Abstract
The effects of artificial light at night (ALAN) on human health have drawn increased attention in the last two decades. Numerous studies have discussed the effects of ALAN on human health on diverse topics. A broader scope of how ALAN may affect human health is thus urgently needed. This paper depicts a systematic evidence map in a multi-component framework to link ALAN with human health through a comprehensive literature review of English research articles in the past two decades. A three-phase systematic review was conducted after a generalized search of relevant articles from three publication databases, namely Scopus, the Web of Science, and PubMed. In total, 552 research articles were found in four categories and on numerous topics within our framework. We cataloged the evidence that shows direct and indirect as well as positive and negative effects of ALAN on human physical and mental health. We also summarized the studies that consider ALAN as a social determinant of human health. Based on our framework and the systematic evidence map, we also suggest several promising directions for future studies, including method design, co-exposure and exposome studies, and social and environmental justice.
1. Introduction
Humans have a tremendously long history of fighting against darkness at night, and the earliest use of artificial light at night (ALAN) (i.e., fire) can be dated back to more than 450,000 years ago [1]. In the agrarian age, humans developed controllable ALAN, e.g., candles and torches. The industrial revolution then equipped humans with safer electricity-based ALAN, e.g., incandescent lamps. The invention and prevalence of light-emitting diodes (LEDs) led humans to the age of cool and efficient lighting [2] and it is tightly bonded with the progress of the information revolution. By the end of the 20th century, a group of studies started to link ALAN with human health. This novel association has drawn increasing attention in the last two decades since it may contribute to a new era of healthy lighting in human history. The era of healthy lighting could be another notable phase in the relationship between humans and ALAN after the era of illumination extension, the era of controllable lighting, the era of safe lighting, and the era of efficient lighting [3].
Health is essential for humans, but it is so complex and thus is difficult to give a complete definition. The World Health Organization (WHO) has given the most general definition of human health as a state of complete physical, mental, and social well-being [4], which emphasizes at least three components of human health. Physical health is a state of being free from illness and injury. Mental health is a state of well-being in which people realize their abilities, can deal with normal stresses of life, can work productively and fruitfully, and can contribute to their community [5]. The rest can be categorized as social determinants of health. They are non-medical factors that influence health outcomes [6] and have triggered a lot of discussion on social equality and justice. A comprehensive discussion of ALAN’s effects on human health should include these three components and the fundamental mechanisms. Previous studies have observed associations between ALAN and human physical and mental health. ALAN is also considered a social determinant of health. However, the pertinent causal pathways are still under discussion. For example, numerous studies have indicated that ALAN may be associated with cancers [7,8,9] and sleep disorders [10,11]. These associations are primarily supported by epidemiological investigations. Some other studies discussed the concrete biochemical pathways, but they are still incomplete and may need further exploration.
The effects of ALAN on human health are complex and diversified. Three primary reasons contribute to the complexity involved. First, various human health concerns may be relevant to ALAN but the attributable risk from ALAN is not easy to characterize because the etiology of human health is multifactorial, particularly in chronic diseases. Second, the response of the human body to the same level of exposure to ALAN may be different between different cohorts since their resilience and vulnerability may vary. Finally, the different ways of using ALAN may also lead to different or even opposite health effects. Most studies to date emphasize the negative health effects of ALAN such as cancers [12,13], chronic diseases such as diabetes [14], and mental disorders [15,16]. However, short exposure to ALAN and acute disturbance of human biological processes do not necessarily lead to adverse human health conditions. Available studies also indicate that the proper use of ALAN may help phase adaptation [17], promote alertness and reduce work-related risks [18], and avoid the injury and death of older adults from falling at night [19]. The complexity and diversity of these issues keep growing with more studies in the last two decades. There is thus an urgent need to have a big picture of the effects of ALAN on human health to inform policy- and decision-making in practice.
We chose to fulfill this urgent need by drawing a systematic evidence map in a comprehensive multi-component framework. A systematic evidence map aims at providing a comprehensive summary of the characteristics and availability of evidence rather than synthesizing them through a universal measurement. We found it particularly suitable for this issue due to the diversified topics and non-interconvertible measurements from diverse studies. We defined the scope of this review through a PECO statement (Population, Exposure, Comparator, and Outcome), and then depicted such a systematic evidence map through a literature review of the relevant research articles in the past two decades based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. In the rest of this review paper, we first introduce the method we used to search and classify relevant research articles. We then identify the prominent topics concerning the health effects of ALAN and analyze the spatial and temporal evolution of relevant research articles. We summarize the direct and indirect effects of ALAN on human physical and mental health as well as ALAN’s role as a social determinant of health. We then describe the multi-component framework for linking ALAN with human health and discuss how to use it before presenting the conclusions in the last section.
2. Materials and Methods
2.1. Study Objectives and Scope
This review seeks to provide an overview of the evidence on the effects of ALAN on human health. Our primary foci are the pathways through which ALAN may affect human health and the physical, mental, and social well-being outcomes. We developed a PECO statement to articulate the scope of this review:
Population: any population group or particular cohort with well-defined characteristics (e.g., women, white/Caucasian, older adults, ICU patients, and shift workers).
Exposure: any sort of light exposure only if it is artificial, during the nighttime, and involved in the consideration of analysis, for either the purpose of illumination or information (i.e., reading from a screen).
Comparator: any sort of contrast in ALAN (e.g., the intensity of ALAN as a control variable in case-control studies and as an independent variable in regression analysis) in either cross-sectional design or longitudinal design of experiments.
Outcome: any sort of human health concerns regarding human physical, mental, and social well-being through either direct or indirect pathways.
According to our PECO statement, a three-phase systematic review was conducted after a generalized search for relevant research articles from multiple publication databases.
2.2. Initial Generalized Search and Literature Screening
In the first phase of our literature review, three experienced researchers used the keywords “nighttime light” and “human health” to identify all possible publications by scanning the full text of publications from three databases, namely Scopus, the Web of Science, and PubMed, respectively. A publication in any of the three databases was included if it mentioned any of the following words: “nighttime light”, “artificial light”, “night light”, “nocturnal light”, “screen light”, “street light”, “outdoor light”, “indoor light”, “room light”, “*light*”, “illuminat*” and other light-relevant words together with “human health” in any section of its text, e.g., title, abstract, introduction, materials and methods, results, discussions, conclusions, back matters, and references. The search period is confined to the last two decades, from 2000 to 2021. After removing duplications, the inquiry results returned a collection of publications, namely a raw pool of literature. We further excluded literature reviews that have no data-based analysis and a few irrelevant articles from newspapers, which produced an initial collection of data-based research articles.
In the second phase of the literature review, three steps were taken to remove irrelevant research articles. In the first step, we examined the titles of the research articles to remove works on animals, which include mice, hamsters, zebrafish, bacteria, and others. In the second step, we read the abstracts of the remaining articles and removed papers that are not about light, such as those on research design and methodological issues, pure discussion on sleep without using light conditions as a control variable, and other irrelevant articles involving the word “light”, e.g., light sleep and light exercise. In the final step, we assessed the full text of the remaining articles and removed several that do not fit the scope of this review (e.g., the health effects of daytime light and polar region midnight sunlight). In the second phase, three experienced reviewers were in charge of one-third of the initial collection of research articles, respectively. The results from one reviewer were examined by the other reviewer until they reached an agreement. The result yields a collection of 552 relevant research articles, all the materials were stored as a digital library using EndNote 20.
2.3. Data Extraction and Study Quality Assessment
In the last phase of the review, the included research articles were categorized into four domains, namely the mechanisms of how ALAN may affect human health, ALAN’s effects on human physical health, ALAN’s effects on human mental health, and ALAN as a social determinant of human health. A codebook was developed by the primary reviewer with the guidance of two senior members of the research team. The information includes the year of publication, the country where the study was conducted, the primary topic of research (e.g., breast cancer and depression), the cohorts (e.g., women, shift workers, and patients in ICUs), and study results (e.g., significant or not). Generally, the exposure to ALAN is digitized as an independent variable in controlled groups, whereby the differences in the concerned health outcomes and the effect sizes of ALAN are tested using statistical methods in the included research articles. With respect to the different definitions of the significance of tests in diverse topics, we decided to include and report the same results and significance as the original studies. This review aims at a systematic evidence map and does not aim at a meta-analysis since the meta-analysis is impossible regarding our scope. Reporting the original results will not yield a misleading conclusion since we are not doing the synthesis. Each article was further coded with a group of essential attributes according to this codebook and the assessment of the full text. To be consistent throughout the data extraction procedure, the data extraction was conducted by only the primary reviewer and then examined by other reviewers in the research team. These attributes were used to classify the articles into distinct groups. All these essential attributes are stored in Excel sheets, please see Supplementary File S1 for more details.
Given the scope of our systematic evidence map, we did not conduct a conventional quality assessment for each included article. By their nature, systematic evidence maps are broad in scope but limited in depth in order to catalog diverse or even contradictory evidence, which provides comprehensive perspectives for policy- and decision-making. Topics and the number of studies in each topic are easy to derive from our initial data extraction, and we found several intensively discussed research topics concerning ALAN’s effects on human health. However, a higher frequency of studies on a topic means higher academic interest and more study efforts rather than a stronger confidence in ALAN’s health effects. A lower frequency of studies on a topic may indicate an essential issue that has long been overlooked, and that is one of the reasons why a systematic evidence map is necessary.
We further extracted the details of the experimental design for research articles on four intensively discussed research topics that concern ALAN’s effects on human physical and mental health, namely ALAN’s association with breast cancer, obesity and diabetes, sleep disorders, and depression. The extracted details include study design (e.g., case-control, cross-sectional, and longitudinal), sample size, cohort types, measurements of ALAN, measurements of health outcomes, effect size types (e.g., odd ratio, relative risk, mean of difference, Cohen’s d, correlation coefficient, and regression coefficient), mean estimation of effect sizes, 95% confidence intervals if available or derivable, and p-values. We further assessed the certainties of research articles on these four topics according to the guidance from the Grades of Recommendation, Assessment, Development, and Evaluation Working Group (GRADE Working Group). These extracted details are stored in Excel sheets, please also see Supplementary File S1 for more details. It turns out that there are no consistent measurements and interconvertible effect sizes for even these intensively discussed research topics. Consequentially, a conventional meta-analysis using I2 or other statistics and forest plots does not apply to our study objectives, and a systematic evidence map with broad scope but limited depth is proper and adequate for our study objectives. A revolutionary methodology may advance the quality assessment of a multi-component research concern, but it is beyond the scope of this study.
3. Results
In this section, we present our systematic evidence map in detail. In the following subsections, we first summarize the development of this research in the last two decades according to the attributes of the included research articles. The effects of ALAN on human physical and mental health are then summarized. We also summarize the role of ALAN as an environmental factor and social determinant of human health, including both its direct and indirect effects. Finally, we propose a multi-component framework to systematically classify these diversified studies.
3.1. Research on ALAN’s Health Effects on Human Health in the Last Two Decades
Through our generalized search and systematic screening, we finally selected 552 research articles on ALAN’s health effects (Figure 1). Since the early papers that link ALAN with breast cancer in the 1980s [20] works on how ALAN may affect human health keep drawing attention from multiple disciplines, especially in the last two decades (Figure 2). These studies are mostly concentrated in countries with well-developed nightscapes (Figure 3), such as the United States (217 studies), Japan (36), Australia (33), Canada (32), the United Kingdom (30), China (27), and South Korea (17).
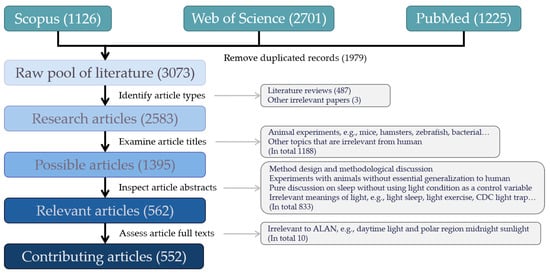
Figure 1.
Flow chart of the article screening scheme.
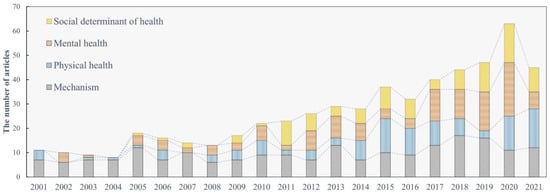
Figure 2.
The temporal evolution of research on ALAN’s effects on human health.
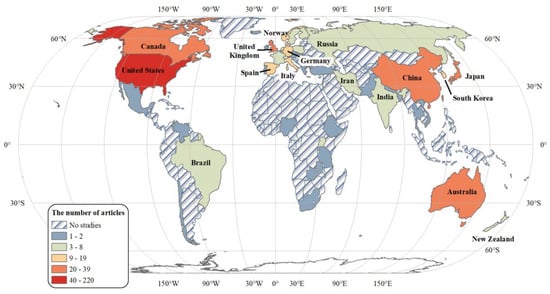
Figure 3.
The global distribution of research on ALAN’s effects on human health.
These studies mainly focused on four domains (Figure 4). About 36.59% of them studied the mechanisms of how ALAN may affect human health. The discussions are mainly on the photobiological effects of ALAN on human circadian rhythms, especially melatonin secretion and the sleep-wake cycle. Articles on the effects on human physical and mental health comprise 21.01% and 23.91% of these studies, respectively. The intensively discussed topics on human physical health include breast cancer, obesity, diabetes, and cardiovascular diseases, while there are also some discussions on how vector-borne diseases are tightly linked with ALAN. On the other hand, the intensively discussed topics on mental health include changes in cognition, sleep disorders, and depression. Finally, considering ALAN as a social determinant of health, studies on health care and traffic safety are also very common. Each of these four domains is detailed in the following subsections.
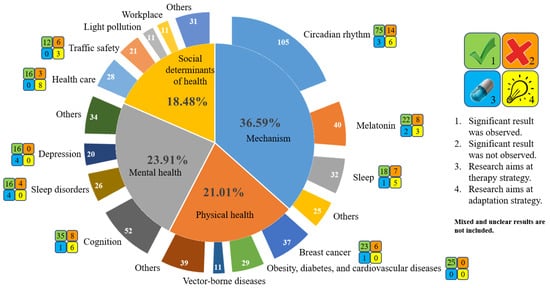
Figure 4.
The intensively discussed topics on ALAN’s effects on human health and the number of relevant research articles. The symbol following each research topic is explained by the legend on the right.
3.2. How ALAN May Affect Human Health
How ALAN may affect human physical and mental health has been well studied. The mechanisms involve multiple human organ systems and numerous biological processes. These organs and biological processes are tightly linked to each other, and it is thus difficult to simply attribute the effects of ALAN on human health to a single or a few internal factors. However, after the active exploration and accumulation of evidence for more than 20 years, past research tends to agree that human circadian rhythms play fundamental roles in the health effects of ALAN.
Human circadian rhythms refer to the diurnal changes in human physiological functions and metabolisms in approximately 24 h. These rhythms are externally regulated by the natural daily light-dark cycle and internally regulated by the suprachiasmatic nucleus (SCN), which is a bilateral structure located in the anterior part of the hypothalamus [21]. Human circadian rhythms have been observed in many physiological functions at multiple levels, including the expression of genes [22,23,24,25], the regulation of body temperature [26,27,28], the secretion of hormones [29], and sleepiness and wakefulness [30]. ALAN imposes extra light at night on the retina through the eyes and triggers nerve impulses to stimulate the SCN through the optic nerves. These extra stimulations to SCN disturb the ordinary human circadian rhythms and then lead to a series of unusual performances of the human body. These unusual performances could be roughly categorized into two groups: one is the disturbance of human hormone secretions and the other is the disturbance of human sleep. We also acknowledge that there may be other essential pathways, e.g., the disturbance of human immune functions. However, relevant studies on ALAN’s disturbance of immune functions are primarily on animals and are few on humans, probably due to the morality issues in human experiments. According to our review scheme, we decide to start with the two disturbances that have intensive discussions on humans.
Many human hormone secretions show an apparent circadian rhythm. Melatonin is one of them and one that is examined the earliest and most studied [31,32], and melatonin is the best indicator of circadian phases in humans. The secretion of melatonin naturally peaks during dark nights and is suspended during bright days, while previous studies consistently indicate that ALAN may well lead to melatonin suppression during nights [33,34,35]. Due to such a strong relationship, the concentration of melatonin from either serum [36], plasma [37], salivary [38], or urinary [39] samples is also generally used as an indicator to inspect the change in human circadian rhythms. Moreover, light exposure history and daytime/nighttime light contrast may also affect melatonin secretion, and relevant studies have observed that less light intensity during the day makes a stronger decrease in melatonin levels during the night [40,41]. Similar disturbance has also been observed in the circadian rhythms of other hormones (e.g., cortisol [42], other steroid hormones, leptin [43,44], and some sex hormones [45,46]). The disturbance of the circadian rhythms by these hormones may lead to abnormal metabolisms, chronic stress, and elevated risks of some diseases such as breast cancer [47].
The other essential human activity regulated by circadian rhythms and melatonin is sleep. The mechanism of sleep has not yet been fully understood but it is generally agreed that sleep is critical to the human nervous system and human cognition. The diurnal sleep-wake cycle is supposed to well-match the dark-light cycle but may also be disturbed by ALAN. The results may include phase shift (e.g., phase advance and phase delay) [48,49,50], the interruption of sleep [51], daytime sleepiness [52], and even insomnia [53,54]. Low quality of sleep may have negative impacts on human mental health and may lead to sleep disorders and trigger or enhance particular mental disorders, e.g., depression [55]. One factor to note is that it is difficult to claim that ALAN may affect human health through a single pathway since all the relevant biological processes such as hormone secretions and sleep are tightly bonded with each other. Attributing the health effects of ALAN to a single pathway may be an oversimplification, and most studies thus have a low level of certainty (Please see Supplementary File S1 for more details).
The acute disturbance of human hormone secretions and sleep by ALAN does not necessarily lead to physical diseases and mental disorders since the human body has a very strong resilience, but the risks are higher in some vulnerable groups with chronic and regular exposure. Three classification systems help us categorize these vulnerable groups (Figure 5). The primary one is the phases in the human life cycle from birth to death. Infants are at their early phase of life, where light may play an essential role to build circadian rhythms, especially the sleep-wake cycle [56]. ALAN may disturb circadian rhythms during childhood, and it may be relevant to some neurodevelopmental problems [57]. Adolescence is a particular phase when adolescents go through rapid changes in their body and cognition and ALAN may have a strong disturbance of the sleep-wake cycle [58] and the endocrine system [59]. Young adults have the strongest resilience, but biological differences should be acknowledged between males and females, including underlying differences in hormones, as well as age-dependent hormonal changes. For example, women at menstruation may have a higher susceptibility to sleep disturbances and working shifts may increase menstrual cycle irregularities and painful menstruation [60], and that may be the reason why the studies of ALAN’s health effects on women have drawn more attention. Menopause is another particular phase when women go through rapid changes in the endocrine and reproductive systems, and ALAN may impose extra stress on women’s physical and mental health [61]. Finally, aging people face the decline of body functions in multiple organ systems. The disturbance of ALAN on sleep then may increase the risks of various physical diseases and mental disorders in older adults, e.g., coronary heart disease [62], diabetes [14] and depression [15].
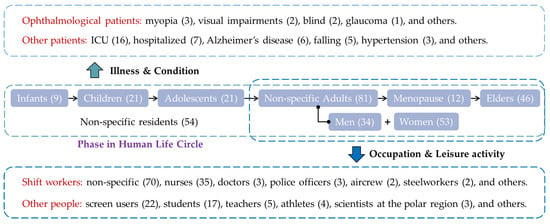
Figure 5.
Differentiated vulnerable groups to ALAN’s effects. The number of relevant articles is enclosed in parentheses.
The second classification system emphasizes people’s jobs and leisure activities. Although adults have the strongest resilience to the disturbance of ALAN and its health effects, some groups with sleep deprivation and poor sleep qualities also need attention. The first group comprises various shift workers, whose jobs may require partial or complete sleep deprivation during nighttime work. Nurses are the earliest and most studied shift workers. This group also includes resident physicians, police officers, flight crew, and steelworkers. The other group comprises nighttime screen users, who do not experience sleep deprivation but may face degradation of sleep quality due to ALAN. Studies on nighttime screen users have recently emerged but have drawn increasing attention. The ALAN from computers [63], smartphones [64], iPads [65], or other electronic devices all may lead to the disturbance of people’s circadian rhythms and a phase shift in sleep, which consequentially imposes stress on mental health. Moreover, sleep quality degradation caused by ALAN is also discussed for students [66], teachers [67], athletes [68], scientists in polar regions [69], and other cohorts. For a complete list of relevant studies, please see Supplementary File S1.
While the first and second classification systems emphasize healthy people, the last one emphasizes people with particular health conditions. On one hand, ALAN may directly increase the health and injury risks of ophthalmological patients since they have a deficient visual capability, e.g., those with glaucoma [70]. On the other hand, ALAN may also have indirect health effects on patients in recovery since ALAN may degrade their sleep quality, e.g., those in ICUs [71]. However, these health effects depend on particular diseases and health conditions. For example, less ALAN for those in ICUs may lead to less disturbance and better sleep quality [71], while sufficient ALAN may reduce injury and death due to falling during the night for older adults [72].
On the other side of this issue, many studies have also discussed the photobiological effects of different types of ALAN. These differences cover light wavelength (e.g., red light or blue light), light intensity (e.g., bright light or dim light), light pause frequency (e.g., millisecond flash, 100 Hz flicker, or 10 min break), the phase of giving light (e.g., early or late in the night), and the light source (e.g., incandescent light or LED light). It has been found that blue-enriched light and bright light have a stronger disturbance of human circadian rhythms [73,74], while dim light may only have minor or insignificant effects on human circadian rhythms [75]. Consequentially, LED light has recently drawn increasing attention since it is rich in blue light and has high intensity.
3.3. Exposure to ALAN and Human Physical Health
Considering the disturbance of human circadian rhythms as an essential pathway, associations have been found between ALAN and various physical health issues. These physical health issues include multiple cancers and chronic health conditions, and they involve almost all the organ systems of the human body. These associations were supported by epidemiological investigations that are summarized in the following paragraphs. Pertinent causal pathways were examined in numerous animal models, but few causal pathways were examined in the human body.
Cancers are commonly studied as one possible consequence of ALAN’s effects on human physical health. Numerous studies have indicated that chronic, long-term, regular, or cumulative exposure to ALAN is significantly associated with certain cancers. Particularly, breast cancer is the most discussed cancer in this context, accounting for about one-third of the physical health studies. The “light-at-night” hypothesis argues that exposure to ALAN is considered to disturb the secretion of melatonin and some sex hormones (e.g., estrogen), thereby imposing extra stress on the reproductive system and increasing the risk of breast cancer [47,76,77]. However, due to morality issues and the difficulties in human experiments, solid pathways and strong evidence have not yet been observed for humans. Recent progress mainly relies on epidemiological investigations and statistical analysis, while numerous statistical associations between the incidence of breast cancer and exposure to both indoor and outdoor ALAN have been observed. For instance, exposure to home ALAN, e.g., sleeping with lights or a TV on, can increase the risks of breast cancer [78,79]. Women who live in areas with high ambient ALAN may also have an elevated risk of breast cancer [61]. However, some studies have also shown that exposure to ALAN may have weak or no association with breast cancer [80,81,82]. Other factors, e.g., geographic contexts, race, and health conditions, could be confounders on this issue. For instance, studies on Caucasian or Asian, premenopausal, or postmenopausal women in different regions showed inconsistent results [7,82,83].
Some studies discussed the associations between exposure to ALAN and other cancers. Prostate cancer is drawing increasing interest since many studies found a significant positive association between exposure to ALAN and prostate cancer incidence [84,85,86]. More extended night shift work duration along with more exposure to ALAN has been found to be associated with a lower risk of skin cancer [87,88]. However, this association may indeed indicate an unusual sleep-wake cycle that significantly reduces surveyed participants’ daytime exposure to outdoor high-energy light (e.g., ultraviolet light). No significant association had been observed between the exposure to ALAN and other intensively discussed cancers until now, including cancers of the cervix, lung and trachea, bladder, larynx, esophagus, stomach, liver, pancreas, colon, brain and central nervous system, and lymphatic system [12,85,89,90].
The associations between exposure to ALAN and chronic health conditions involving some organs also triggered a lot of discussions. The first group of discussions is about the digestive and endocrine systems. ALAN is considered an essential factor in promoting obesity [91,92], and associations have been observed for exposure to both outdoor and indoor ALAN [93,94,95]. The disturbance of relevant hormone secretion such as leptin may play an essential role in this issue [96]. ALAN may also increase the risk of other chronic diseases such as diabetes and alcoholic liver diseases. For example, higher nighttime light intensity and later sleep time are strongly associated with a higher incidence rate of diabetes [14,97]. The second group of discussions is on the cardiopulmonary system, targeting cardiovascular diseases and apnea [98]. Older adults attracted more attention since they are more vulnerable to the malfunction of the cardiopulmonary system. For example, a significant association has been found between exposure to ALAN and elevated nocturnal blood pressure among older adults [62,99,100]. The third group of discussions concerns the nervous system. Exposure to bright ALAN can alter the optimum refractive state of the accommodating eye [101] and affect the diurnal variation in choroidal thickness [102], thus increasing the risk of myopia. Current studies also provide some insights into ALAN’s health effects on other organ systems, covering renal dysfunction [103,104], fibromyalgia [105], sperm quality [106], bone fracture [107], and COVID-19 infections [108]. However, these are pilot studies that need more subsequent support.
3.4. Exposure to ALAN and Human Mental Health
Disturbing human sleep is the other essential pathway linking ALAN with human health, the outcomes are also diversified. To comply with WHO’s definition of human health, we made a simplification and roughly categorized these direct outcomes into cognitive impairments and mental disorders. The primary change in cognition as a result of exposure to ALAN is impaired alertness. People with disrupted circadian rhythms may exhibit reduced cognitive flexibility and diminished memory [109,110,111]. Shift workers are more vulnerable to impaired alertness because of the potential circadian rhythm maladaptation during their night shift work [112,113], which increases their work-related risks. Similarly, excessive exposure to LED light at night can also lead to reduced alertness the next day and increased daytime sleepiness [114,115], which imposes an extra health burden on those who use digital media devices at nighttime. Chronic sleep deprivation resulting from excessive ALAN exposure may also lead to progressive degradation of people’s neurobehavioral performance [116] and may even trigger violence and discrimination [117].
Mental disorders are also found to have associations with exposure to ALAN, while the most discussed is the relationship between sleep disorders and ALAN. ALAN is considered to cause sleep disorders [118], especially insomnia [119]. For example, increased light exposure during sleep has been found to be associated with poor sleep quality and mood disorders, especially in nursing homes, ICUs, or regions with high levels of ALAN [67,120]. The relationship between depression and ALAN has also been widely examined. The prevalence of depression and anxiety is higher in areas with higher outdoor light levels at night [58,121]. Exposure to ALAN in the home environment can also increase the risk of depression [15,122]. As an important hormone that regulates sleep and human circadian rhythms, melatonin is considered to have an essential role in this issue [55]. There are also similar discussions on the relationships between ALAN and other mental disorders, including bipolar disorder, dementia, seasonal affective disorder, autism, anxiety disorder, and night-eating syndrome. Exposure to ALAN generally shows a positive association with the incidence of these mental disorders through epidemiological investigations [58,123], and poor sleep quality is considered the primary trigger [11,124].
While chronic and regular exposure to ALAN may adversely affect human cognition and mental well-being, some studies also indicate that the proper usage of ALAN may be considered a non-pharmacological intervention to treat some of these cognitive impairments, maladaptation, and mental disorders. For example, bright light can improve performance and alertness during simulated night shifts and effectively reduces fatigue among shift workers [37,125,126]. In addition, controlling exposure to ALAN (e.g., controlling light intensity in different phases of the night or depriving light during the night) and controlling the day/night light contrast is also common in treating many mental disorders. Relevant attempts and suggestions cover anxiety and depression [127,128], increasing sleep time [129], insomnia [130], Alzheimer’s disease [131], and bipolar disorders [132]. Some of these mentioned studies indicate that controlling the exposure to ALAN may promote participants’ mental health, but others indicate that controlling the exposure to ALAN may not work well alone. The primary research interest of light therapy for mental disorders targets depression. The most updated literature synthesis indicates that 23 relevant studies particularly discussed the effects of light therapy on non-seasonal depression symptoms in the last three decades [133]. The synthesis indicates a significantly mild-to-moderate treatment effect of controlling diversified lights of different phases of the day (including night) to reduce depression symptoms.
3.5. ALAN as a Social Determinant of Human Health and Safety
Since ALAN is an artificial entity, exposure to ALAN is highly regulated by urban planning and environmental design. The inadequate and improper design of ALAN in return raises the discussion on its health effects while considering ALAN as a social determinant of health. Newly emerged studies have drawn interest to the modification of ALAN, traffic safety at night, perception of local environments, and environmental justice. Though numerous studies have indicated that chronic and regular exposure to ALAN is very likely associated with chronic physical and mental conditions, especially for shift workers, the effects of acute exposure to ALAN may also be important for some specific groups of people, with respect to improving nighttime visibility, promoting the perception of neighborhoods, and temporally increasing alertness. Please note that this trade-off between acute and chronic exposure to ALAN may not be equivalently necessary for all groups of people, and it makes this issue more complicated in practice since a specific group of people may need a different emphasis on the need for either acute or chronic exposure to ALAN.
Many studies have started to discuss the modification of ALAN for specific aims and specific groups of people, particularly in office environments, healthcare environments, and bedroom environments. For example, some studies suggest altering ALAN settings and schedules to improve the living quality and work efficiency of shift workers. Particularly in indoor office environments, it is suggested to gain exposure to bluer light with shorter wavelengths during the day to mimic daylight, and dimmer light with longer wavelengths at night to mimic moonlight and stars [134]. Regarding the implementation, a particular design of LED can be helpful [135]. With respect to the effects of acute exposure to ALAN, improved lighting in industrial environments could reduce the sleepiness of shift workers and increase their adaptation to night work [136,137]. Lighting intervention, which requires a proper schedule of light exposure and shifts, can also improve the sleep quality and health status of shift workers [138,139]. ALAN has also been studied to decrease the falling risks of older adults in nursing homes. People’s gait becomes slower, and their stride length becomes shorter while aging, which increases their falling risks, especially during midnight awakening in dim environments [19,72,140]. Proper ALAN settings will help stabilize the steps of older adults and reduce their falling risks [140]. Moreover, the current version of sleep hygiene would always suggest less ALAN throughout the night, but it may need more elaborated discussions for different cases. Proper usage of ALAN may improve people’s sleep hygiene through more effective phase adaptation, and indirectly promote human health.
Being free from injury is also an essential component of good human physical health. ALAN improves visibility and provides signals for traffic at night, which brings up intensive discussions on this issue. ALAN may fundamentally decrease traffic collision risks by increasing the visibility and contrast of pedestrians/vehicles as well as reducing the visual reaction time of drivers [141,142,143,144]. On the contrary, an improper set of ALAN may have negative effects [145]. Excessive ALAN can hinder visibility rather than improve it. For instance, vehicle headlights create glare that temporarily obstructs drivers’ vision and reduces their visual sensitivity, which may lead to collision [146]. Particularly, glare at night is currently considered a fatal issue for older drivers with age-related visual deficiencies [147].
The perception of safety in the neighborhood is another interesting pathway linking ALAN with human health while considering ALAN as a social determinant of health. ALAN is originally adopted to increase visibility at night and thus contributes to the sense of comfort and safety at night. Studies have observed that more ALAN can improve the sense of security, reduce fear of crime, and arouse more pleasant feelings [148,149]. These positive senses brought by ALAN foster people’s willingness to take part in nighttime outdoor activities. For older adults and women, ALAN is effective in promoting walking and other kinds of physical activity at night [150,151]. Women are more sensitive to poor ALAN conditions, which leads to fewer nighttime outdoor physical activities [152]. However, an ALAN-based perception of security does not necessarily mean actual safety. Studies also found that ALAN does not reduce crimes at night compared to that during the day, while the role of ALAN is to increase the optimistic sense of neighborhood environments rather than directly increasing surveillance and reducing crime motives [153,154].
3.6. Indirect Effects of ALAN on Human Health
We have also found a group of interesting studies that consider ALAN as an indirect factor that affects human health. These studies have recently emerged. They provided valuable insights but have not yet attracted sufficient attention and adequate discussion.
The first group of studies provides valuable insights into how ALAN may be related to other environmental factors that might affect human health (e.g., air pollutants, heat waves, and noise). Air pollution has drawn increasing attention to this issue, as emerging studies have found a positive association between ALAN and the aerosol concentration of air pollutants, e.g., secondary inorganic aerosols (SIAs), volatile organic compounds (VOCs), and PM2.5 [155,156]. One role of ALAN is to yield photochemical smog by disturbing the natural chemical reactions that help clean the air throughout the night. For instance, the reaction of with to form is very unstable when it is disturbed by ALAN [157,158,159]. Measurements show that ALAN inhibits the formation of , thereby increasing and levels in urban areas [158,160]. Moreover, ALAN shows a significant positive association with greenhouse gas emissions and urban heat island intensity because of its relationship with energy consumption and urban built environment characteristics [146,160,161].
The other group of studies is interested in ALAN’s effects on the behavioral pattern of vectors and wild animals, which indirectly changes the risks of vector-borne and zoonotic diseases. The primary discussion targets mosquitoes since they have phototaxis and they are vectors of multiple infectious diseases, e.g., malaria, dengue, and West Nile fever [162,163,164]. ALAN may motivate mosquitoes to actively bite humans and spread diseases [165]. In addition, exposure to dim ALAN helps female mosquitoes skip diapause and forces them to be more actively reproductive [166]. Similar discussions are also applicable to other vectors and vector-borne diseases if they have nighttime activity patterns and may be disturbed by ALAN, e.g., sand fly and visceral leishmaniasis [167]. ALAN may also be used to map the risks of zoonotic diseases since the activities of these intermediate hosts may be regulated by ALAN, e.g., bats and bat-borne rabies [168]. However, these discussions are still pilot studies and demand further development.
3.7. A Framework for Linking ALAN with Human Health
Based on the above systemic review of the literature published in the last two decades, we developed a multi-component framework for drawing a big picture to link ALAN with human health (Figure 6). This framework includes both the direct and indirect effects of ALAN on both the physical and mental health of humans, as well as considering ALAN as a social determinant of human health. Meanwhile, this framework also divides these effects into positive and negative health effects to cover diversified studies.
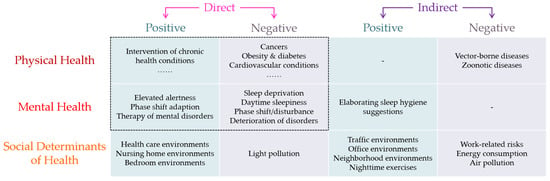
Figure 6.
The multi-component framework to link ALAN with human health.
The proposed framework would encourage neither the immoderate development of ALAN nor the return to complete darkness at night. On the opposite, this framework would encourage the proper use of ALAN to promote human health. However, conflicts in demands are unavoidable in this framework. For example, intensifying outdoor ALAN in highly populated neighborhoods may raise the residents’ perception of neighborhood safety for outdoor vigorous physical activity [151], which promotes both physical and mental health. On the other hand, more outdoor ALAN in the neighborhood may disturb sleep and human neuroendocrine circadian rhythms and may increase the risks of cancers and mental disorders such as breast cancer [81] and depression [121]. Another concrete example would be the nursing of older adults. Avoiding ALAN is suggested to improve the sleep quality of older adults and mitigate the syndromes of Alzheimer’s disease [169], but adequate ALAN may also be necessary to avoid the injury and death of older adults by preventing falling at night [19]. The trade-off between the positive effects of acute exposure to ALAN and the negative effects of chronic exposure to ALAN may not be equivalent for everyone, and elaborated discussions are necessary for particular groups of people, in specific contexts, and targeting special aims. This framework makes it easier to trace the different health effects of ALAN on a concrete issue and helps design the research to find the balance in the trade-off. Meanwhile, this framework indicates that there is more space for exploration of the health effects of ALAN, and it is also flexible to embrace new topics and insights in the future.
4. Discussion
Based on the above systematic evidence map and the proposed framework, we suggest several promising directions for future research in this area. These directions cover the methodological shift, the necessity of a framework, and social and environmental justice. We also discussed the limitations of this systematic evidence map in the last subsection.
4.1. From Lab Experiments to Epidemiological Investigations
Since the first attempt to associate ALAN with human health through breast cancer in the 1980s, scientists and doctors keep accumulating evidence concerning this interesting relationship, and it has never shown a trend to stop (Figure 2). Based on these findings, it is clear that ALAN does affect human neuroendocrine functions and sleep, which in turn may lead to changes in the use of ALAN. Further, we notice a boom of studies in recent years that consider ALAN as an environmental factor and a social determinant of health. Together with this boom, we notice several essential shifts in the studies of ALAN’s health effects. Primarily, there is a shift of study objectives from the human body’s responses to ALAN to people’s exposure to ALAN. Correspondingly, study designs have changed from well-controlled laboratory experiments to field surveys. Last and most importantly, discussions on this issue become more interdisciplinary. In addition to biomedical and health scientists, this research area started to attract the attention of scholars in geography, social science, and data science. A more interdisciplinary study requires the modification of the study paradigm since well-designed laboratory experiments with controlled variables and controlled groups are difficult to reproduce in real life. The study paradigm must be tailored according to the existing shape of sociodemographic profiles and human everyday life.
This paradigm shift faces many challenges in study methodology. The first challenge is the measurement of exposure to ALAN in residents’ daily life. Existing studies used field surveys [170], interviews and questionnaires [76,171,172], and environmental approximations through remote sensing techniques [173]. Field surveys provide more accurate and reliable data, but they are time-consuming, cost-inefficient, and thus are limited to small-sample studies. Interviews and questionnaires may provide unstructured data to challenge the design of the analysis. Remote sensing approximations are the most applicable strategy for large-scope investigations, but they only provide exposure to ambient outdoor ALAN, while the difference between outdoor and indoor ALAN should not be ignored. Since most people’s nighttime activities, especially sleep, are indoor, results based on remote sensing approximations may be biased. Moreover, conventional measurements of exposure to outdoor ALAN from remote sensing approaches mainly rely on nighttime light (NTL) imagery of a coarse spatial resolution due to the limited capability of sensors [174]. The spatial resolution of these NTL images is too coarse to match human nighttime activities. This mismatch may lead to inaccurate measurements of people’s exposure to outdoor ALAN and misleading conclusions [175,176]. Recently emerged NTL data sources with a higher spatial resolution (e.g., Luojia1-01 imagery at the 130 m spatial resolution and SDGSAT-1 imagery at the 10 m spatial resolution) may help mitigate these issues but the relevant academic efforts are still in demand.
The second challenge is embedded in conventional geographic and social studies. Considering the scale of study design, we could have multiple levels of studies on this issue, from case reports, laboratory experiments, cohort studies, and individual-based studies, to population-based studies. Individual-based studies are recently booming since they currently have strong support from the first three sorts of studies, and they can provide practical implications for public health. Stratified sampling is necessary for individual-based studies to use a small sample to represent the community, while the results would emphasize the disparities along the axes of age, gender, socio-economic status, etc., as well as in different geographic contexts. However, individual-based studies face two important methodological problems, namely the uncertain geographic context problem (UGCoP) [177] and the neighborhood effect averaging problem (NEAP) [178]. The UGCoP indicates that it is very difficult to accurately identify (1) the appropriate contextual units for capturing people’s exposure to ALAN and (2) the timing and duration in which individuals are exposed to ALAN. The NEAP indicates that the disparity of people’s exposure to ALAN may be averaged out when their mobility is considered. These problems challenge the validity and generalizability of the conclusions from individual-based studies that ignore the UGCoP, the NEAP, and human daily mobility. The use of portable smart devices, e.g., GENEActiv devices and light meters, to collect real-time exposure to ALAN [179] and the integration of multi-source data may help mitigate these challenges [180].
The last factor to emphasize is the vulnerable groups. As summarized in Section 3.2 and Figure 5, people may have a different vulnerability to ALAN’s health effects in different phases of life, health conditions, and occupations. Young adults have the strongest resilience to the health effects of ALAN while older adults may be more vulnerable. Investigations that include different social or age groups may average out the health impacts and obscure certain groups of vulnerable people. That may be a reason some of these individual-based studies showed insignificant results. Hence, while the sampling for epidemiological investigations must be consistent with the sociodemographic profile, the emphasis on clearly defined and embedded cohorts in the profile (e.g., women, older adults, and shift workers) is also necessary for future studies.
4.2. From Overemphasizing Negative Effects to a Multi-Component Framework
While the attention to the negative health effects of chronic exposure to ALAN is currently dominant in the literature, a few other studies also show the positive health effects of acute exposure to ALAN. These studies yielded valuable findings on ALAN’s health effects. However, people’s actual use of ALAN may be more complex than the study settings, and it may involve multiple health effects of ALAN in both the short and long term. For example, one of the indirect health effects of ALAN is through the bright spaces for nighttime physical exercise. Proper nighttime exercise is similar to daytime exercise, which directly promotes people’s cardiopulmonary system, muscular system, and skeletal system without disturbing sleep [181]. However, extra nighttime light may disturb human circadian rhythms, e.g., the sleep-wake cycle, and impair the human nervous system. These arguments may both be true in their own scope but together make a complicated pathway for the ultimate outcome of human health. This scenario is particularly important for sedentary office workers and some students. Their activities are constrained in offices and schools during the workdays, while their leisure time and bright spaces for nighttime exercise are highly regulated by ALAN after work and school. The answers to these issues are still unclear, and long-term longitudinal studies may help provide insightful perspectives. This multi-component framework has summarized all the relevant health effects of ALAN on humans given up-to-date studies, which makes it easier to have a comprehensive design of the investigation from all relevant aspects.
The framework is also useful for including ALAN in co-exposure or exposome research on an even larger scope. For example, the spatial distribution of ALAN has recently been found to be negatively associated with the spatial distribution of green space in urban areas [182]. As mentioned in this framework, ALAN provides bright spaces for nighttime physical exercise. Similarly, green space is one of the public places for daytime physical exercise. The integration of green space and ALAN-regulated bright spaces yields a comprehensive space-time for physical exercise and the promotion of human health. The co-exposure and joint accessibility to both green space and ALAN-regulated bright spaces are thus essential to discuss their overall promotion of human health. The proposed framework contains numerous topics to be joined with another environmental impact factor, which helps stimulate the development of co-exposure and exposome studies.
4.3. ALAN as a Social Determinant of Human Health and Social Justice
In recent years, there is an increasing interest in considering ALAN as a social determinant of human health. These studies are based on the premise that ALAN has either direct or indirect effects on human health, as well as acknowledging the disparity of exposure to ALAN across various sociodemographic axes. These studies provide valuable insights into the policy-making of urban planning, the suggestions for healthcare and nursing homes, and the discussion on environmental and social justice. We argue that more efforts and elaborated discussions are still in demand since different groups of people may need different settings of ALAN, and either the positive effects of acute exposure to ALAN or the negative effects of chronic exposure to ALAN matters for different groups of people, in different places, and regarding different aims.
The nighttime mobility of humans should not be ignored in research on environmental and social justice. Human nighttime activities are regulated by ALAN-determined bright spaces at night. Low illumination in the neighborhood may discourage nighttime outdoor activities while good lighting in the neighborhood may improve residents’ perception of security and encourage nighttime outdoor activities. This contrast may result in activity space-based social segregation [183], which in return further amplifies the disparity of exposure to ALAN across multiple axes, e.g., age, race, gender, and socio-economic status. These disparities in activity space and accumulated exposure to ALAN may chronically affect people’s physical, mental, and social well-being through either direct or indirect pathways. This topic is currently lacking discussion but is worth attention.
4.4. Limitations of This Systematic Evidence Map
We must acknowledge that whilst we tried our best efforts to draw a big picture on how ALAN may affect human health in a comprehensive framework, it is impossible to have exhaustive details in confined research. A few restrictions may lead to limited biases in our systematic evidence map. The first is the restriction of language. We aimed at only these English publications, which may skip the research that is purely documented in other languages, particularly for mainland Europe and East Asia. However, since English is currently the most generally used language for international academic communications, we believe that the search of only English publications will not lead to a significant absence of high-quality relevant research articles. It may also be a restriction to search only a limited number of academic publication databases. Regarding this restriction, we seriously chose the most popular academic publication databases that have very broad coverage of academic interests to minimize the bias, and it is less likely to miss a significant volume of relevant research articles, either. The last restriction is the search period. We determined the search period as the last two decades. Several pilot studies before this search period are excluded, but they are repeatedly reexamined in the later studies and thus will not lead to a bias in our systematic evidence map. On the other side, this topic is drawing increasing academic interest and new evidence will also keep accumulating soon after this search period. However, it is a very low probability that some breaking through will entirely reshape our systematic evidence map in the very short term. In a summary, with limited biases in spatial and temporal dimensions, our systematic evidence map can serve as the big picture to show how ALAN may affect human health for policy-making and decision-making.
By the nature of systematic evidence maps and the fact that ALAN may affect human health in tremendously diverse pathways, it is impossible to have a meta-analysis using a universal statistic such as Q or I2. Our quality assessments of the research articles on the four intensively discussed topics further strengthened this claim, please see Supplementary File S1 for more details. Downscaling to a smaller inner-homogenous question will mismatch the scope of this study, even though it is worth further effort. Our systematic evidence map can provide a road map for numerous such small questions and also remind researchers of other less popular but enlightening questions.
5. Conclusions
Since ALAN has been considered a human health impact factor, much research has been conducted in the last two decades. With diverse emphases and experiment settings, these studies have shown a complex picture, with even contradictory conclusions. This paper tries to clarify this big picture through a systematic evidence map of English research articles published in the three most popular publication databases. We then proposed a multi-component framework for conceptualizing the health effects of ALAN and organizing these diverse studies. The proposed framework includes both direct and indirect effects as well as both positive and negative effects of ALAN on human physical and mental health. It also considers ALAN as a social determinant of human health. ALAN imposes extra stress on human health by disturbing human circadian rhythms, where the disturbance of hormone secretions may have various effects on physical health and the disturbance of sleep may have various effects on mental health. The concerned outcomes are mainly cancers, chronic health conditions, cognitive impairments, and mental disorders. Inversely, the light-based intervention is also discussed to assist the therapy for multiple diseases. Because ALAN may affect human health, numerous studies have considered ALAN as a social determinant of health and suggest the modification of ALAN in offices, bedrooms, nursing homes, nighttime traffic, and so on. We also found that ALAN can be considered as an indirect environmental impact factor in affecting human health through other pathways, e.g., air pollution and vector-borne and zoonotic diseases.
In addition to the summary of rich evidence that links ALAN to human health, we also suggest some directions for further studies. Since individual-based studies are booming, we emphasize the importance of human mobility when measuring exposure to ALAN. Moreover, since the topics in our framework are also common to other human health impact factors, the proposed framework shows a clear picture to encourage co-exposure and exposome studies that include ALAN. Finally, considering ALAN as a social determinant of human health, it is also important to research ALAN-determined environmental justice, which is currently lacking in the discussion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments10030039/s1. Supplementary File S1. The attribute codes of the included literature.
Author Contributions
Conceptualization, Y.L. and M.-P.K.; methodology, data collection, formal analysis, investigation, and visualization, Y.L., C.Y. and K.W.; writing-original draft preparation, all authors; writing—review and editing, Y.L. and M.-P.K.; supervision, M.-P.K. and L.A.T.; funding acquisition, M.-P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Hong Kong Research Grants Council (General Research Fund Grant no. 14605920, 14606922; Collaborative Research Fund Grant no. C4023-20GF; Research Matching Grants RMG 8601219, 8601242), and a grant from the Research Committee on Research Sustainability of Major Research Grants Council Funding Schemes (3133235) of the Chinese University of Hong Kong.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- James, S.R.; Dennell, R.; Gilbert, A.S.; Lewis, H.T.; Gowlett, J.; Lynch, T.F.; McGrew, W.; Peters, C.R.; Pope, G.G.; Stahl, A.B. Hominid use of fire in the Lower and Middle Pleistocene: A review of the evidence [and comments and replies]. Curr. Anthropol. 1989, 30, 1–26. [Google Scholar] [CrossRef]
- Sanderson, S.W.; Simons, K.L. Light emitting diodes and the lighting revolution: The emergence of a solid-state lighting industry. Res. Policy 2014, 43, 1730–1746. [Google Scholar] [CrossRef]
- DiLaura, D. A brief history of lighting. Opt. Photonics News 2008, 19, 22–28. [Google Scholar] [CrossRef]
- WHO. Constitution of the World Health Organization. 2020. Available online: https://apps.who.int/gb/bd/PDF/bd47/EN/constitution-en.pdf?fbclid=IwAR3bSPmEMO5GR9oP9tFp5lD0BZFJM-fAtc12ogjAp8kFTD2t_fahFbfFeNY (accessed on 14 January 2022).
- WHO. Mental health: Strengthening our Response. 30 March 2018. Available online: https://cdn.ymaws.com/www.safestates.org/resource/resmgr/connections_lab/glossary_citation/mental_health_strengthening_.pdf (accessed on 20 January 2022).
- WHO. Social Determinants of Health. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 12 January 2022).
- Xiao, Q.; James, P.; Breheny, P.; Jia, P.; Park, Y.; Zhang, D.; Fisher, J.A.; Ward, M.H.; Jones, R.R. Outdoor light at night and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int. J. Cancer 2020, 147, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef]
- Rybnikova, N.; Portnov, B.A. Population-level study links short-wavelength nighttime illumination with breast cancer incidence in a major metropolitan area. Chronobiol. Int. 2018, 35, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.A.; Phillips, A.J.K.; Hosken, I.T.; McGlashan, E.M.; Anderson, C.; Lack, L.C.; Lockley, S.W.; Rajaratnam, S.M.W.; Cain, S.W. Increased sensitivity of the circadian system to light in delayed sleep-wake phase disorder. J. Physiol. 2018, 596, 6249–6261. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Abbott, S.M.; Reid, K.J.; Wu, D.; Kang, J.; Wilson, J.; Zee, P.C. Timing of light exposure and activity in adults with delayed sleep-wake phase disorder. Sleep Med. 2017, 32, 259–265. [Google Scholar] [CrossRef]
- Kloog, I.; Haim, A.; Stevens, R.G.; Portnov, B.A. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol. Int. 2009, 26, 108–125. [Google Scholar] [CrossRef]
- Srour, B.; Plancoulaine, S.; Andreeva, V.A.; Fassier, P.; Julia, C.; Galan, P.; Hercberg, S.; Deschasaux, M.; Latino-Martel, P.; Touvier, M. Circadian nutritional behaviours and cancer risk: New insights from the NutriNet-sante prospective cohort study. Int. J. Cancer 2018, 143, 2369–2379. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol. Int. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Exposure to light at night and risk of depression in the elderly. J. Affect. Disord. 2013, 151, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Association between light exposure at night and manic symptoms in bipolar disorder: Cross-sectional analysis of the APPLE cohort. Chronobiol. Int. 2020, 37, 887–896. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Harris, M.P.; Kim, M.J.; Wang, W.; Duffy, J.F. Scheduled evening sleep and enhanced lighting improve adaptation to night shift work in older adults. Occup. Environ. Med. 2016, 73, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, M.G.; Sahin, L.; Wood, B.; Plitnick, B. Light at night and measures of alertness and performance: Implications for shift workers. Biol. Res. Nurs. 2016, 18, 90–100. [Google Scholar] [CrossRef]
- Lim, Y.M.; Sung, M.H. Home environmental and health-related factors among home fallers and recurrent fallers in community dwelling older Korean women. Int. J. Nurs. Pract. 2012, 18, 481–488. [Google Scholar] [CrossRef]
- Stevens, R.G.; Davis, S. The melatonin hypothesis: Electric power and breast cancer. Environ. Health Perspect. 1996, 104, 135–140. [Google Scholar]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzinska, J.; Lie, J.-A.; Kjuus, H.; Wasowicz, W. Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses. Scand. J. Work Environ. Health 2013, 39, 187–194. [Google Scholar] [CrossRef]
- Cheng, P.; Tallent, G.; Burgess, H.J.; Tran, K.M.; Roth, T.; Drake, C.L. Daytime sleep disturbance in night shift work and the role of PERIOD3. J. Clin. Sleep Med. 2018, 14, 393–400. [Google Scholar] [CrossRef]
- Kervezee, L.; Cuesta, M.; Cermakian, N.; Boivin, D.B. The phase-shifting effect of bright light exposure on circadian rhythmicity in the human transcriptome. J. Biol. Rhythm. 2019, 34, 84–97. [Google Scholar] [CrossRef]
- Resuehr, D.; Wu, G.; Johnson, R.L., Jr.; Young, M.E.; Hogenesch, J.B.; Gamble, K.L. Shift work disrupts circadian regulation of the transcriptome in hospital nurses. J. Biol. Rhythm. 2019, 34, 167–177. [Google Scholar] [CrossRef]
- Aoki, K.; Yokoi, M.; Masago, R.; Iwanaga, K.; Kondo, N.; Katsuura, T. Modification of internal temperature regulation for cutaneous vasodilation and sweating by bright light exposure at night. Eur. J. Appl. Physiol. 2005, 95, 57–64. [Google Scholar] [CrossRef] [PubMed]
- de Blasiis, K.; Mauvieux, B.; Elsworth-Edelsten, C.; Peze, T.; Jouffroy, R.; Hurdiel, R. Photoperiod impact on a sailor’s sleep-wake rhythm and core body temperature in polar environment. Wilderness Environ. Med. 2019, 30, 343–350. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D.B. Skin temperature rhythms in humans respond to changes in the timing of sleep and light. J. Biol. Rhythm. 2017, 32, 257–273. [Google Scholar] [CrossRef] [PubMed]
- James, F.O.; Cermakian, N.; Boivin, D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep 2007, 30, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Galina, S.D.; Souza, J.C.; Valdez, P.; Azevedo, C.V.M. Daily light exposure, sleep-wake cycle and attention in adolescents from different urban contexts. Sleep Med. 2021, 81, 410–417. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocrinol. 2004, 25, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.S.; Rajaratnam, S.M.W.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef]
- Kayumov, L.; Casper, R.F.; Hawa, R.J.; Perelman, B.; Chung, S.A.; Sokalsky, S.; Shapiro, C.M. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J. Clin. Endocrinol. Metab. 2005, 90, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Nagare, R.; Plitnick, B.; Figueiro, M.G. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light. Res. Technol. 2019, 51, 530–543. [Google Scholar] [CrossRef]
- Wen, P.; Tan, F.; Wu, M.; Cai, Q.; Xu, R.; Zhang, X.; Wang, Y.; Khan, M.S.A.; Chen, W.; Hu, X. The effects of different bedroom light environments in the evening on adolescents. Build. Environ. 2021, 206, 108321. [Google Scholar] [CrossRef]
- Nie, J.; Zhou, T.; Chen, Z.; Dang, W.; Jiao, F.; Zhan, J.; Chen, Y.; Chen, Y.; Pan, Z.; Kang, X.; et al. The effects of dynamic daylight-like light on the rhythm, cognition, and mood of irregular shift workers in closed environment. Sci. Rep. 2021, 11, 13059. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, T.; Hidaka, Y.; Takakura, J.; Kusano, Y. Salivary melatonin suppression under 100-Hz flickering blue light and non-flickering blue light conditions. Neurosci. Lett. 2020, 722, 134857. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Devore, E.E.; Bajaj, A.; Lockley, S.W.; Figueiro, M.G.; Ricchiuti, V.; Gauderman, W.J.; Hankinson, S.E.; Willett, W.C.; Schernhammer, E.S. Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Hébert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 2002, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, T.; Taketomi, R.; Hidaka, Y.; Ide, N.; Yasuda, T. Preventive effect of morning bluish LED light on light-induced melatonin suppression at night. J. Sci. Technol. Light. 2018, 41, 206–210. [Google Scholar] [CrossRef]
- Jung, C.M.; Khalsa, S.B.S.; Scheer, F.; Cajochen, C.; Lockley, S.W.; Czeisler, C.A.; Wright, K.P. Acute effects of bright light exposure on cortisol levels. J. Biol. Rhythm. 2010, 25, 208–216. [Google Scholar] [CrossRef]
- Driller, M.W.; Jacobson, G.; Uiga, L. Hunger hormone and sleep responses to the built-in blue-light filter on an electronic device: A pilot study. Sleep Sci. 2019, 12, 171–177. [Google Scholar] [CrossRef]
- Nguyen, J.; Wright, K.P., Jr. Influence of weeks of circadian misalignment on leptin levels. Nat. Sci. Sleep 2010, 2, 9–18. [Google Scholar]
- Papantoniou, K.; Pozo, O.J.; Espinosa, A.; Marcos, J.; Castano-Vinyals, G.; Basagana, X.; Juanola Pages, E.; Mirabent, J.; Martin, J.; Such Faro, P.; et al. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 854–863. [Google Scholar] [CrossRef]
- Gómez-Acebo, I.; Dierssen-Sotos, T.; Papantoniou, K.; García-Unzueta, M.T.; Santos-Benito, M.F.; Llorca, J. Association between exposure to rotating night shift versus day shift using levels of 6-sulfatoxymelatonin and cortisol and other sex hormones in women. Chronobiol. Int. 2015, 32, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.K.; Stevens, R.G. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001, 93, 1557–1562. [Google Scholar] [CrossRef]
- Lee, C.; Smith, M.; Eastman, C. A compromise phase position for permanent night shift workers: Circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol. Int. 2006, 23, 859–875. [Google Scholar] [CrossRef]
- Smith, M.R.; Revell, V.L.; Eastman, C.I. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009, 10, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Khodasevich, D.; Tsui, S.; Keung, D.; Skene, D.J.; Revell, V.; Martinez, M.E. Characterizing the modern light environment and its influence on circadian rhythms. Proc. Biol. Sci. 2021, 288, 20210721. [Google Scholar] [CrossRef] [PubMed]
- Yelden, K.; Duport, S.; Kempny, A.; Playford, E.D. A rehabilitation unit at night: Environmental characteristics of patient rooms. Disabil. Rehabil. 2015, 37, 91–96. [Google Scholar] [CrossRef]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol. Int. 2017, 34, 855–865. [Google Scholar] [CrossRef]
- Mantua, J.; Ritland, B.M.; Naylor, J.A.; Simonelli, G.; Mickelson, C.A.; Choynowski, J.J.; Bessey, A.F.; Sowden, W.J.; Burke, T.M.; McKeon, A.B. Physical sleeping environment is related to insomnia risk and measures of readiness in US army special operations soldiers. BMJ Mil. Health 2021. [Google Scholar] [CrossRef]
- Fossum, I.N.; Nordnes, L.T.; Storemark, S.S.; Bjorvatn, B.; Pallesen, S. The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav. Sleep Med. 2014, 12, 343–357. [Google Scholar] [CrossRef]
- Rahman, S.A.; Marcu, S.; Kayumov, L.; Shapiro, C.M. Altered sleep architecture and higher incidence of subsyndromal depression in low endogenous melatonin secretors. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Mayes, L.; Jacobs, H.; Gross, I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics 2004, 113, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P.; Gamble, C.; Jones, A.P.; Wiggs, L.; Williamson, P.R.; Sutcliffe, A.; Montgomery, P.; Whitehouse, W.P.; Choonara, I.; Allport, T.; et al. Melatonin for sleep problems in children with neurodevelopmental disorders: Randomised double masked placebo-controlled trial. BMJ 2012, 345, e6664. [Google Scholar] [CrossRef]
- Paksarian, D.; Rudolph, K.E.; Stapp, E.K.; Dunster, G.P.; He, J.; Mennitt, D.; Hattar, S.; Casey, J.A.; James, P.; Merikangas, K.R. Association of outdoor artificial light at night with mental disorders and sleep patterns among US adolescents. JAMA Psychiatry 2020, 77, 1266–1275. [Google Scholar] [CrossRef]
- Crowley, S.J.; Cain, S.W.; Burns, A.C.; Acebo, C.; Carskadon, M.A. Increased sensitivity of the circadian system to light in early/mid-puberty. J. Clin. Endocrinol. Metab. 2015, 100, 4067–4073. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Driver, H.S. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Nelson, D.; Hertz, A.; Horn-Ross, P.L.; Bernstein, L.; Reynolds, P. Light at night and breast cancer risk among California teachers. Epidemiology 2014, 25, 697–706. [Google Scholar] [CrossRef]
- Sun, S.; Cao, W.; Ge, Y.; Ran, J.; Sun, F.; Zeng, Q.; Guo, M.; Huang, J.; Lee, R.S.; Tian, L.; et al. Outdoor light at night and risk of coronary heart disease among older adults: A prospective cohort study. Eur. Heart J. 2021, 42, 822–830. [Google Scholar] [CrossRef]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Comparing the response to acute and chronic exposure to short wavelength lighting emitted from computer screens. Chronobiol. Int. 2018, 35, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Bartel, K.; Scheeren, R.; Gradisar, M. Altering adolescents’ pre-bedtime phone use to achieve better sleep health. Health Commun. 2019, 34, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Grønli, J.; Byrkjedal, I.K.; Bjorvatn, B.; Nødtvedt, Ø.; Hamre, B.; Pallesen, S. Reading from an iPad or from a book in bed: The impact on human sleep. A randomized controlled crossover trial. Sleep Med. 2016, 21, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Lin, Y.; Qiu, J.; Zhang, Y.; Ohashi, M.; Lee, S.-i.; Kitamura, S.; Yasukouchi, A. Is the use of high correlated color temperature light at night related to delay of sleep timing in university students? A cross-country study in Japan and China. J. Physiol. Anthropol. 2021, 40, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Franklin, M.; Wiemels, J.; Chung, N.; Benbow, J.; Wang, S.; Lacey, J.; Longcore, T. Outdoor artificial light at night, sleep duration, and sleep quality in the California teachers study cohort. Sleep 2020, 43, A147. [Google Scholar] [CrossRef]
- Monma, T.; Ando, A.; Asanuma, T.; Yoshitake, Y.; Yoshida, G.; Miyazawa, T.; Ebine, N.; Takeda, S.; Omi, N.; Satoh, M.; et al. Sleep disorder risk factors among student athletes. Sleep Med. 2018, 44, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Bishop, L.; Luke, C.; Middleton, B.; Williams, P.; Arendt, J. Sleep during the Antarctic winter: Preliminary observations on changing the spectral composition of artificial light. J. Sleep Res. 2008, 17, 354–360. [Google Scholar] [CrossRef]
- Janz, N.K.; Musch, D.C.; Gillespie, B.W.; Wren, P.A.; Niziol, L.M. Evaluating clinical change and visual function concerns in drivers and nondrivers with glaucoma. Invest. Ophthalmol. Vis. Sci. 2009, 50, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Ackerman, R.C.; Lira-Trujillo, M.; Gollaz-Cervantez, A.C.; Cortés-Flores, A.O.; Zuloaga-Fernández Del Valle, C.J.; García-González, L.A.; Morgan-Villela, G.; Barbosa-Camacho, F.J.; Pintor-Belmontes, K.J.; Guzmán-Ramírez, B.G.; et al. Associations between stressors and difficulty sleeping in critically ill patients admitted to the intensive care unit: A cohort study. BMC Health Serv. Res. 2020, 20, 631. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, Q.; Zhou, P.; Jiang, S.; Diwan, V.K.; Xu, B. Environmental hazards increase the fall risk among residents of long-term care facilities: A prospective study in Shanghai, China. Age Ageing 2021, 50, 875–881. [Google Scholar] [CrossRef]
- Sunde, E.; Pedersen, T.; Mrdalj, J.; Thun, E.; Gronli, J.; Harris, A.; Bjorvatn, B.; Waage, S.; Skene, D.J.; Pallesen, S. Alerting and circadian effects of short-wavelength vs. long-wavelength narrow-bandwidth light during a simulated night shift. Clocks Sleep 2020, 2, 502–522. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, E.; Moon, J.-H.; Kim, Y.; Lee, H.-J. Circadian disruption and increase of oxidative stress in male and female volunteers after bright light exposure before bed time. Mol. Cell. Toxicol. 2019, 15, 221–229. [Google Scholar] [CrossRef]
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.M.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- O’Leary, E.S.; Schoenfeld, E.R.; Stevens, R.G.; Kabat, G.C.; Henderson, K.; Grimson, R.; Gammon, M.D.; Leske, M.C. Shift work, light at night, and breast cancer on Long Island, New York. Am. J. Epidemiol. 2006, 164, 358–366. [Google Scholar] [CrossRef]
- Stevens, R.G. Artificial lighting in the industrialized world: Circadian disruption and breast cancer. Cancer Causes Control 2006, 17, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, T.; Holford, T.R.; Boyle, P.; Zhang, Y.; Dai, M. Light at night and breast cancer risk: Results from a population-based case-control study in Connecticut, USA. Cancer Causes Control 2010, 21, 2281–2285. [Google Scholar] [CrossRef]
- White, A.J.; Weinberg, C.R.; Park, Y.-M.; D’Aloisio, A.A.; Vogtmann, E.; Nichols, H.B.; Sandler, D.P. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int. J. Cancer 2017, 141, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Ritonja, J.; McIsaac, M.A.; Sanders, E.; Kyba, C.C.M.; Grundy, A.; Cordina-Duverger, E.; Spinelli, J.J.; Aronson, K.J. Outdoor light at night at residences and breast cancer risk in Canada. Eur. J. Epidemiol. 2020, 35, 579–589. [Google Scholar] [CrossRef]
- Clarke, R.B.; Amini, H.; James, P.; von Euler-Chelpin, M.; Jørgensen, J.T.; Mehta, A.; Cole-Hunter, T.; Westendorp, R.; Mortensen, L.H.; Loft, S.; et al. Outdoor light at night and breast cancer incidence in the Danish nurse cohort. Environ. Res. 2021, 194, 110631. [Google Scholar] [CrossRef]
- Johns, L.E.; Jones, M.E.; Schoemaker, M.J.; McFadden, E.; Ashworth, A.; Swerdlow, A.J. Domestic light at night and breast cancer risk: A prospective analysis of 105 000 UK women in the generations sudy. Br. J. Cancer 2018, 118, 600–606. [Google Scholar] [CrossRef]
- Li, W.; Ray, R.M.; Thomas, D.B.; Davis, S.; Yost, M.; Breslow, N.; Gao, D.L.; Fitzgibbons, E.D.; Camp, J.E.; Wong, E.; et al. Shift work and breast cancer among women textile workers in Shanghai, China. Cancer Causes Control 2015, 26, 143–150. [Google Scholar] [CrossRef]
- Papantoniou, K.; Castano-Vinyals, G.; Espinosa, A.; Aragones, N.; Perez-Gomez, B.; Burgos, J.; Gomez-Acebo, I.; Llorca, J.; Peiro, R.; Jimenez-Moleon, J.J.; et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer 2015, 137, 1147–1157. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y.J.; Kim, J. The association between artificial light at night and prostate cancer in Gwangju city and South Jeolla Province of South Korea. Chronobiol. Int. 2017, 34, 203–211. [Google Scholar] [CrossRef]
- Garcia-Saenz, A.; de Miguel, A.S.; Espinosa, A.; Valentin, A.; Aragones, N.; Llorca, J.; Amiano, P.; Sanchez, V.M.; Guevara, M.; Capelo, R.; et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study). Environ. Health Perspect. 2018, 126, 047011. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Razavi, P.; Li, T.Y.; Qureshi, A.A.; Han, J. Rotating night shifts and risk of skin cancer in the nurses’ health study. J. Natl. Cancer Inst. 2011, 103, 602–606. [Google Scholar] [CrossRef]
- Heckman, C.J.; Kloss, J.D.; Feskanich, D.; Culnan, E.; Schernhammer, E.S. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup. Environ. Med. 2017, 74, 169–175. [Google Scholar] [CrossRef]
- Kloog, I.; Stevens, R.G.; Haim, A.; Portnov, B.A. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 2010, 21, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, E.; Lee, H.S.; Kim, M.; Park, M.S. High prevalence of breast cancer in light polluted areas in urban and rural regions of South Korea: An ecologic study on the treatment prevalence of female cancers based on National Health Insurance data. Chronobiol. Int. 2015, 32, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.S.; Song, J.-Y.; Joo, E.-Y.; Lee, H.-J.; Lee, E.; Lee, S.-k.; Jung, K.-Y. Outdoor artificial light at night, obesity, and sleep health: Cross-sectional analysis in the KoGES study. Chronobiol. Int. 2016, 33, 301–314. [Google Scholar] [CrossRef]
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int. J. Obes. 2016, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Zhang, A.H.; Yu, Y.; Wan, Y.H.; Tao, F.B.; Sun, Y. Sex-specific association of exposure to bedroom light at night with general and abdominal adiposity in young adults. Ecotoxicol. Environ. Saf. 2021, 223, 112561. [Google Scholar] [CrossRef]
- Park, Y.-M.M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef]
- Zhang, D.; Jones, R.R.; Powell-Wiley, T.M.; Jia, P.; James, P.; Xiao, Q. A large prospective investigation of outdoor light at night and obesity in the NIH-AARP diet and health study. Environ. Health 2020, 19, 74. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P., Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Wu, D.; Patel, S.R.; Loredo, J.S.; Redline, S.; Cai, J.; Gallo, L.C.; Mossavar-Rahmani, Y.; Ramos, A.R.; Teng, Y.; et al. Association between sleep timing, obesity, diabetes: The hispanic community health study/study of Latinos (HCHS/SOL) cohort study. Sleep 2017, 40, zsx014. [Google Scholar] [CrossRef]
- Butler, M.P.; Smales, C.; Wu, H.; Hussain, M.V.; Mohamed, Y.A.; Morimoto, M.; Shea, S.A. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep 2015, 38, 1793–1801. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol. Int. 2014, 31, 779–786. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Light exposure at night is associated with subclinical carotid atherosclerosis in the general elderly population: The HEIJO-KYO cohort. Chronobiol. Int. 2015, 32, 310–317. [Google Scholar] [CrossRef]
- Lopez-Gil, N.; Peixoto-De-Matos, S.C.; Thibos, L.N.; Gonzalez-Meijome, J.M. Shedding light on night myopia. J. Vis. 2012, 12, 4. [Google Scholar] [CrossRef]
- Ahn, J.; Ahn, S.-E.; Yang, K.-S.; Kim, S.-W.; Oh, J. Effects of a high level of illumination before sleep at night on chorioretinal thickness and ocular biometry. Exp. Eye Res. 2017, 164, 157–167. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Song, Y.; Cui, S.; Xue, C.; Wang, L.; Wu, J.; Yuan, J. An association between cumulative exposure to light at night and the prevalence of hyperuricemia in steel workers. Int. J. Occup. Med. Environ. Health 2021, 34, 385–401. [Google Scholar] [CrossRef]
- Zhang, S.K.; Wang, Y.B.; Zhu, Y.; Li, X.M.; Song, Y.; Yuan, J.X. Rotating night shift work, exposure to light at night, and glomerular filtration rate: Baseline results from a Chinese occupational cohort. Int. J. Environ. Res. Public Health 2020, 17, 9035. [Google Scholar] [CrossRef]
- Burgess, H.J.; Park, M.; Ong, J.C.; Shakoor, N.; Williams, D.A.; Burns, J. Morning versus evening bright light treatment at home to improve function and pain sensitivity for women with fibromyalgia: A pilot study. Pain Med. 2017, 18, 116–123. [Google Scholar] [CrossRef]
- Green, A.; Barak, S.; Shine, L.; Kahane, A.; Dagan, Y. Exposure by males to light emitted from media devices at night is linked with decline of sperm quality and correlated with sleep quality measures. Chronobiol. Int. 2020, 37, 414–424. [Google Scholar] [CrossRef]
- Feskanich, D.; Hankinson, S.E.; Schernhammer, E.S. Nightshift work and fracture risk: The nurses’ health study. Osteoporos. Int. 2009, 20, 537–542. [Google Scholar] [CrossRef]
- Meng, Y.; Zhu, V.; Zhu, Y. Co-distribution of light at night (LAN) and COVID-19 incidence in the United States. BMC Public Health 2021, 21, 1509. [Google Scholar] [CrossRef]
- Cheng, P.; Tallent, G.; Bender, T.J.; Tran, K.M.; Drake, C.L. Shift work and cognitive flexibility: Decomposing task performance. J. Biol. Rhythm. 2017, 32, 143–153. [Google Scholar] [CrossRef]
- Manousakis, J.E.; Scovelle, A.J.; Rajaratnam, S.M.W.; Naismith, S.L.; Anderson, C. Advanced circadian timing and sleep fragmentation differentially impact on memory complaint subtype in subjective cognitive decline. J. Alzheimers Dis. 2018, 66, 565–577. [Google Scholar] [CrossRef]
- Namita; Ranjan, D.P.; Shenvi, D.N. Effect of shift working on reaction time in hospital employees. Indian J. Physiol. Pharmacol 2010, 54, 289–293. [Google Scholar]
- Ganesan, S.; Magee, M.; Stone, J.E.; Mulhall, M.D.; Collins, A.; Howard, M.E.; Lockley, S.W.; Rajaratnam, S.M.W.; Sletten, T.L. The impact of shift work on sleep, alertness and performance in healthcare workers. Sci. Rep. 2019, 9, 4635. [Google Scholar] [CrossRef]
- McHill, A.W.; Wright, K.P., Jr. Cognitive impairments during the transition to working at night and on subsequent night shifts. J. Biol. Rhythm. 2019, 34, 432–446. [Google Scholar] [CrossRef]
- Chang, A.-M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Green, A.; Dagan, Y.; Haim, A. Exposure to screens of digital media devices, sleep, and concentration abilities in a sample of Israel adults. Sleep Biol. Rhythm. 2018, 16, 273–281. [Google Scholar] [CrossRef]
- Anderson, C.; Sullivan, J.P.; Flynn-Evans, E.E.; Cade, B.E.; Czeisler, C.A.; Lockley, S.W. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. Sleep 2012, 35, 1137–1146. [Google Scholar] [CrossRef]
- Scullin, M.K.; Hebl, M.R.; Corrington, A.; Nguyen, S. Experimental sleep loss, racial bias, and the decision criterion to shoot in the Police Officer’s Dilemma task. Sci. Rep. 2020, 10, 20581. [Google Scholar] [CrossRef]
- Gazenkampf, K.A.; Omelenchuk, R.K.; Emelyanova, V.N.; Shnayder, N.A.; Alekseeva, A.N.; Alekseeva, O.V.; Kazantsev, A.D.; Fadeeva, E.P.; Dmitrenko, D.V. Circadian sleep disorders in schoolchildren of countryside Siberia. Rus. Zhunal Det. Nevrol. 2017, 12, 40–42. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Association between light exposure at night and insomnia in the general elderly population: The HEIJO-KYO cohort. Chronobiol. Int. 2014, 31, 976–982. [Google Scholar] [CrossRef]
- Herrmann, W.J.; Flick, U. External barriers to good sleep from the nursing home residents’ perspective. Pflege Z. 2012, 65, 744–748. [Google Scholar]
- Min, J.-y.; Min, K.-b. Outdoor light at night and the prevalence of depressive symptoms and suicidal behaviors: A cross-sectional study in a nationally representative sample of Korean adults. J. Affect. Disord. 2018, 227, 199–205. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Bedroom light exposure at night and the incidence of depressive symptoms: A longitudinal study of the HEIJO-KYO cohort. Am. J. Epidemiol. 2018, 187, 427–434. [Google Scholar] [CrossRef]
- Esaki, Y.; Kitajima, T.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N. Light exposure at night and sleep quality in bipolar disorder: The APPLE cohort study. J. Affect. Disord. 2019, 257, 314–320. [Google Scholar] [CrossRef]
- Gumenyuk, V.; Howard, R.; Roth, T.; Korzyukov, O.; Drake, C.L. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep 2014, 37, 545–556. [Google Scholar] [CrossRef]
- Olson, J.A.; Artenie, D.Z.; Cyr, M.; Raz, A.; Lee, V. Developing a light-based intervention to reduce fatigue and improve sleep in rapidly rotating shift workers. Chronobiol. Int. 2020, 37, 573–591. [Google Scholar] [CrossRef]
- Sunde, E.; Mrdalj, J.; Pedersen, T.; Thun, E.; Bjorvatn, B.; Grønli, J.; Harris, A.; Waage, S.; Pallesen, S. Role of nocturnal light intensity on adaptation to three consecutive night shifts: A counterbalanced crossover study. Occup. Environ. Med. 2020, 77, 249–255. [Google Scholar] [CrossRef]
- Bjorvatn, B.; Waage, S. Bright light improves sleep and psychological health in shift working nurses. J. Clin. Sleep Med. 2013, 9, 647–648. [Google Scholar] [CrossRef]
- Moscovici, L.; Kotler, M. A multistage chronobiologic intervention for the treatment of depression: A pilot study. J. Affect. Disord. 2009, 116, 201–207. [Google Scholar] [CrossRef]
- Kaplan, K.A.; Mashash, M.; Williams, R.; Batchelder, H.; Starr-Glass, L.; Zeitzer, J.M. Effect of light flashes vs sham therapy during sleep with adjunct cognitive behavioral therapy on sleep quality among adolescents: A randomized clinical trial. JAMA Netw. Open 2019, 2, e1911944. [Google Scholar] [CrossRef]
- Suhner, A.G.; Murphy, P.J.; Campbell, S.S. Failure of timed bright light exposure to alleviate age-related sleep maintenance insomnia. J. Am. Geriatr. Soc. 2002, 50, 617–623. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Plitnick, B.A.; Lok, A.; Jones, G.E.; Higgins, P.; Hornick, T.R.; Rea, M.S. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin. Interv. Aging 2014, 9, 1527–1537. [Google Scholar] [CrossRef]
- Henriksen, T.E.; Skrede, S.; Fasmer, O.B.; Schoeyen, H.; Leskauskaite, I.; Bjørke-Bertheussen, J.; Assmus, J.; Hamre, B.; Grønli, J.; Lund, A. Blue-blocking glasses as additive treatment for mania: A randomized placebo-controlled trial. Bipolar Disord. 2016, 18, 221–232. [Google Scholar] [CrossRef]
- Tao, L.; Jiang, R.; Zhang, K.; Qian, Z.; Chen, P.; Lv, Y.; Yao, Y. Light therapy in non-seasonal depression: An update meta-analysis. Psychiatry Res. 2020, 291, 113247. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Moore-Ede, M.; Heitmann, A.; Guttkuhn, R. Circadian potency spectrum with extended exposure to polychromatic white LED light under workplace conditions. J. Biol. Rhythm. 2020, 35, 405–415. [Google Scholar] [CrossRef]
- Sadeghniiat-Haghighi, K.; Yazdi, Z.; Jahanihashemi, H.; Aminian, O. The effect of bright light on sleepiness among rapid-rotating 12-hour shift workers. Scand. J. Work Environ. Health 2011, 37, 77–79. [Google Scholar] [CrossRef]
- Karchani, M.; Kakooei, H.; Yazdi, Z.; Zare, M. Do bright-light shock exposures during breaks reduce subjective sleepiness in night workers? Sleep Biol. Rhythm. 2011, 9, 95–102. [Google Scholar] [CrossRef]
- Harrison, E.M.; Schmied, E.A.; Easterling, A.P.; Yablonsky, A.M.; Glickman, G.L. A hybrid effectiveness-implementation study of a multi-component lighting intervention for hospital shift workers. Int. J. Environ. Res. Public Health 2020, 17, 9141. [Google Scholar] [CrossRef]
- Postnova, S.; Robinson, P.A.; Postnov, D.D. Adaptation to shift work: Physiologically based modeling of the effects of lighting and shifts’ start time. PLoS ONE 2013, 8, e53379. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Plitnick, B.; Rea, M.S.; Gras, L.Z.; Rea, M.S. Lighting and perceptual cues: Effects on gait measures of older adults at high and low risk for falls. BMC Geriatr. 2011, 11, 49. [Google Scholar] [CrossRef]
- Wood, J.M.; Isoardi, G.; Black, A.; Cowling, I. Night-time driving visibility associated with LED streetlight dimming. Accid. Anal. Prev. 2018, 121, 295–300. [Google Scholar] [CrossRef]
- Abegaz, T.; Berhane, Y.; Worku, A.; Assrat, A.; Assefa, A. Effects of excessive speeding and falling asleep while driving on crash injury severity in Ethiopia: A generalized ordered logit model analysis. Accid. Anal. Prev. 2014, 71, 15–21. [Google Scholar] [CrossRef]
- Chang, F.; Li, M.; Xu, P.; Zhou, H.; Haque, M.M.; Huang, H. Injury severity of motorcycle riders involved in traffic crashes in Hunan, China: A mixed ordered logit approach. Int. J. Environ. Res. Public Health 2016, 13, 714. [Google Scholar] [CrossRef]
- Plainis, S.; Murray, I.J.; Pallikaris, I.G. Road traffic casualties: Understanding the night-time death toll. Inj. Prev. 2006, 12, 125–128. [Google Scholar] [CrossRef]
- Marchant, P.; Hale, J.D.; Sadler, J.P. Does changing to brighter road lighting improve road safety? Multilevel longitudinal analysis of road traffic collision frequency during the relighting of a UK city. J. Epidemiol. Community Health 2020, 74, 467–472. [Google Scholar] [CrossRef]
- Claudio, L. Switch on the night: Policies for smarter lighting. Environ. Health Perspect. 2009, 117, A28–A31. [Google Scholar] [CrossRef]
- Kimlin, J.A.; Black, A.A.; Wood, J.M. Nighttime driving in older adults: Effects of glare and association with mesopic visual function. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Petticrew, M.; Whitehead, M.; Neary, D.; Clayton, S.; Wright, K.; Thomson, H.; Cummins, S.; Sowden, A.; Renton, A. Fear of crime and the environment: Systematic review of UK qualitative evidence. BMC Public Health 2013, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Innes, M.; Jones, V. Neighbourhood Security and Urban Change: Risk, Resilience and Recovery; Joseph Rowntree Foundation: York, UK, 2006. [Google Scholar]
- Lee, C.; Lee, C.; Stewart, O.T.; Carlos, H.A.; Adachi-Mejia, A.; Berke, E.M.; Doescher, M.P. Neighborhood environments and utilitarian walking among older vs. younger rural adults. Front. Public Health 2021, 9, 634751. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Scott, M.M.; Cohen, D.A.; Voorhees, C.C. Girls’ perception of neighborhood factors on. physical activity, sedentary behavior, and BMI. Obesity 2007, 15, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Burton, N.W.; Khan, A. Perceived environmental barriers to physical activity in young adults in Dhaka City, Bangladesh-does gender matter? Int. Health 2018, 10, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Welsh, B.C.; Farrington, D.P. Effects of improved street lighting on crime. Campbell Syst. Rev. 2008, 4, 1–51. [Google Scholar] [CrossRef]
- Green, J.; Perkins, C.; Steinbach, R.; Edwards, P. Reduced street lighting at night and health: A rapid appraisal of public views in England and Wales. Health Place 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Larkin, A.; van Donkelaar, A.; Geddes, J.A.; Martin, R.V.; Hystad, P. Relationships between changes in urban characteristics and air quality in East Asia from 2000 to 2010. Environ. Sci. Technol. 2016, 50, 9142–9149. [Google Scholar] [CrossRef]
- Huss, A.; van Wel, L.; Bogaards, L.; Vrijkotte, T.; Wolf, L.; Hoek, G.; Vermeulen, R. Shedding some light in the dark—A comparison of personal measurements with satellite-based estimates of exposure to light at night among children in the Netherlands. Env. Health Perspect. 2019, 127, 67001. [Google Scholar] [CrossRef]
- Wong, K.W.; Stutz, J. Influence of nocturnal vertical stability on daytime chemistry: A one-dimensional model study. Atmos. Environ. 2010, 44, 3753–3760. [Google Scholar] [CrossRef]
- Stark, H.; Brown, S.S.; Wong, K.W.; Stutz, J.; Elvidge, C.D.; Pollack, I.B.; Ryerson, T.B.; Dube, W.P.; Wagner, N.L.; Parrish, D.D. City lights and urban air. Nat. Geosci. 2011, 4, 730–731. [Google Scholar] [CrossRef]
- Brown, S.; Ryerson, T.; Wollny, A.; Brock, C.; Peltier, R.; Sullivan, A.; Weber, R.; Dube, W.; Trainer, M.; Meagher, J.F. Variability in nocturnal nitrogen oxide processing and its role in regional air quality. Science 2006, 311, 67–70. [Google Scholar] [CrossRef]
- Bedi, T.K.; Puntambekar, K.; Singh, S. Light pollution in India: Appraisal of artificial night sky brightness of cities. Environ. Dev. Sustain. 2021, 23, 18582–18597. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Wang, Y. Estimating local-scale urban heat island intensity using nighttime light satellite imageries. Sustain. Cities Soc. 2020, 57, 102125. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Labb, L.F.; Benefiel, O.M.; Duffield, G.E. Artificial light at night increases Aedes aegypti mosquito biting behavior with implications for arboviral disease transmission. Am. J. Trop. Med. Hyg. 2020, 103, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Duffield, G.E.; Acri, D.J.; George, G.F.; Sheppard, A.D.; Beebe, N.W.; Ritchie, S.A.; Burkot, T.R. Diel flight activity of wild-caught Anopheles farauti (s.s.) and An. hinesorum malaria mosquitoes from northern Queensland, Australia. Parasites Vectors 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Osório, H.C.; Zé-Zé, L.; Amaro, F.; Alves, M.J. Mosquito surveillance for prevention and control of emerging mosquito-borne diseases in Portugal—2008–2014. Int. J. Environ. Res. Public Health 2014, 11, 11583–11596. [Google Scholar] [CrossRef]
- Sheppard, A.D.; Rund, S.S.C.; George, G.F.; Clark, E.; Acri, D.J.; Duffield, G.E. Light manipulation of mosquito behaviour: Acute and sustained photic suppression of biting activity in the Anopheles gambiae malaria mosquito. Parasites Vectors 2017, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Fyie, L.R.; Gardiner, M.M.; Meuti, M.E. Artificial light at night alters the seasonal responses of biting mosquitoes. J. Insect Physiol. 2021, 129, 104194. [Google Scholar] [CrossRef]
- Aklilu, E.; Gebresilassie, A.; Yared, S.; Kindu, M.; Tekie, H.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-Michael, T. Comparative study on the nocturnal activity of phlebotomine sand flies in a highland and lowland foci of visceral leishmaniasis in north-western Ethiopia with special reference to Phlebotomus orientalis. Parasites Vectors 2017, 10, 393. [Google Scholar] [CrossRef]
- Escobar, L.E.; Peterson, A.T.; Papeş, M.; Favi, M.; Yung, V.; Restif, O.; Qiao, H.; Medina-Vogel, G. Ecological approaches in veterinary epidemiology: Mapping the risk of bat-borne rabies using vegetation indices and night-time light satellite imagery. Vet. Res. 2015, 46, 92. [Google Scholar] [CrossRef]
- Song, Y.; McCurry, S.M.; Lee, D.; Josephson, K.R.; McGowan, S.K.; Fung, C.H.; Irwin, M.R.; Teng, E.; Alessi, C.A.; Martin, J.L. Development of a dyadic sleep intervention for Alzheimer’s disease patients and their caregivers. Disabil. Rehabil. 2021, 43, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Bedroom light exposure at night and obesity in individuals with bipolar disorder: A cross-sectional analysis of the APPLE cohort. Physiol. Behav. 2021, 230, 113281. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Yablonsky, A.M.; Powell, A.L.; Ancoli-Israel, S.; Glickman, G.L. Reported light in the sleep environment: Enhancement of the sleep diary. Nat. Sci. Sleep 2019, 11, 11. [Google Scholar] [CrossRef]
- Siraji, M.A.; Lazar, R.R.; van Duijnhoven, J.; Schlangen, L.J.; Haque, S.; Kalavally, V.; Vetter, C.; Glickman, G.; Smolders, K.C.; Spitschan, M. Light exposure behaviour assessment (LEBA): A novel self-reported instrument to capture light exposure-related behaviour. In Proceedings of the CIE Australia Lighting Research Conference, Online, 8 February 2022; p. 1. [Google Scholar]
- Katz, Y.; Levin, N. Quantifying urban light pollution—A comparison between field measurements and EROS-B imagery. Remote Sens. Environ. 2016, 177, 65–77. [Google Scholar] [CrossRef]
- Levin, N.; Kyba, C.C.; Zhang, Q.; de Miguel, A.S.; Román, M.O.; Li, X.; Portnov, B.A.; Molthan, A.L.; Jechow, A.; Miller, S.D. Remote sensing of night lights: A review and an outlook for the future. Remote Sens. Environ. 2020, 237, 111443. [Google Scholar] [CrossRef]
- Kyba, C.C. Defense meteorological satellite program data should no longer be used for epidemiological studies. Chronobiol. Int. 2016, 33, 943–945. [Google Scholar] [CrossRef]
- McIsaac, M.A.; Sanders, E.; Kuester, T.; Aronson, K.J.; Kyba, C.C. The impact of image resolution on power, bias, and confounding: A simulation study of ambient light at night exposure. Environ. Epidemiol. 2021, 5, e145. [Google Scholar] [CrossRef]
- Kwan, M.-P. The uncertain geographic context problem. Ann. Assoc. Am. Geogr. 2012, 102, 958–968. [Google Scholar] [CrossRef]
- Kwan, M.-P. The neighborhood effect averaging problem (NEAP): An elusive confounder of the neighborhood effect. Int. J. Environ. Res. Public Health 2018, 15, 1841. [Google Scholar] [CrossRef]
- Stone, J.E.; McGlashan, E.M.; Facer-Childs, E.R.; Cain, S.W.; Phillips, A.J. Accuracy of the GENEActiv device for measuring light exposure in sleep and circadian research. Clocks Sleep 2020, 2, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kou, L.; Kwan, M.-P.; Shakespeare, R.M.; Lee, K.; Park, Y.M. An integrated individual environmental exposure assessment system for real-time mobile sensing in environmental health studies. Sensors 2021, 21, 4039. [Google Scholar] [CrossRef] [PubMed]
- Buman, M.P.; Phillips, B.A.; Youngstedt, S.D.; Kline, C.E.; Hirshkowitz, M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Med. 2014, 15, 755–761. [Google Scholar] [CrossRef]
- Stanhope, J.; Liddicoat, C.; Weinstein, P. Outdoor artificial light at night: A forgotten factor in green space and health research. Environ. Res. 2021, 197, 111012. [Google Scholar] [CrossRef] [PubMed]
- Ta, N.; Kwan, M.-P.; Lin, S.; Zhu, Q. The activity space-based segregation of migrants in suburban Shanghai. Appl. Geogr. 2021, 133, 102499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).