Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water
Abstract
1. Introduction
2. Classification and Properties of PAHs
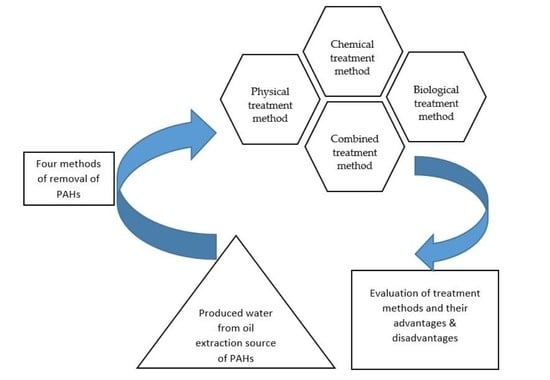
3. Methods of Removal of PAHs
3.1. Physical Treatment Method
3.2. Membrane Filtration
3.3. Floatation
3.3.1. Adsorption
3.3.2. Chemical Treatment Method
3.3.3. Chemical Precipitation
3.3.4. Chemical Oxidation
3.3.5. Electrochemical Technologies
3.3.6. Advanced Oxidation Process
3.4. Biological Treatment Method
Phytoremediation and Bioremediation
3.5. Combined Treatment Method
4. Conclusions
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
References
- Vela, N.; Martínez-Menchón, M.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. Removal of polycyclic aromatic hydrocarbons (PAHs) from groundwater by heterogeneous photocatalysis under natural sunlight. J. Photochem. Photobiol. A Chem. 2012, 232, 32–40. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Zhang, B.; Zheng, J.; Liu, B. Naphthalene degradation in seawater by UV irradiation: The effects of fluence rate, salinity, temperature and initial concentration. Mar. Pollut. Bull. 2014, 81, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Haneef, T.; Mustafa, M.R.U.; Wan Yusof, K.; Isa, M.H.; Bashir, M.J.; Ahmad, M.; Zafar, M. Removal of polycyclic aromatic hydrocarbons (PAHs) from produced water by ferrate (VI) oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef]
- Mandal, S.K.; Das, N. Microbial remediation of high molecular weight PAHs from environment: An overview. Int. J. ChemTech Res. 2015, 8, 36–43. [Google Scholar]
- Abdel-Gawad, S.A.; Baraka, A.M.; El-Shafei, M.M.; Mahmoud, A.S. Effects of nano zero valent iron and entrapped nano zero valent iron in alginate polymer on poly aromatic hydrocarbons removal. J. Environ. Biotechnol. Res. 2016, 5, 18–28. [Google Scholar]
- Rubio-Clemente, A.; Torres-Palma, R.A.; Peñuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Catalfo, A.; Finocchiaro, G.; Vagliasindi, F.G.; Romano, S.; De Guidi, G. Microwave heating coupled with UV-A irradiation for PAH removal from highly contaminated marine sediments and subsequent photo-degradation of the generated vaporized organic compounds. Chem. Eng. J. 2018, 334, 172–183. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Gaurav, G.K.; Mehmood, T.; Kumar, M.; Cheng, L.; Sathishkumar, K.; Kumar, A.; Yadav, D. Review on polycyclic aromatic hydrocarbons (PAHs) migration from wastewater. J. Contam. Hydrol. 2021, 236, 103715. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dutta, R.; Das, P. A critical review on plant biomonitors for determination of polycyclic aromatic hydrocarbons (PAHs) in air through solvent extraction techniques. Chemosphere 2020, 251, 126441. [Google Scholar] [CrossRef]
- Kargar, N.; Amani-Ghadim, A.R.; Matin, A.A.; Matin, G.; Buyukisik, H.B. Abatement efficiency and fate of EPA-Listed PAHs in aqueous medium under simulated solar and UV-C irradiations, and combined process with TiO2 and H2O2. Ege J. Fish. Aquat. Sci. 2020, 37, 15–27. [Google Scholar]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An overview and evaluation of highly porous adsorbent materials for polycyclic aromatic hydrocarbons and phenols removal from wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Ates, H.; Argun, M.E. Fate of PAHs under subcritical and supercritical conditions in landfill leachate: Removal or formation? Chem. Eng. J. 2021, 414, 128762. [Google Scholar] [CrossRef]
- Sigmund, G.; Poyntner, C.; Piñar, G.; Kah, M.; Hofmann, T. Influence of compost and biochar on microbial communities and the sorption/degradation of PAHs and NSO-substituted PAHs in contaminated soils. J. Hazard. Mater. 2018, 345, 107–113. [Google Scholar] [CrossRef]
- Amodu, O.S.; Ojumu, T.V.; Ntwampe, S.K.O. Bioavailability of high molecular weight polycyclic aromatic hydrocarbons using renewable resources. Environ. Biotechnol.-New Approaches Prospect. Appl. 2013, 171. [Google Scholar] [CrossRef]
- Tian, W.; Bai, J.; Liu, K.; Sun, H.; Zhao, Y. Occurrence and removal of polycyclic aromatic hydrocarbons in the wastewater treatment process. Ecotoxicol. Environ. Saf. 2012, 82, 1–7. [Google Scholar] [CrossRef]
- Klemz, A.C.; Weschenfelder, S.E.; de Carvalho Neto, S.L.; Damas, M.S.P.; Viviani, J.C.T.; Mazur, L.P.; Marinho, B.A.; dos Santos Pereira, L.; da Silva, A.; Valle, J.A.B.; et al. Oilfield produced water treatment by liquid-liquid extraction: A review. J. Pet. Sci. Eng. 2021, 199, 108282. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R. Green one-spot synthesis of hydrochar supported zero-valent iron for heterogeneous Fenton-like discoloration of dyes at neutral pH. J. Mol. Liq. 2020, 320, 114421. [Google Scholar] [CrossRef]
- Saeed, M.O.; Azizli, K.; Isa, M.H.; Bashir, M.J. Application of CCD in RSM to obtain optimize treatment of POME using Fenton oxidation process. J. Water Process Eng. 2015, 8, e7–e16. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Dong, J.; Zhao, J.; Gong, X.; Liu, S. Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol. Environ. Saf. 2018, 149, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, B.; Zhang, B.; Song, X.; Zeng, G.; Lee, K. Photocatalytic ozonation of offshore produced water by TiO2 nanotube arrays coupled with UV-LED irradiation. J. Hazard. Mater. 2021, 402, 123456. [Google Scholar] [CrossRef]
- Qi, Y.B.; Wang, C.Y.; Lv, C.Y.; Lun, Z.M.; Zheng, C.G. Removal capacities of polycyclic aromatic hydrocarbons (PAHs) by a newly isolated strain from oilfield produced water. Int. J. Environ. Res. Public Health 2017, 14, 215. [Google Scholar] [CrossRef]
- Mortazavi, M.; Baghdadi, M.; Javadi, N.H.S.; Torabian, A. The black beads produced by simultaneous thermal reducing and chemical bonding of graphene oxide on the surface of amino-functionalized sand particles: Application for PAHs removal from contaminated waters. J. Water Process Eng. 2019, 31, 100798. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Comfort, S.; Chokejaroenrat, C.; Harris, C.; Li, X. A combined chemical and biological approach to transforming and mineralizing PAHs in runoff water. Chemosphere 2014, 117, 1–9. [Google Scholar] [CrossRef]
- Lai, X.; Ning, X.A.; Zhang, Y.; Li, Y.; Li, R.; Chen, J.; Wu, S. Treatment of simulated textile sludge using the Fenton/Cl− system: The roles of chlorine radicals and superoxide anions on PAHs removal. Environ. Res. 2021, 197, 110997. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Wick, L.Y.; Duran, R. Fungi in PAH-contaminated marine sediments: Cultivable diversity and tolerance capacity towards PAH. Mar. Pollut. Bull. 2021, 164, 112082. [Google Scholar] [CrossRef]
- Sbani, N.H.A.; Abdullah, S.R.S.; Idris, M.; Hasan, H.A.; Halmi, M.I.E.; Jehawi, O.H. PAH-degrading rhizobacteria of Lepironia articulata for phytoremediation enhancement. J. Water Process Eng. 2021, 39, 101688. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhang, D.; Feng, T.; Zhang, L. Nitrate-assisted biodegradation of polycyclic aromatic hydrocarbons (PAHs) in the water-level-fluctuation zone of the three Gorges Reservoir, China: Insights from in situ microbial interaction analyses and a microcosmic experiment. Environ. Pollut. 2021, 268, 115693. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Wang, L.; Zhao, J.; Song, T.; Chu, M. Removal of high-molecular-weight polycyclic aromatic hydrocarbons by a microbial consortium immobilized in magnetic floating biochar gel beads. Mar. Pollut. Bull. 2020, 159, 111489. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Liao, X. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, C.; Wang, Z.; Zhao, S.; Fan, X.; Jia, H. Performance and potential mechanism of transformation of polycyclic aromatic hydrocarbons (PAHs) on various iron oxides. J. Hazard. Mater. 2021, 403, 123993. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Li, S.; Zhu, G. Air-assisted liquid-liquid microextraction based on the solidification of floating deep eutectic solvents for the simultaneous determination of bisphenols and polycyclic aromatic hydrocarbons in tea infusions via HPLC. Food Chem. 2021, 348, 129106. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Wang, X.; Wei, C. Efficient adsorption of phenanthrene by simply synthesized hydrophobic MCM-41 molecular sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Chowdhury, Z.Z.; Shemsi, A.M.; Saleh, T.A. Effect of the oxidation process on the molecular interaction of polyaromatic hydrocarbons (PAH) with carbon nanotubes: Adsorption kinetic and isotherm study. J. Mol. Liq. 2019, 289, 111107. [Google Scholar] [CrossRef]
- Hung, C.M.; Huang, C.P.; Lam, S.S.; Chen, C.W.; Dong, C.D. The removal of polycyclic aromatic hydrocarbons (PAHs) from marine sediments using persulfate over a nano-sized iron composite of magnetite and carbon black activator. J. Environ. Chem. Eng. 2020, 8, 104440. [Google Scholar] [CrossRef]
- Wickramasinghe, A.D.; Shukla, S.P. Performance evaluation of a pellet based column bed for removal of a potentially carcinogenic Polycyclic Aromatic Hydrocarbon (PAH) from water. J. Environ. Chem. Eng. 2018, 6, 6012–6020. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Modified μ-QuEChERS coupled to diethyl carbonate-based liquid microextraction for PAHs determination in coffee, tea, and water prior to GC–MS analysis: An insight to reducing the impact of caffeine on the GC–MS measurement. J. Chromatogr. B 2021, 1171, 122555. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Investigating the degradation of nC12 to nC23 alkanes and PAHs in petroleum-contaminated water by electrochemical advanced oxidation process using an inexpensive Ti/Sb-SnO2/PbO2 anode. Chem. Eng. J. 2021, 404, 125268. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Qari, H.; Basahi, J.M.A.B.; Godon, J.J.; Dhavamani, J. Role of a halothermophilic bacterial consortium for the biodegradation of PAHs and the treatment of petroleum wastewater at extreme conditions. Int. Biodeterior. Biodegrad. 2017, 121, 44–54. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Chen, J.; Huang, Y.; Lu, H.; Yuan, W.; Yang, Q.; Hu, J.; Yu, B.; Wang, D.; et al. Physical pretreatment of petroleum refinery wastewater instead of chemicals addition for collaborative removal of oil and suspended solids. J. Clean. Prod. 2021, 278, 123821. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Hang, X.; Zhao, S.; Wan, Y. Removal of polycyclic aromatic hydrocarbons by nanofiltration membranes: Rejection and fouling mechanisms. J. Membr. Sci. 2019, 582, 264–273. [Google Scholar] [CrossRef]
- González-Pérez, D.M.; Garralón, G.; Plaza, F.; Pérez, J.I.; Moreno, B.; Gómez, M.A. Removal of low concentrations of phenanthrene, fluoranthene and pyrene from urban wastewater by membrane bioreactors technology. J. Environ. Scie 2012, 47, 2190–2197. [Google Scholar] [CrossRef]
- Hosseini, P.K.; Liu, L.; Hosseini, M.K.; Bhattacharyya, A.; Miao, J.; Wang, F. Treatment of a synthetic decanted oily seawater in a pilot-scale hollow fiber membrane filtration process: Experimental investigation. J. Hazard. Mater. 2023, 441, 129928. [Google Scholar] [CrossRef]
- Fatone, F.; Di Fabio, S.; Bolzonella, D.; Cecchi, F. Fate of aromatic hydrocarbons in Italian municipal wastewater systems: An overview of wastewater treatment using conventional activated-sludge processes (CASP) and membrane bioreactors (MBRs). Water Res. 2011, 45, 93–104. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M.; Mielczarek, K.; Bohdziewicz, J.; Włóka, D. The use of reverse osmosis in the removal of PAHs from municipal landfill leachate. Polycycl. Aromat. Compd. 2016, 36, 20–39. [Google Scholar] [CrossRef]
- Gong, C.; Huang, H.; Qian, Y.; Zhang, Z.; Wu, H. Integrated electrocoagulation and membrane filtration for PAH removal from realistic industrial wastewater: Effectiveness and mechanisms. Rsc Adv. 2017, 7, 52366–52374. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M. Effectiveness in the removal of Polycyclic Aromatic Hydrocarbons from industrial wastewater by ultrafiltration technique. Arch. Environ. Prot. 2012, 38, 49–58. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, H.H.; Wang, L.; Guo, X.F. Study of effects of ionic strength and pH on PAHs removal by nanofiltration. In Proceedings of the 2nd Annual Congress on Advanced Engineering and Technology II (CAET 2015), Hong Kong, China, 4–5 April 2015; pp. 4–5. [Google Scholar]
- Chen, X.; Huang, G.; An, C.; Feng, R.; Wu, Y.; Huang, C. Superwetting polyethersulfone membrane functionalized with ZrO2 nanoparticles for polycyclic aromatic hydrocarbon removal. J. Mater. Sci. Technol. 2022, 98, 14–25. [Google Scholar] [CrossRef]
- da Silva, S.S.; Chiavone-Filho, O.; de Barros Neto, E.L.; Foletto, E.L. Oil removal from produced water by conjugation of flotation and photo-Fenton processes. J. Environ. Manag. 2015, 147, 257–263. [Google Scholar] [CrossRef]
- Moosai, R.; Dawe, R.A. Gas attachment of oil droplets for gas flotation for oily wastewater cleanup. Sep. Purif. Technol. 2003, 33, 303–314. [Google Scholar] [CrossRef]
- Guerra, K.; Dahm, K.; Dundorf, S. Oil and Gas Produced Water Management and Beneficial Use in the Western United States; US Department of the Interior, Bureau of Reclamation: Washington, DC, USA, 2011; pp. 1–113.
- Consulting, A.L.L. Handbook on coal bed methane produced water: Management and beneficial use alternatives. In Prepared for: Groundwater Protection Research Foundation; US Department of Energy, National Petroleum Technology Ofce, Bureau of Land Management: Washington, DC, USA, 2003. [Google Scholar]
- Beyer, A.H.; Palmer, L.L.; Stock, J. Biological oxidation of dissolved compounds in oilfield-produced water by a pilot aerated lagoon. J. Pet. Technol. 1979, 31, 241–245. [Google Scholar] [CrossRef]
- Chebbi, S.; Allouache, A.; Schwarz, M.; Belkacemi, H.; Merabet, D. Treating Produced Water Using Induced Air Flotation: The Effect of Ethanol on Conditioning and Flotation of PAHs in the Presence of Tween 80. Pol. J. Environ. Stud. 2019, 28, 1–9. [Google Scholar] [CrossRef]
- Yahya, M.S.; Lau, E.V. Graphene oxide (GO)-coated microbubble flotation for polycyclic aromatic hydrocarbon (PAH) removal from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106508. [Google Scholar] [CrossRef]
- Pal, P.; Corpuz, A.G.; Hasan, S.W.; Sillanpää, M.; Banat, F. Treatment of polycyclic aromatic hydrocarbons (PAHs) from aqueous solutions by flotation using colloidal gas aphrons. Sep. Purif. Technol. 2022, 285, 120367. [Google Scholar] [CrossRef]
- Kumar, J.A.; Amarnath, D.J.; Jabasingh, S.A.; Kumar, P.S.; Anand, K.V.; Narendrakumar, G.; Namasivayam, S.K.R.; Krithiga, T.; Sunny, S.; Pushkala, S.P.; et al. One pot Green Synthesis of Nano magnesium oxide-carbon composite: Preparation, characterization and application towards anthracene adsorption. J. Clean. Prod. 2019, 237, 117691. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K.; Vankayala, R.; Kalluru, P.; Pamanji, S.R. Bioinspired reduced graphene oxide nanosheets using Terminalia chebula seeds extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Simultaneous removal of multiple polycyclic aromatic hydrocarbons (PAHs) from urban stormwater using low-cost agricultural/industrial byproducts as sorbents. Chemosphere 2021, 274, 129812. [Google Scholar] [CrossRef]
- Cheng, H.; Bian, Y.; Wang, F.; Jiang, X.; Ji, R.; Gu, C.; Yang, X.; Song, Y. Green conversion of crop residues into porous carbons and their application to efficiently remove polycyclic aromatic hydrocarbons from water: Sorption kinetics, isotherms, and mechanism. Bioresour. Technol. 2019, 284, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Abdel-Shafy, H.I.; Mansour, M.S. Removal of pyrene and benzo (a) pyrene micropollutant from the water via adsorption by green synthesized iron oxide nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015006. [Google Scholar] [CrossRef]
- Xi, Z.; Chen, B. Removal of polycyclic aromatic hydrocarbons from aqueous solution by raw and modified plant residue materials as biosorbents. J. Environ. Sci. 2014, 26, 737–748. [Google Scholar] [CrossRef]
- Radwan, A.M.Y.; Magram, S.F.; Zubair, A. Adsorption of acenaphthene using date seed activated carbon. J. Environ. Sci. Technol. 2018, 11, 10–15. [Google Scholar] [CrossRef]
- Afsheen, S.; Tahir, M.B.; Iqbal, T.; Liaqat, A.; Abrar, M. Green synthesis and characterization of novel iron particles by using different extracts. J. Alloys Compd. 2018, 732, 935–944. [Google Scholar] [CrossRef]
- Shanker, U.; Jassal, V.; Rani, M. Green synthesis of iron hexacyanoferrate nanoparticles: Potential candidate for the degradation of toxic PAHs. J. Environ. Chem. Eng. 2017, 5, 4108–4120. [Google Scholar] [CrossRef]
- Han, N.; Wang, S.; Rana, A.K.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Long, J.; Thakur, V.K.; Zhao, X. Rational design of boron nitride with different dimensionalities for sustainable applications. Renew. Sustain. Energy Rev. 2022, 170, 112910. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Metal oxide-chitosan based nanocomposites for efficient degradation of carcinogenic PAHs. J. Environ. Chem. Eng. 2020, 8, 103810. [Google Scholar] [CrossRef]
- Abbasi, M.; Saeed, F.; Rafique, U. Preparation of silver nanoparticles from synthetic and natural sources: Remediation model for PAHs. IOP Conf. Ser. Mater. Sci. Eng. 2014, 60, 012061. [Google Scholar] [CrossRef]
- Adeola, A.O.; Forbes, P.B. Advances in water treatment technologies for removal of polycyclic aromatic hydrocarbons: Existing concepts, emerging trends, and future prospects. Water Environ. Res. 2021, 93, 343–359. [Google Scholar] [CrossRef]
- Abbas, S.; Nasreen, S.; Haroon, A.; Ashraf, M.A. Synhesis of silver and copper nanoparticles from plants and application as adsorbents for naphthalene decontamination. Saudi J. Biol. Sci. 2020, 27, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Crisafully, R.; Milhome, M.A.L.; Cavalcante, R.M.; Silveira, E.R.; De Keukeleire, D.; Nascimento, R.F. Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin. Bioresour. Technol. 2008, 99, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total Environ. 2014, 466, 210–213. [Google Scholar] [CrossRef]
- Martínez-Cabanas, M.; López-García, M.; Barriada, J.L.; Herrero, R.; de Vicente, M.E.S. Green synthesis of iron oxide nanoparticles. Development of magnetic hybrid materials for efficient As (V) removal. Chem. Eng. J. 2016, 301, 83–91. [Google Scholar] [CrossRef]
- Prasad, K.S.; Gandhi, P.; Selvaraj, K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As (III) and As (V) from aqueous solution. Appl. Surf. Sci. 2014, 317, 1052–1059. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Heterogeneous Fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles. J. Colloid Interface Sci. 2013, 410, 67–73. [Google Scholar] [CrossRef]
- Rafique, M.; Shafiq, F.; Gillani, S.S.A.; Shakil, M.; Tahir, M.B.; Sadaf, I. Eco-friendly green and biosynthesis of copper oxide nanoparticles using Citrofortunella microcarpa leaves extract for efficient photocatalytic degradation of Rhodamin B dye form textile wastewater. Optik 2020, 208, 164053. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, M.B.; Irshad, M.; Nabi, G.; Gillani, S.S.A.; Iqbal, T.; Mubeen, M. Novel Citrus aurantifolia leaves based biosynthesis of copper oxide nanoparticles for environmental and wastewater purification as an efficient photocatalyst and antibacterial agent. Optik 2020, 219, 165138. [Google Scholar] [CrossRef]
- Prasad, C.; Yuvaraja, G.; Venkateswarlu, P. Biogenic synthesis of Fe3O4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and Methyl orange dye degradation studies. J. Magn. Magn. Mater. 2017, 424, 376–381. [Google Scholar] [CrossRef]
- Harshiny, M.; Iswarya, C.N.; Matheswaran, M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. 2015, 286, 744–749. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef]
- Prasad, C.; Karlapudi, S.; Venkateswarlu, P.; Bahadur, I.; Kumar, S. Green arbitrated synthesis of Fe3O4 magnetic nanoparticles with nanorod structure from pomegranate leaves and Congo red dye degradation studies for water treatment. J. Mol. Liq. 2017, 240, 322–328. [Google Scholar] [CrossRef]
- Rao, A.; Bankar, A.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles. J. Contam. Hydrol. 2013, 146, 63–73. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Kumar, B.N.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys. B Condens. Matter 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Raj, G.A. Bio-approach: Plant mediated synthesis of ZnO nanoparticles and their catalytic reduction of methylene blue and antimicrobial activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Photocatalytic degradation of toxic phenols from water using bimetallic metal oxide nanostructures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 546–561. [Google Scholar] [CrossRef]
- Weng, X.; Lin, Z.; Xiao, X.; Li, C.; Chen, Z. One-step biosynthesis of hybrid reduced graphene oxide/iron-based nanoparticles by eucalyptus extract and its removal of dye. J. Clean. Prod. 2018, 203, 22–29. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M. Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions. Chin. J. Chem. Eng. 2017, 25, 924–930. [Google Scholar] [CrossRef]
- Prasad, A.S. Iron oxide nanoparticles synthesized by controlled bio-precipitation using leaf extract of Garlic Vine (Mansoa alliacea). Mater. Sci. Semicond. Process. 2016, 53, 79–83. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Kumar, K.S.; Reddy, P.S.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Prasad, T.N.V.K.V.; Reddy, A.V.B.; Reddy, B.R.; Madhavi, G. Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jothi, V.K.; Natarajan, A.; Rajaram, A.; Ravichandran, S.; Ramalingam, S. Green synthesis of Copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab. J. Chem. 2020, 13, 6802–6814. [Google Scholar] [CrossRef]
- Pandey, N.; Gusain, R.; Suthar, S. Exploring the efficacy of powered guar gum (Cyamopsis tetragonoloba) seeds, duckweed (Spirodela polyrhiza), and Indian plum (Ziziphus mauritiana) leaves in urban wastewater treatment. J. Clean. Prod. 2020, 264, 121680. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (Kw). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Nazir, N.A.M.; Raoov, M.; Mohamad, S. Spent tea leaves as an adsorbent for micro-solid-phase extraction of polycyclic aromatic hydrocarbons (PAHs) from water and food samples prior to GC-FID analysis. Microchem. J. 2020, 159, 105581. [Google Scholar] [CrossRef]
- Muthukumar, H.; Shanmugam, M.K.; Gummadi, S.N. Caffeine degradation in synthetic coffee wastewater using silverferrite nanoparticles fabricated via green route using Amaranthus blitum leaf aqueous extract. J. Water Process Eng. 2020, 36, 101382. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 801–804. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Kadir, D.H.; Balaky, S.M.; Perot, E.M. An Eco-friendly nanocatalyst for removal of some poisonous environmental pollutions and statistically evaluation of its performance. Surf. Interfaces 2021, 23, 100908. [Google Scholar] [CrossRef]
- Halpegama, J.U.; Bandara, P.M.C.J.; Jayarathna, L.; Bandara, A.; Yeh, C.Y.; Chen, J.Y.; Kuss, C.; Dahanayake, U.; Herath, A.C.; Weragoda, S.K.; et al. Facile fabrication of nano zerovalent iron–Reduced graphene oxide composites for nitrate reduction in water. Environ. Adv. 2021, 3, 100024. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Dolan, F.C.; Cath, T.Y.; Hogue, T.S. Assessing the feasibility of using produced water for irrigation in Colorado. Sci. Total Environ. 2018, 640, 619–628. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- de Jesús Treviño-Reséndez, J.; Medel, A.; Meas, Y. Electrochemical technologies for treating petroleum industry wastewater. Curr. Opin. Electrochem. 2021, 27, 100690. [Google Scholar] [CrossRef]
- Nyström, F.; Nordqvist, K.; Herrmann, I.; Hedström, A.; Viklander, M. Removal of metals and hydrocarbons from stormwater using coagulation and flocculation. Water Research 2020, 182, 115919. [Google Scholar] [CrossRef]
- Shabeer, T.A.; Saha, A.; Gajbhiye, V.T.; Gupta, S.; Manjaiah, K.M.; Varghese, E. Removal of poly aromatic hydrocarbons (PAHs) from water: Effect of nano and modified nano-clays as a flocculation aid and adsorbent in coagulation-flocculation. Polycycl. Aromat. Compd. 2014, 34, 452–467. [Google Scholar] [CrossRef]
- Gong, C.; Shen, G.; Huang, H.; He, P.; Zhang, Z.; Ma, B. Removal and transformation of polycyclic aromatic hydrocarbons during electrocoagulation treatment of an industrial wastewater. Chemosphere 2017, 168, 58–64. [Google Scholar] [CrossRef]
- Rosińska, A.; Dąbrowska, L. Influence of type and dose of coagulants on effectiveness of PAH removal in coagulation water treatment. Water Sci. Eng. 2021, 14, 193–200. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, W.K.; Hu, X.J.; Ma, X.H.; Sun, Y.J.; Wen, Y. Oxidative degradation of polycyclic aromatic hydrocarbons in contaminated industrial soil using chlorine dioxide. Chem. Eng. J. 2020, 394, 124857. [Google Scholar] [CrossRef]
- Brown, G.S.; Barton, L.L.; Thomson, B.M. Permanganate oxidation of sorbed polycyclic aromatic hydrocarbons. Waste Manag. 2003, 23, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Makuła, M. Changes of PAHs content in wastewater during oxidation process. Rocz. Ochr. Sr. 2011, 13, 1093–1104. [Google Scholar]
- Chen, Y.; Sun, Z.; Yang, Y.; Ke, Q. Heterogeneous photocatalytic oxidation of polyvinyl alcohol in water. J. Photochem. Photobiol. A Chem. 2001, 142, 85–89. [Google Scholar] [CrossRef]
- Ehssan, M.R.N. Removal of polyaromatic hydrocarbons from waste water by electrocoagulation. J. Pet. Gas Eng. 2014, 5, 32–42. [Google Scholar]
- Herrada, R.A.; Medel, A.; Manríquez, F.; Sirés, I.; Bustos, E. Preparation of IrO2-Ta2O5|Ti electrodes by immersion, painting and electrophoretic deposition for the electrochemical removal of hydrocarbons from water. J. Hazard. Mater 2016, 319, 102–110. [Google Scholar] [CrossRef]
- Yaqub, A.; Isa, M.H.; Ajab, H.; Kutty, S.R.; Ezechi, E.H. Polycyclic aromatic hydrocarbons removal from produced water by electrochemical process optimization. Ecol. Chem. Eng. S 2017, 24, 397–404. [Google Scholar] [CrossRef]
- Ajab, H.; Isa, M.H.; Yaqub, A. Electrochemical oxidation using Ti/RuO2 anode for COD and PAHs removal from aqueous solution. Sustain. Mater. Technol. 2020, 26, e00225. [Google Scholar] [CrossRef]
- Herrada, R.A.; Acosta-Santoyo, G.; Sepúlveda-Guzmán, S.; Brillas, E.; Sirés, I.; Bustos, E. IrO2-Ta2O5|Ti electrodes prepared by electrodeposition from different Ir: Ta ratios for the degradation of polycyclic aromatic hydrocarbons. Electrochim. Acta 2018, 263, 353–361. [Google Scholar] [CrossRef]
- Treviño-Reséndez, J.D.J.; Mijaylova Nacheva, P.; García-Espinoza, J.D. Influencia de los parámetros de operación en la degradación de naftaleno y fenantreno mediante electrooxidación. Rev. Int. De Contam. Ambient. 2020, 36, 2. [Google Scholar] [CrossRef]
- Han, N.; Feng, S.; Guo, W.; Mora, O.M.; Zhao, X.; Zhang, W.; Xie, S.; Zhou, Z.; Liu, Z.; Liu, Q.; et al. Rational design of Ruddlesden–Popper perovskite electrocatalyst for oxygen reduction to hydrogen peroxide. SusMat 2022, 2, 456–465. [Google Scholar] [CrossRef]
- Butkovskyi, A.; Bruning, H.; Kools, S.A.; Rijnaarts, H.H.; Van Wezel, A.P. Organic pollutants in shale gas flowback and produced waters: Identification, potential ecological impact, and implications for treatment strategies. Environ. Sci. Technol. 2017, 51, 4740–4754. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Duan, X.; Wang, S.; Wang, Y. Carbocatalytic ozonation toward advanced water purification. J. Mater. Chem. A 2021, 9, 18994–19024. [Google Scholar] [CrossRef]
- Ke, Y.; Ning, X.A.; Liang, J.; Zou, H.; Sun, J.; Cai, H.; Lin, M.; Li, R.; Zhang, Y. Sludge treatment by integrated ultrasound-Fenton process: Characterization of sludge organic matter and its impact on PAHs removal. J. Hazard. Mater. 2018, 343, 191–199. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, W.; Yuan, M.; Feng, C.; Ren, Y.; Wei, C. Degradation of polycyclic aromatic hydrocarbons in a coking wastewater treatment plant residual by an O3/ultraviolet fluidized bed reactor. Environ. Sci. Pollut. Res. 2014, 21, 10329–10338. [Google Scholar] [CrossRef]
- Vilhunen, S.; Vilve, M.; Vepsäläinen, M.; Sillanpää, M. Removal of organic matter from a variety of water matrices by UV photolysis and UV/H2O2 method. J. Hazard. Mater. 2010, 179, 776–782. [Google Scholar] [CrossRef]
- Shemer, H.; Linden, K.G. Aqueous photodegradation and toxicity of the polycyclic aromatic hydrocarbons fluorene, dibenzofuran, and dibenzothiophene. Water Res. 2007, 41, 853–861. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of polycyclic aromatic hydrocarbons in a heterogeneous Fenton like oxidation system using nanoscale zero-valent iron as a catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Vaferi, B.; Bahmani, M.; Keshavarz, P.; Mowla, D. Experimental and theoretical analysis of the UV/H2O2 advanced oxidation processes treating aromatic hydrocarbons and MTBE from contaminated synthetic wastewaters. J. Environ. Chem. Eng. 2014, 2, 1252–1260. [Google Scholar] [CrossRef]
- Ates, H.; Argun, M.E. Removal of PAHs from leachate using a combination of chemical precipitation and Fenton and ozone oxidation. Water Sci. Technol. 2018, 78, 1064–1070. [Google Scholar] [CrossRef]
- Isaac, P.; Martínez, F.L.; Bourguignon, N.; Sánchez, L.A.; Ferrero, M.A. Improved PAHs removal performance by a defined bacterial consortium of indigenous Pseudomonas and actinobacteria from Patagonia, Argentina. Int. Biodeterior. Biodegrad. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Janbandhu, A.; Fulekar, M.H. Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J. Hazard. Mater. 2011, 187, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Pasandideh, Y.; Razmi, H. Introduction of a biowaste/graphene oxide nanocomposite as a coating for a metal alloy based SPME fiber: Application to screening of polycyclic aromatic hydrocarbons. Arab. J. Chem. 2020, 13, 8499–8512. [Google Scholar] [CrossRef]
- Lu, X.Y.; Li, B.; Zhang, T.; Fang, H.H. Enhanced anoxic bioremediation of PAHs-contaminated sediment. Bioresour. Technol. 2012, 104, 51–58. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Bioremediation potential of natural polyphenol rich green wastes: A review of current research and recommendations for future directions. Environ. Technol. Innov. 2015, 4, 17–28. [Google Scholar] [CrossRef]
- Lu, L.; Chai, Q.; He, S.; Yang, C.; Zhang, D. Effects and mechanisms of phytoalexins on the removal of polycyclic aromatic hydrocarbons (PAHs) by an endophytic bacterium isolated from ryegrass. Environ. Pollut. 2019, 253, 872–881. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.H.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Parrish, Z.D.; White, J.C.; Isleyen, M.; Gent, M.P.; Iannucci-Berger, W.; Eitzer, B.D.; Kelsey, J.W.; Mattina, M.I. Accumulation of weathered polycyclic aromatic hydrocarbons (PAHs) by plant and earthworm species. Chemosphere 2006, 64, 609–618. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Xie, H.; Hu, Z.; Liang, S.; Ngo, H.H.; Guo, W.; Chen, X.; Fan, J.; Zhao, C. Intensive removal of PAHs in constructed wetland filled with copper biochar. Ecotoxicol. Environ. Saf. 2020, 205, 111028. [Google Scholar] [CrossRef]
- Dominguez, J.J.A.; Bacosa, H.P.; Chien, M.F.; Inoue, C. Enhanced degradation of polycyclic aromatic hydrocarbons (PAHs) in the rhizosphere of sudangrass (Sorghum× drummondii). Chemosphere 2019, 234, 789–795. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, M.; Dureja, P.; Sarma, P.M.; Lal, B. Bioelectrochemical approaches for removal of sulfate, hydrocarbon and salinity from produced water. Chemosphere 2017, 166, 96–108. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced water treatment: Application of air gap membrane distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Wang, M.; He, W.; Hua, Y.; Xie, X.; Chen, S.; Zhou, L.; Zhang, Y.; Hou, Y.; Lin, S.; Xia, H.; et al. Alternative photothermal/electrothermal hierarchical membrane for hypersaline water treatment. SusMat 2022, 2, 679–688. [Google Scholar] [CrossRef]
- Dowaidar, A.M.; El-Shahawi, M.S.; Ashour, I. Adsorption of polycyclic aromatic hydrocarbons onto activated carbon from non-aqueous media: 1. the influence of the organic solvent polarity. Sep. Sci. Technol. 2007, 42, 3609–3622. [Google Scholar] [CrossRef]
- Hidayat, D.; Supriyanto, R.; Permana, D.F. Adsorption of polycyclic aromatic hydrocarbons using low-cost activated carbon derived from rice husk. J. Phys. Conf. Ser. 2019, 1338, 012005. [Google Scholar]
- Millar, G.J.; Lin, J.; Arshad, A.; Couperthwaite, S.J. Evaluation of electrocoagulation for the pre-treatment of coal seam water. J. Water Process Eng. 2014, 4, 166–178. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef]
- Bisht, S.; Pandey, P.; Bhargava, B.; Sharma, S.; Kumar, V.; Sharma, K.D. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz. J. Microbiol. 2015, 46, 7–21. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Riley, S.M.; Oliveira, J.M.; Regnery, J.; Cath, T.Y. Hybrid membrane bio-systems for sustainable treatment of oil and gas produced water and fracturing flowback water. Sep. Purif. Technol. 2016, 171, 297–311. [Google Scholar] [CrossRef]
- Riley, S.M.; Ahoor, D.C.; Regnery, J.; Cath, T.Y. Tracking oil and gas wastewater-derived organic matter in a hybrid biofilter membrane treatment system: A multi-analytical approach. Sci. Total Environ. 2018, 613, 208–217. [Google Scholar] [CrossRef] [PubMed]

| Name of LMW PAHs | Abbreviation | Formula | Structure | Molecular Weight (g/mol) | Source |
|---|---|---|---|---|---|
| Acenaphthylene | ACY | C12 H8 | 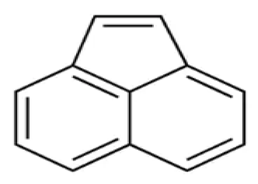 | 152.1 | [9] |
| Acenaphthene | ACE | C12 H10 | 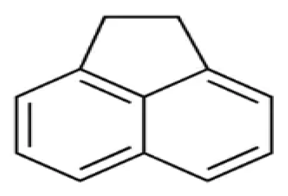 | 154.2 | [10] |
| Anthracene | ANT | C14 H10 |  | 178.2 | [11] |
| Fluorene | FL | C13 H10 |  | 166.2 | [12] |
| Naphthalene | NAP | C10 H8 | 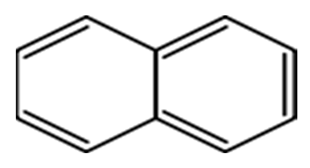 | 128.91 | [13] |
| Phenanthrene | PHE | C14 H10 | 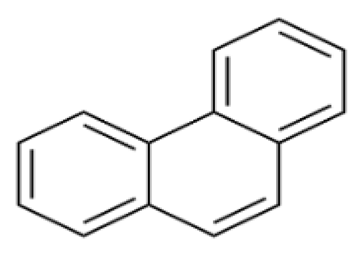 | 174.2 | [8] |
| Name of HMW PAHs | Abbreviation | Formula | Structure | Molecular Weight (g/mol) | Source |
|---|---|---|---|---|---|
| Benzo (a) anthracene | BaA | C18 H12 |  | 228.3 | [14] |
| Benzo (b) fluoranthene | BbF | C20 H12 | 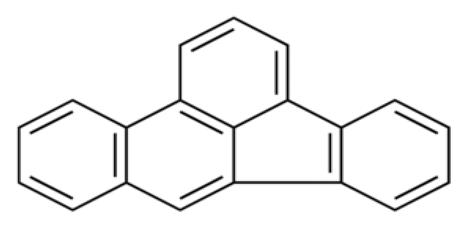 | 252.3 | [14] |
| Benzo (k) fluoranthene | BkF | C20 H12 | 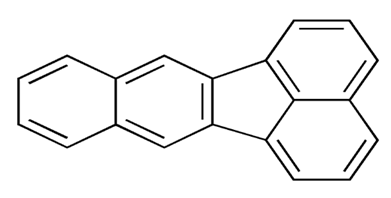 | 252.3 | [13] |
| Benzo (a) pyrene | BaP | C20 H12 | 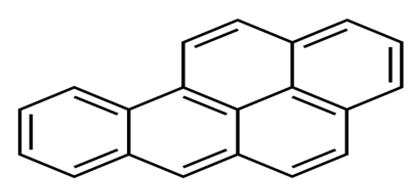 | 252.3 | [15] |
| Benzo (ghi) perylene | BhP | C22 H12 |  | 276.3 | [16] |
| Chrysene | CHY | C18 H12 |  | 228.2 | [17] |
| Dibenz (a,h) anthracene | DahA | C22 H14 |  | 278.3 | [9] |
| Fluoranthene | FLU | C16 H10 |  | 202.2 | [11] |
| Indeno (1,2,3-cd) pyrene | IcdP | C22 H12 | 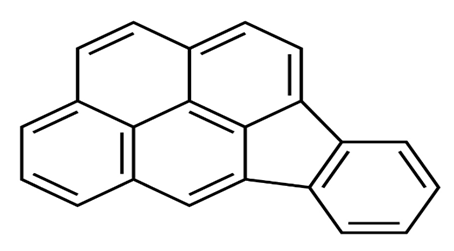 | 276.3 | [16] |
| Pyrene | PYR | C16 H10 | 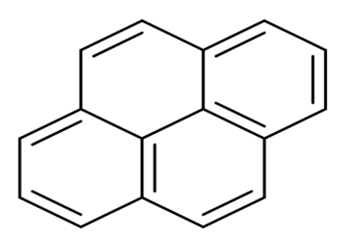 | 202.2 | [18] |
| PAHs | Method | Efficiency of Removal | Sample | Source |
|---|---|---|---|---|
| Naphthalene | Ultraviolent irradiation | 62% | Sea water | [2] |
| Pyrene & benzo (a) pyrene | Adsorption | 40% & 48 % | Synthetic wastewater | [24] |
| 16 PAHs | Photocatalyst ozonation & ultraviolent light-emitting diode irradiation | 57% | Offshore produced water | [25] |
| Naphthalene, phenanthrene, anthracene & pyrene | Bioremediation | 100%, 95.4%, 73.8% & 53.4% respectively | Oil fields produced water | [26] |
| Naphthalene & acenaphthene | Adsorption | 100% to 97% | Water treatment plant | [27] |
| 16 PAHs | Ozonation & biological approach | 42% to 63% | Urban runoff water | [28] |
| Anthracene, phenanthrene & fluoranthene | Fenton process | 85.47%, 63.16% & 62.95 respectively | Textile dying sludge | [29] |
| Phenanthrene, pyrene, & benzo (a) pyrene | Degradation and sorption | 80% to 65% | Oil polluted sediment | [30] |
| 16 PAHs | Phytoremediation | 89% | Wastewater | [31] |
| 16 PAHs | Biodegradation | 67.27% | River | [32] |
| Pyrene, benzo (a) pyrene & indenopyrene | Magnetic floatation | 89.9%, 66.9% & 78.2 respectively | Sea water | [33] |
| 16 PAHs | Biodegradation | 77.38% | Agricultural soil | [34] |
| Phenanthrene, naphthalene, anthracene & benzo (a) pyrene | Oxidation | 90.1%, 97.5%, 55.4% & 26.7% respectively | Soil | [35] |
| Naphthalene, phenanthrene, anthracene biphenyl & fluorene | Air-assisted liquid-liquid microextraction | 82.0% to 116.6% | Water | [36] |
| Phenanthrene | Adsorption | 90% | Wastewater | [37] |
| Naphthalene & fluorene | Oxidation adsorption | 92% to 100% | Produced water | [38] |
| Pyrene, fluoranthene, chrysene & phenanthrene | Precipitation method | 99%, 98%, 87% & 97% respectively | Marine sediments | [39] |
| Naphthalene, anthracene & fluorene | Oxidations | 97%, 95%, & 87% respectively | Landfill leachate | [17] |
| Benzo (b) fluoranthene | Adsorption | 59% to 91% | Water in the treatment cycle | [40] |
| 15 PAHs | Dispersive liquid–liquid microextraction | >90% | Four Rivers and tape water | [41] |
| PAHs | Electrochemical advanced oxidation | 99.9 % | Petroleum contaminated water | [42] |
| Low & high molecular weight PAHs | Biodegradation | 86% | Petroleum wastewater | [43] |
| PAHs Compound | Adsorbent | Adsorption Model | Adsorption Capacity | Source |
|---|---|---|---|---|
| Pyrene, naphthalene, phenanthrene, & acenaphthylene | Waste tire crumb rubber, coconut coir fibre & blast furnace slag | Linear, Freundlich, isotherm & Pseudo second order kinematic model | - | [66] |
| Naphthalene, phenanthrene, & acenaphthene | Rape straw & corn cob | Kinematic model & isotherm model | 592.97 mg/g, 480.27 mg/g & 692.27 mg/g | [67] |
| Benzo (a) pyrene & pyrene | Iron oxide nanoparticles | Pseudo second-order kinematic model | 0.96 mg/g & 0.99 mg/g | [68] |
| Naphthalene, phenanthrene, acenaphthene & pyrene | Bamboo wood, pine wood, pine needles & pine bark | Pseudo second-order kinematic model | 0.008 mg/L to 1 mg/L | [69] |
| Acenaphthene | Granular activated carbon | Langmuir isotherm | - | [70] |
| Plant Name | Chemical Compound Used for Nanoparticles | Nanoparticles Size/Shape | Application | Characterization Technique | Source |
|---|---|---|---|---|---|
| Eucalyptus leaves extract | Ferrous sulfate heptahydrate (FeSO4·7H2O) | Spheroid with diameter 20–80 nm | Removal of Nitrogen, phosphorus & chemical oxygen demand | EDS, SEM, XRD & FTIR | [79] |
| Castanea sativa, eucalyptus globulus, ulex europaeus & pinus pinaster extract | Iron (III) nitrate nine hydrate (Fe (NO3)3·9H2O) with Chitosan matrix | Beads with a diameter of 01 mm | Removal of Arsenic (V) | - | [80] |
| Mint plant leaves extract | Ferric nitrate (Fe NO3) 3 | Diameter 20–45 nm with face centre cubic | Removal of Arsenic (III), Arsenic (V) | TEM, EDX, XRD & FTIR | [81] |
| Green, black & oolong tea leaves extract | FeSO4 solution | Spherical with particle size 20–40 nm | Removal of Monochloro benzene & chemical oxygen demand | SEM, XRD & EDS | [82] |
| Citrofortunellamicrocarpa Leaves extract | Cu (NO3)2·3H2O | Spherical with particles size 54–68 nm | Removal of Rhodamin (B) | XRD, EDS, SEM & FTIR | [83] |
| Citrus aurantifolia leaves extract | Copper sulfate pentahydrate (CuSO4·5H2O) | Crystalline & average size approximately 22 nm | Removal of Rhodamin (B) & bacteria (S. aureus & E. coli) | XRD, EDS & FTIR | [84] |
| Pisum sativum peel extract | Ferric chloride hexahydrate (FeCl3·6H2O) | Spherical & particle size 20–30 nm | Removal of Methyl orange | XRD, FTIR, TEM, BET, & RSM | [85] |
| Amaranthus dubius leaves extract | FeCl3 & NaBH4 | Spherical with cubic shape & diameter 43–220 nm | Removal of Methyl orange 1-diphenyl-2-picrylhydrazyl | XRD, SEM & FTIR | [86] |
| Peltophorumpterocarpum leaves extract | Zinc acetate dehydrate | Average crystalline & size 11.64 nm | Removal of Methylene blue | XRD, EDS, SEM & FTIR | [87] |
| Pomegranate leaves extract | FeCl3 | Rod shape & average size 45–60 nm | Removal of Congo red | XRD, SEM, FTIR & EDS | [88] |
| Pomegranate leaves extract | Ammonium ferrous sulfate and ammonium ferric sulfate | Diameter 100–200 nm | Removal of Chromium (V) | XRD, SEM, VSM & FTIR | [89] |
| Syzygiumcumini seed extract | FeCl3·6H2O & sodium acetate | Crystalline & size approximately 14 nm | - | XRD, SEM, EDS, VSM, & FTIR | [90] |
| Vitex trifolia leaves extract | Zinc nitrate hexahydrate | Spherical & size 15–46 nm | Removal of Methylene blue | XRD, SEM, EDS & FTIR | [91] |
| Aegle marmelos leaves extract | Nickel nitrate, copper nitrate & chromium nitrate | Nanorods, nanosphere & nanoflower with an average size of 50 nm | Removal of phenol, 2, 4-dinitrophenol & 3-aminophenol | EDS, TEM & SEM | [92] |
| Eucalyptus leaves extract | Ferric chloride (FeCl3) & Graphene oxide | Spherical with a diameter of 4–7 nm | Removal of Methylene blue | XPS, EDS, TEM & FTIR | [93] |
| Green tea extract | Natural Bentonite & ferrous sulfate heptahydrate | Spherical with an average diameter of 40–60 nm | Removal of Phosphorous | TEM, XRD & FTIR | [94] |
| Cyanometraramiflora leaves extract | Zinc acetate | Hexagonal wurtzite crystalline with a size of 13.33 nm | Removal of Rhodamine (B) | TEM, XRD, FTIR, EDS & BET | [87] |
| Garlic vine leaf extract | FeSO4·7H2O | Crystallite with size 13.82–15.45 nm | - | XRD & FTIR | [95] |
| Terminalia chebula extract | FeSO4·7H2O & PdCl2 | Amorphous iron with a size less than 80 nm & cubic palladium with a size less than 100 nm | - | XRD & TEM | [96] |
| Eucalyptus globulus leaf extract | FeSO4·7 H2O | Spherical with size 50–80 nm | Removal of Chromium (VI) | TEM, XRD & FTIR | [97] |
| Acalypha Indica leaves extract | Copper Sulphate & Graphene oxide | - | Removal of Methylene blue | TEM, XRD, FTIR & EDX | [98] |
| Sapindus-mukorossi extract | K4(Fe(CN)6) & Fe(NO3)3 | Hexagonal, spherical & rod with size 10–60 nm | Removal of PAHs | XRD, TEM & SEM | [99] |
| Penicilliumexpansum | FeCl3·6 H2O | Spherical with size 15–66 nm | Removal of heavy metals such as Cobalt, lead, chromium, nickel, cadmium, chemical oxygen demand, total dissolved solid, & total suspended solids | TEM, XRD, FTIR, XPS & DLS | [100] |
| Spent tea leaves | Tea filter bag polyethylene & polypropylene | 250–211 µm diameter | Removal of PAHs | EDX, SEM & FTIR | [101] |
| Amaranthus blitum leaves extract | Fe(NO3)3∙9 H2O & AgNO3 | Spherical with an average size of 92 nm | Removal of caffeine | XPS & SEM | [102] |
| Oolong tea extract | Ferrous Sulphate | Spherical with a diameter of 40–50 nm | Removal of malachite green | EDX, SEM, XRD & FTIR | [103] |
| Aloe barbedensis, Azadirachta indica & Coriandrum sativum plant extract | Silver nitrate & copper sulphate | - | Removal of PAHs | FTIR | [77] |
| Neem leaves extract | Mg(NO3)2 & palm shells | Average diameter 10 um | Removal of anthracene | SEM, XRD & FTIR | [63] |
| Allium tricoccum extract | FeCl2,FeCl3 & TiO(OH)2 | Spherical with size 40–90 nm | Removal of PAHs | EDX, SEM, XRD & FTIR | [104] |
| Green tea extract | FeSO4·7 H2O & graphene oxide | Spherical with particles diameter of approximately 4–15 mm | Removal of nitrate | XPS, TEM & FTIR, | [105] |
| Pomegranate peel extract | FeSO4 solution | Amorpous with an average particle size of 2.7 nm | Removal of benzo (a) pyrene & pyrene | EDX, SEM, XRD & FTIR | [68] |
| PAHs | Water Sample | Method of Oxidation | Removal Efficacy | Source |
|---|---|---|---|---|
| Point source PAHs | Cooking wastewater treatment plant | Ozone & ultraviolent | 75% | [128] |
| 8PAHs | Coagulant water, Electro coagulated water & groundwater | Hydrogen peroxide & ultraviolent | 76%, 70% & 76% | [129] |
| Fluorene, dibenzofuran & dibenzothiophene | Treated water | Hydrogen peroxide & ultraviolent | 98% to 99% | [130] |
| 15 PAHs | Produced water | Fenton reaction | 89.73% | [131] |
| Hydrocarbons | Synthetic wastewater | Hydrogen peroxide & ultraviolent | 90% | [132] |
| 6 PAHs | Water treatment | Xenon & Hydrogen peroxide | 100% | [14] |
| 6 PAHs | Landfill leachate | Fenton oxidation & ozone oxidation | 70% | [133] |
| PAHs | Water Sample | Biological Method | Removal Efficiency | Source |
|---|---|---|---|---|
| 16 PAHs | Wastewater treatment plant | anaerobic-anoxic-oxic biological treatment | 99% to 100 % | [20] |
| Naphthalene, phenanthrene, acenaphthene, fluoranthene & pyrene | Real sample | Green biomaterial sorbent | 76.20% to 105.60% | [136] |
| 16 PAHs | Marine sediment | Biodegradation | 42% to 77% | [137] |
| PAHs | Sample | Plant | Presence of Bacteria/Substrate | Removal Efficiency | Source |
|---|---|---|---|---|---|
| 5–6 ring PAHs | Gas plant soil | Cucumber | Cucurbita species | 85% | [141] |
| Phenanthrene | Ever bright water treatment plant | Arundo donax | Proteobacteria, Bacteroidetes, Chloroflexi, Actinobacteria & Firmicutes | 94.09% | [142] |
| 16 PAHs | Soil | Sudan grass | Mycobacterium vanbaalenii & bacterial consortium | 98% | [143] |
| Treatment Method | Advantages | Disadvantages | Source |
|---|---|---|---|
| Membrane filtration | Economical, less chances of membrane fouling, condensed modules, suitable for saline water | Mineral scaling, membrane pore wetting and membrane fouling | [144,145,146] |
| Flotation | Simplicity of operation, amalgamation increase the process efficiency, robust and durable, and has no moving parts | 4 to 5 min retention time, maximum amount of air is produced, and skim volume | [108] |
| Adsorption | Low capital cost, condensed modules, ecofriendly, flexible process and reusable and recoverable adsorbent | Frequent regeneration needed, affected by pH, salinity, high temperature, retention time maximum, expensive adsorbent restoration, and harmful excess adsorbent | [147,148] |
| Chemical precipitation | Energy saving process, easy to operate, low cost and maximum recovery | Requirement of chemicals, generation of sludge, and secondary waste | [149] |
| Chemical oxidation | Small treatment time and ecofriendly | Operation and maintained cost maximum | [76] |
| Electrochemical technologies | Beneficial secondary product, eco-friendly and no chemicals required | Skillful labor necessary and scaling up difficulties | [150] |
| Advanced oxidation process | Easy operation, high degradation, dissolve oil mineral and compact | Skillful labor required, optimization, monitoring and pretreatment process required | [151] |
| Bioremediation | Availability of low cost microbes, easy process, whole mineralization leads to production of CO2, H2O and biomass | Lengthy degradation time and time optimization an excessive task | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, S.; Waseem, M.; Leta, M.K. Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water. Environments 2023, 10, 40. https://doi.org/10.3390/environments10030040
Sher S, Waseem M, Leta MK. Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water. Environments. 2023; 10(3):40. https://doi.org/10.3390/environments10030040
Chicago/Turabian StyleSher, Sadaf, Muhammad Waseem, and Megersa Kebede Leta. 2023. "Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water" Environments 10, no. 3: 40. https://doi.org/10.3390/environments10030040
APA StyleSher, S., Waseem, M., & Leta, M. K. (2023). Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water. Environments, 10(3), 40. https://doi.org/10.3390/environments10030040