.014 ± 0.001 a | 0.014 ± 0.001 a |
| Sample | 2018 | 2022 | ||||
|---|---|---|---|---|---|---|
| Roots | Shoots | TF | Roots | Shoots | TF | |
| S1 | 19.50 ± 1.64 b | 15.96 ± 1.33 b | 0.82 | 15.40 ± 1.30 a | 11.74 ± 1.6 a | 0.76 |
| S4 | 18.01 ± 1.22 b | 15.03 ± 1.08 b | 0.83 | 14.10 ± 1.14 a | 11.05 ± 0.95 a | 0.78 |
| S2 | 16.93 ± 1.56 b | 13.39 ± 1.15 b | 0.79 | 12.60 ± 1.04 a | 9.85 ± 0.82 a | 0.78 |
| S5 | 12.10 ± 0.84 b | 9.57 ± 0.59 b | 0.79 | 8.72 ± 0.74 a | 7.04 ± 0.70 a | 0.81 |
| S7 | 10.08 ± 0.65 b | 8.21 ± 0.51 b | 0.81 | 7.68 ± 0.86 a | 6.03 ± 0.53 a | 0.79 |
| S11 | 8.51 ± 0.69 b | 5.74 ± 0.34 b | 0.67 | 6.35 ± 0.46 a | 4.22 ± 0.50 a | 0.66 |
| S12 | 8.07 ± 0.51 b | 5.55 ± 0.45 b | 0.69 | 6.00 ± 0.46 a | 4.08 ± 0.31 a | 0.68 |
| S8 | 7.09 ± 0.40 b | 4.89 ± 0.29 b | 0.69 | 5.48 ± 0.33 a | 3.60 ± 0.22 a | 0.66 |
| S3 | 5.41 ± 0.36 b | 3.60 ± 0.24 b | 0.67 | 3.93 ± 0.26 a | 2.65 ± 0.16 a | 0.67 |
| S9 | 4.72 ± 0.38 b | 2.94 ± 0.11 b | 0.62 | 3.25 ± 0.25 a | 2.16 ± 0.22 a | 0.67 |
| S15 | 4.50 ± 0.21 b | 1.91 ± 0.09 b | 0.42 | 3.15 ± 0.18 a | 1.41 ± 0.07 a | 0.45 |
| S6 | 1.98 ± 0.06 b | 0.81 ± 0.01 b | 0.41 | 1.45 ± 0.04 a | 0.59 ± 0.03 a | 0.41 |
| S13 | 0.75 ± 0.05 b | 0.35 ± 0.02 b | 0.47 | 0.63 ± 0.02 a | 0.26 ± 0.01 a | 0.41 |
| S14 | 0.70 ± 0.04 b | 0.30 ± 0.02 b | 0.43 | 0.58 ± 0.03 a | 0.22 ± 0.01 a | 0.38 |
| S10 | 0.61 ± 0.04 b | 0.24 ± 0.03 b | 0.40 | 0.49 ± 0.02 a | 0.18 ± 0.02 a | 0.36 |
| US | 0.08 ± 0.005 a | 0.03 ± 0.005 a | 0.38 | 0.07 ± 0.005 a | 0.024 ± 0.002 a | 0.40 |
| Sample | Year 2018 | Year 2022 | ||
|---|---|---|---|---|
| Total Uptake | % Removal | Total Uptake | % Removal | |
| S1 | 5.32 | 0.020 | 4.97 | 0.019 |
| S4 | 5.48 | 0.027 | 5.21 | 0.026 |
| S2 | 5.12 | 0.034 | 5.16 | 0.034 |
| S5 | 3.93 | 0.036 | 3.81 | 0.035 |
| S7 | 3.85 | 0.041 | 3.10 | 0.033 |
| S11 | 2.93 | 0.038 | 2.23 | 0.029 |
| S12 | 2.93 | 0.044 | 2.24 | 0.034 |
| S8 | 2.67 | 0.057 | 2.08 | 0.044 |
| S3 | 2.00 | 0.056 | 1.68 | 0.047 |
| S9 | 1.74 | 0.066 | 1.43 | 0.054 |
| S15 | 1.15 | 0.064 | 0.73 | 0.041 |
| S6 | 0.50 | 0.056 | 0.33 | 0.037 |
| S13 | 0.23 | 0.061 | 0.0051 | 0.0014 |
| S14 | 0.20 | 0.060 | 0.0050 | 0.0015 |
| S10 | 0.16 | 0.059 | 0.0050 | 0.0018 |
| US | 0.024 | 0.013 | 0.0094 | 0.0050 |
| Sample | Bioaccessibility | |
|---|---|---|
| 2018 | 2022 | |
| S1 | 31.4 ± 2.30 a | 30.4 ± 2.10 a |
| S4 | 21.8 ± 1.50 a | 20.9 ± 1.10 a |
| S2 | 19.9 ± 1.15 a | 18.6 ± 1.11 a |
| S5 | 11.6 ± 0.88 a | 10.4 ± 0.91 a |
| S7 | 11.5 ± 0.86 a | 10.1 ± 0.79 a |
| S11 | 7.32 ± 0.42 a | 7.82 ± 0.35 a |
| S12 | 6.31 ± 0.38 a | 5.73 ± 0.33 a |
| S8 | 5.29 ± 0.35 a | 4.80 ± 0.37 a |
| S3 | 3.54 ± 0.29 a | 3.21 ± 0.20 a |
| S9 | 2.91 ± 0.24 a | 2.66 ± 0.26 a |
| S15 | 2.26 ± 0.27 a | 1.97 ± 0.25 a |
| S6 | 0.88 ± 0.12 a | 0.68 ± 0.13 a |
| S13 | 0.024 ± 0.08 a | 0.021 ± 0.07 a |
| S14 | 0.025 ± 0.06 a | 0.023 ± 0.05 a |
| S10 | 0.025 ± 0.01 a | 0.024 ± 0.02 a |
| US | 0.022 ± 0.01 a | 0.022 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzelli, G.; Barbafieri, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Pedron, F. Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site. Environments 2023, 10, 105. https://doi.org/10.3390/environments10060105
Petruzzelli G, Barbafieri M, Franchi E, Fusini D, Vocciante M, Pedron F. Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site. Environments. 2023; 10(6):105. https://doi.org/10.3390/environments10060105
Chicago/Turabian StylePetruzzelli, Gianniantonio, Meri Barbafieri, Elisabetta Franchi, Danilo Fusini, Marco Vocciante, and Francesca Pedron. 2023. "Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site" Environments 10, no. 6: 105. https://doi.org/10.3390/environments10060105
APA StylePetruzzelli, G., Barbafieri, M., Franchi, E., Fusini, D., Vocciante, M., & Pedron, F. (2023). Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site. Environments, 10(6), 105. https://doi.org/10.3390/environments10060105