Impact of Polarization Reversal during Photoelectrocatalytic Treatment of WWTP Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of TiO2 Catalyst
2.2. Properties of WWTP Effluents
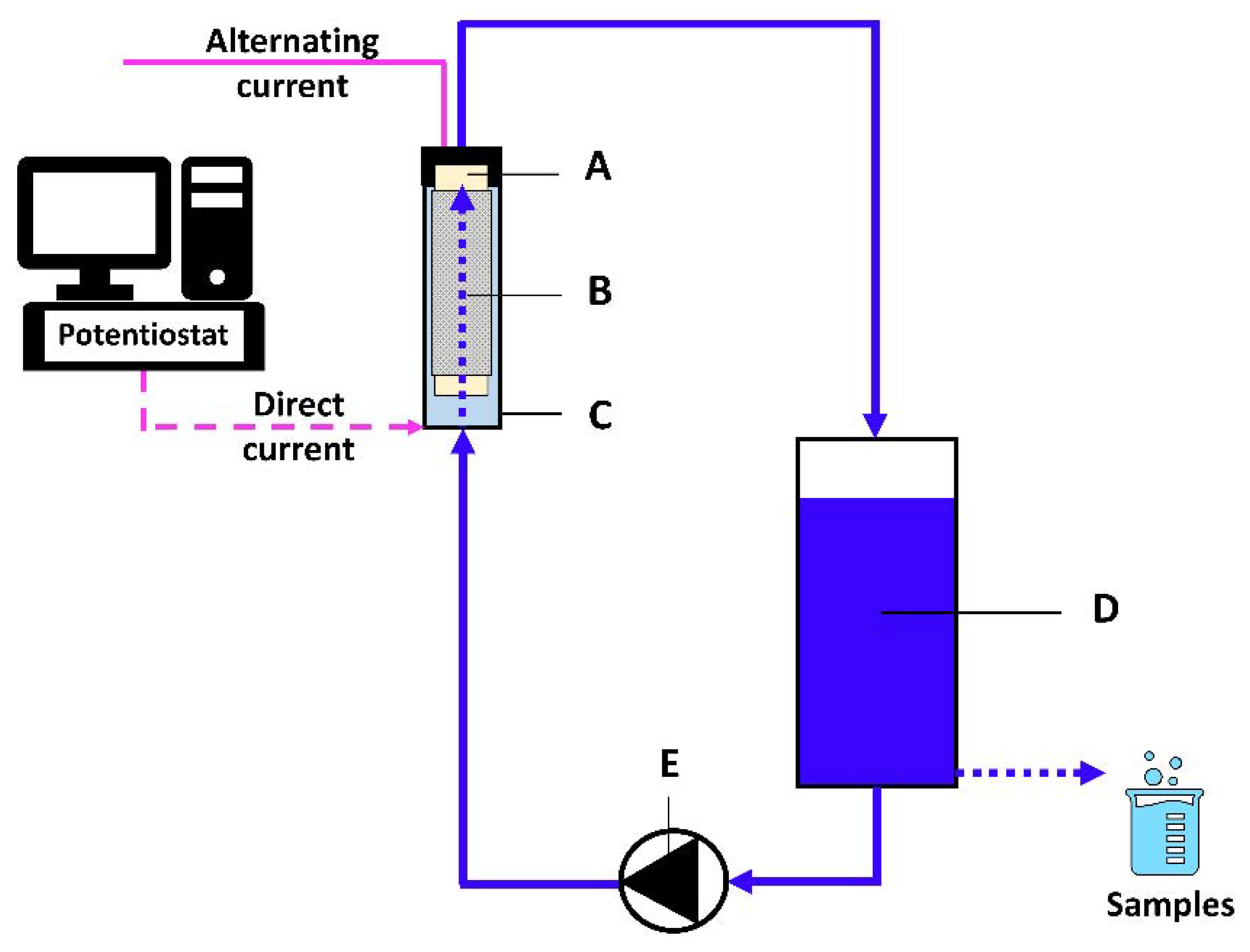
2.3. Experimental Set-Up and Lab-Scale Reactor
- Electrochemical oxidation (EC): TiO2 + bias
- Photolysis (PL): UV
- Photocatalysis (PC): UV + TiO2
- Photoelectrocatalysis (PEC): UV + TiO2 + bias
- Photoelectrocatalysis with polarization reversal (PECr): UV + TiO2 + reverse bias.
2.4. Evaluation of the Treatments Effectiveness
2.4.1. Pollutants Removal
2.4.2. Biodegradability of the Water
3. Results and Discussion
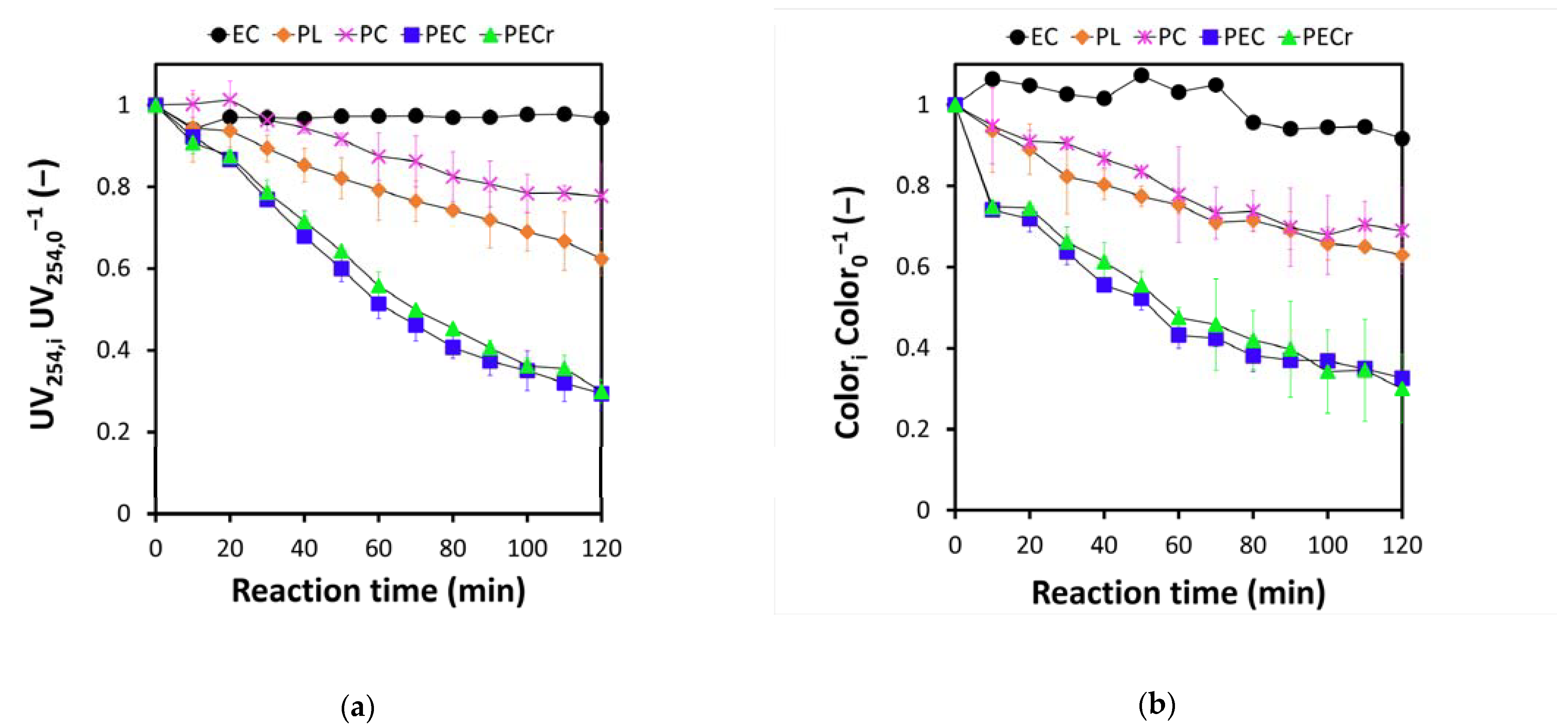
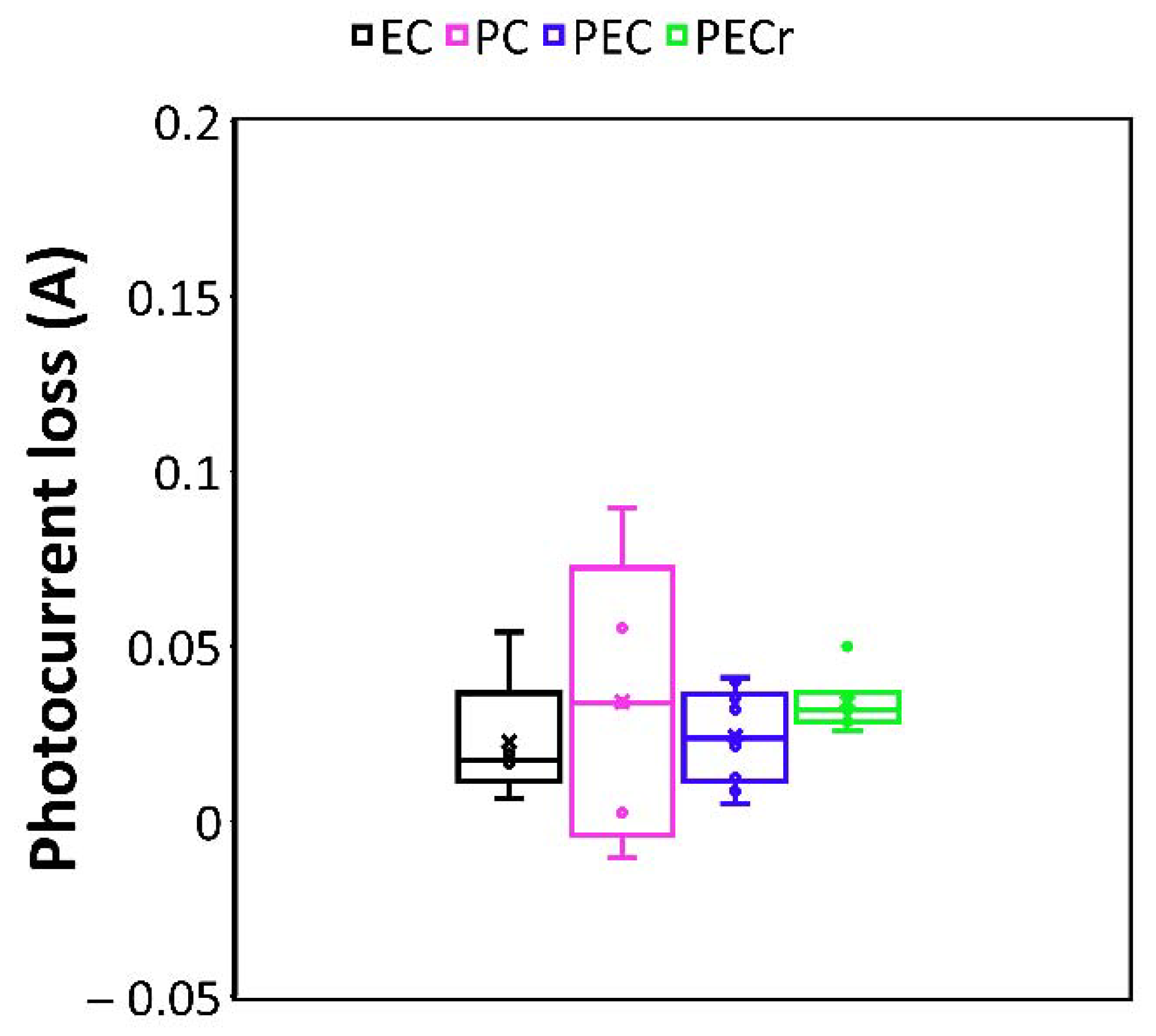
3.1. Pollutants Degradation
3.2. Effects on Biodegradability
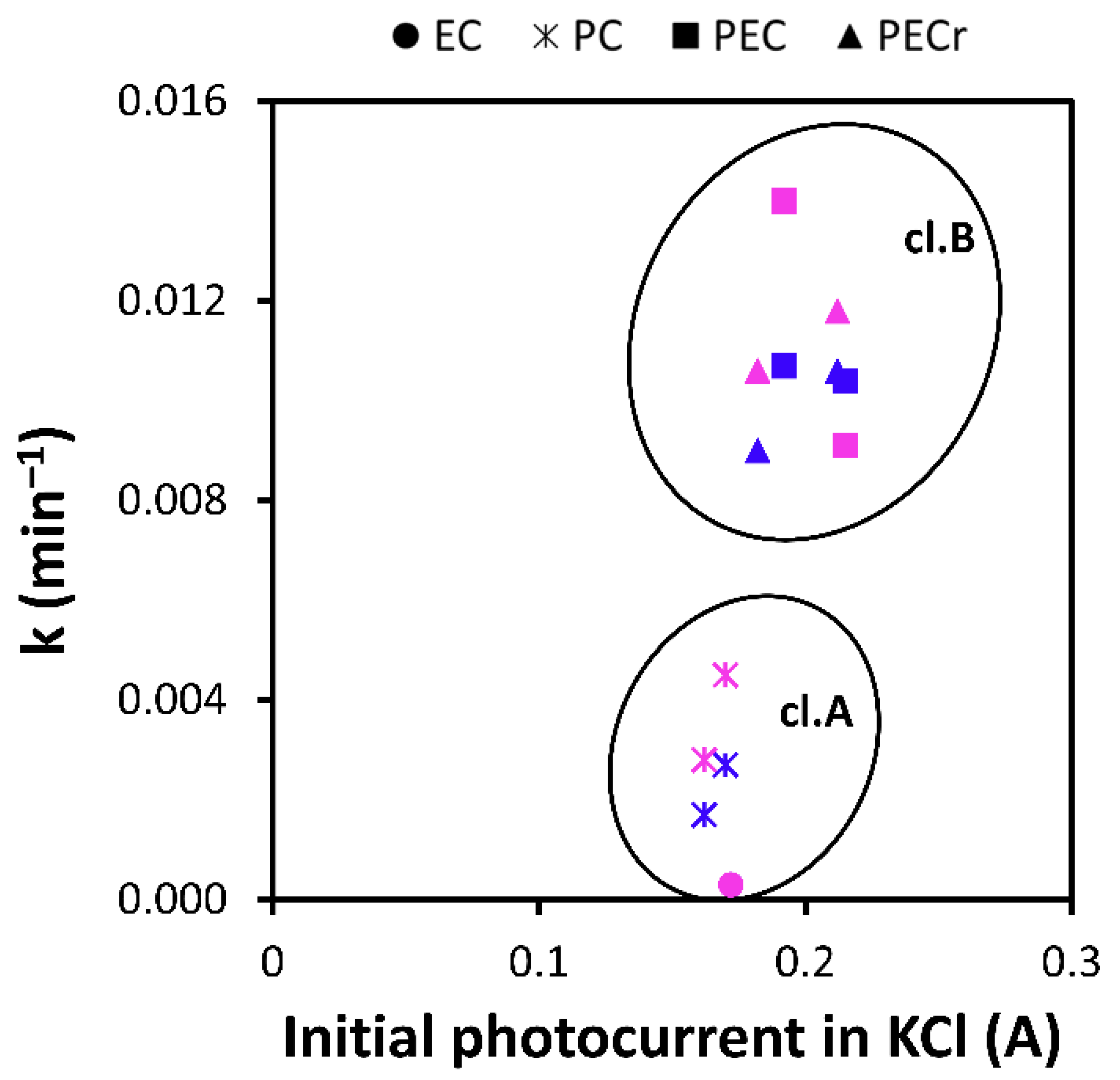
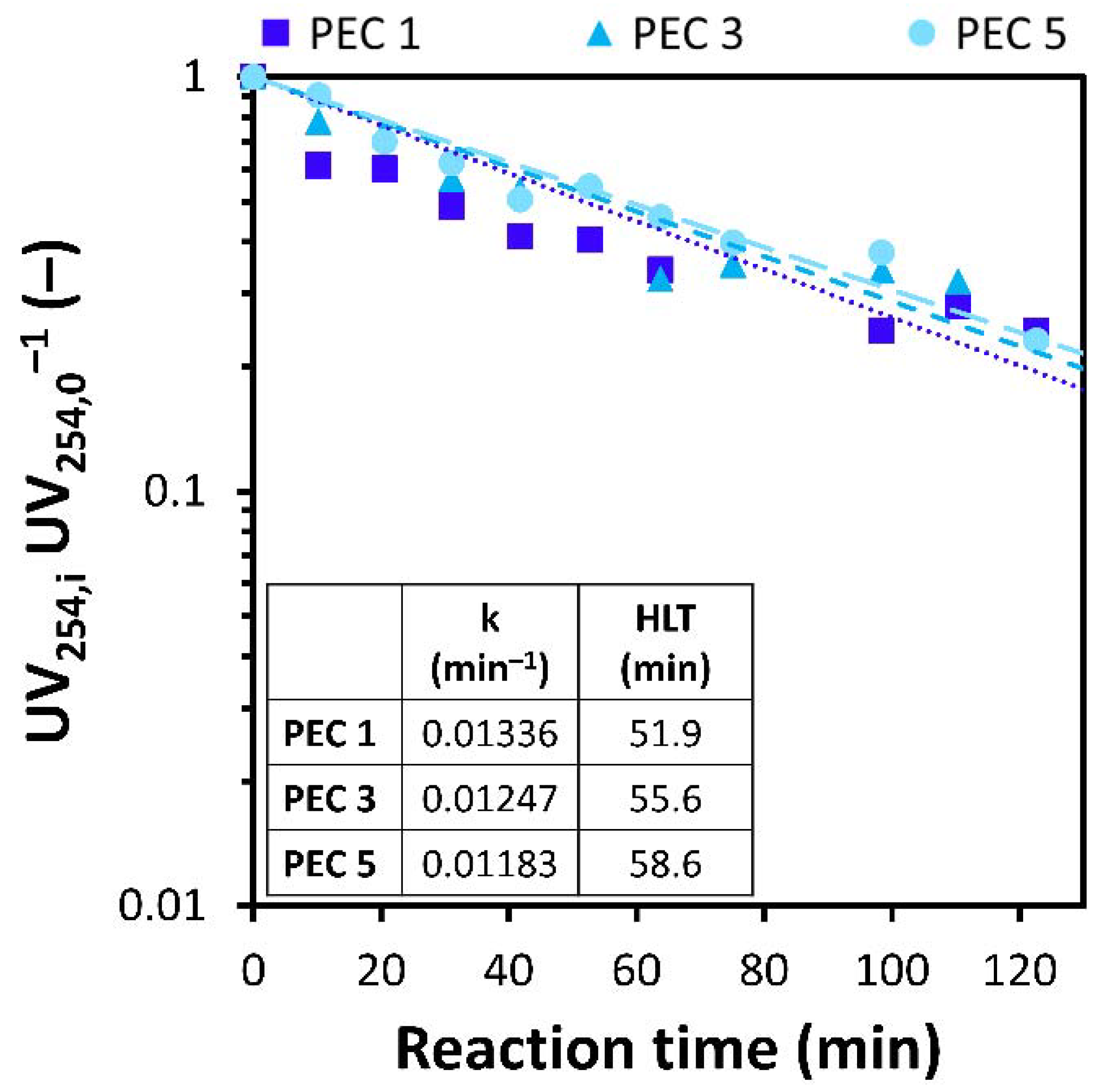
3.3. Mesh Inactivation and Influence of Reverse Polarization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and Biological Treatments for Wastewater Decontamination-A Review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Palmas, S.; Mais, L.; Mascia, M.; Vacca, A. Trend in Using TiO2 Nanotubes as Photoelectrodes in PEC Processes for Wastewater Treatment. Curr. Opin. Electrochem. 2021, 28, 100699. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking Recent Advances and Innovative Technology Approaches of Fenton, Photo-Fenton, Electro-Fenton, and Related Processes: A Review on the Relevance of Phenol as Model Molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar]
- Mills, A.; O’Rourke, C.; Moore, K. Powder Semiconductor Photocatalysis in Aqueous Solution: An Overview of Kinetics-Based Reaction Mechanisms. J. Photochem. Photobiol. A Chem. 2015, 310, 66–105. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Bestetti, M. Photoelectrochemical Advanced Oxidation Processes on Nanostructured TiO2 Catalysts: Decolorization of a Textile Azo-Dye. J. Water Chem. Technol. 2015, 37, 108–115. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Orimolade, B.O.; Masekela, D.; Mamba, B.; Mabuba, N. The Application of Photoelectrocatalysis in the Degradation of Rhodamine B in Aqueous Solutions: A Review. RSC Adv. 2022, 12, 26176–26191. [Google Scholar] [CrossRef]
- Noorjahan, M.; Pratap Reddy, M.; Durga Kumari, V.; Lavédrine, B.; Boule, P.; Subrahmanyam, M. Photocatalytic Degradation of H-Acid over a Novel TiO2 Thin Film Fixed Bed Reactor and in Aqueous Suspensions. J. Photochem. Photobiol. A Chem. 2003, 156, 179–187. [Google Scholar] [CrossRef]
- Baccaro, A.; Gutz, I. Photoelectrocatalysis in Semiconductors: From Basic Principles to Nanoscale Conformation (in Portuguese). Quim. Nova 2018, 41, 326–339. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Fu, Y.; Yan, H.; Qin, L.; Lai, C.; Zhang, C.; Ye, H.; Chen, W.; Qin, F.; et al. Recent Advances in Photoelectrocatalysis for Environmental Applications: Sensing, Pollutants Removal and Microbial Inactivation. Coord. Chem. Rev. 2022, 454, 214341. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Guaraldo, T.T.; de Brito, J.F.; Brugnera, M.F.; Zanoni, M.V.B. Achievements and Trends in Photoelectrocatalysis: From Environmental to Energy Applications. Electrocatalysis 2015, 6, 415–441. [Google Scholar] [CrossRef]
- Olea, M.A.U.; Bueno, J.d.J.P.; Pérez, A.X.M. Nanometric and Surface Properties of Semiconductors Correlated to Photocatalysis and Photoelectrocatalysis Applied to Organic Pollutants—A Review. J. Environ. Chem. Eng. 2021, 9, 106480. [Google Scholar] [CrossRef]
- Komtchou, S.; Dirany, A.; Drogui, P.; Delegan, N.; El Khakani, M.A.; Robert, D.; Lafrance, P. Degradation of Atrazine in Aqueous Solution with Electrophotocatalytic Process Using TiO2−x Photoanode. Chemosphere 2016, 157, 79–88. [Google Scholar] [CrossRef]
- Malato, S. Removal of Emerging Contaminants in Waste-Water Treatment: Removal by Photo-Catalytic Processes. In Emerging Contaminants from Industrial and Municipal Waste. The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 177–197. [Google Scholar]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Bertanza, G.; Sorlini, S.; Damiani, S.; Arab, H.; Bestetti, M.; Franz, S. Photoelectrocatalysis on TiO2 Meshes: Different Applications in the Integrated Urban Water Management. Environ. Sci. Pollut. Res. 2021, 28, 59452–59461. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Nakamura, R.; Tachibana, Y. Primary Photocatalytic Water Reduction and Oxidation at an Anatase TiO2 and Pt-TiO2 Nanocrystalline Electrode Revealed by Quantitative Transient Absorption Studies. Appl. Catal. B 2021, 296, 120226. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Zhou, X.; Lin, H.; Peng, F.; Zhang, S. Designing Efficient TiO2-Based Photoelectrocatalysis Systems for Chemical Engineering and Sensing. Chem. Eng. J. 2020, 381, 122605. [Google Scholar] [CrossRef]
- Egerton, T.A.; Christensen, P.A.; Kosa, S.A.M.; Onoka, B.; Harper, J.C.; Tinlin, J.R. Photoelectrocatalysis by Titanium Dioxide for Water Treatment. Int. J. Environ. Pollut. 2006, 27, 2. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of Emerging Organic Pollutants in Wastewater Effluents by Electrochemical Photocatalysis on Nanostructured TiO2 Meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.; Pham, A.L.-T. Mitigating Electrode Fouling in Electrocoagulation by Means of Polarity Reversal: The Effects of Electrode Type, Current Density, and Polarity Reversal Frequency. Water Res. 2021, 197, 117074. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Efficiency and Energy Demand in Polishing Treatment of Wastewater Treatment Plants Effluents: Photoelectrocatalysis vs. Photocatalysis and Photolysis. Water 2021, 13, 821. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Lucotti, A.; Bestetti, M. Photoactive TiO2 Coatings Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Appl. Surf. Sci. 2016, 385, 498–505. [Google Scholar] [CrossRef]
- Franz, S.; Arab, H.; Chiarello, G.L.; Bestetti, M.; Selli, E. Single-Step Preparation of Large Area TiO2 Photoelectrodes for Water Splitting. Adv. Energy Mater. 2020, 10, 2000652. [Google Scholar] [CrossRef]
- Franz, S.; Arab, H.; Lucotti, A.; Castiglioni, C.; Vicenzo, A.; Morini, F.; Bestetti, M. Exploiting Direct Current Plasma Electrolytic Oxidation to Boost Photoelectrocatalysis. Catalysts 2020, 10, 325. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G.; Damiani, S.; Raboni, M. Resilience of a Combined Chemical-Physical and Biological Wastewater Treatment Facility. J. Environ. Eng. 2019, 145, 05019002. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Todeschini, S.; Abbà, A.; Ricciardi, P.; Carnevale Miino, M.; Torretta, V.; Rada, E.C.; Conti, F.; Cillari, G.; Calatroni, S.; et al. The Performance Evaluation of Wastewater Service: A Protocol Based on Performance Indicators Applied to Sewer Systems and Wastewater Treatment Plants. Environ. Technol. 2022, 43, 3426–3443. [Google Scholar] [CrossRef] [PubMed]
- ISO 8192:2007; Water Quality—Test for Inhibition of Oxygen Consumption by Activated Sludge for Carbonaceous and Ammonium Oxidation. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and Biodegradability of a Real Pharmaceutical Wastewater Treated by H2O2-Assisted Photoelectrocatalysis on TiO2 Meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef] [PubMed]
- Tauchert, E.; Schneider, S.; de Morais, J.L.; Peralta-Zamora, P. Photochemically-Assisted Electrochemical Degradation of Landfill Leachate. Chemosphere 2006, 64, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Durante, A.; Bellazzi, S.; Baldi, M.; Bertanza, G. How to Produce an Alternative Carbon Source for Denitrification by Treating and Drastically Reducing Biological Sewage Sludge. Membranes 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Carnevale Miino, M.; Caccamo, F.M.; Baldi, M.; Abbà, A. Performance of Full-Scale Thermophilic Membrane Bioreactor and Assessment of the Effect of the Aqueous Residue on Mesophilic Biological Activity. Water 2021, 13, 1754. [Google Scholar] [CrossRef]
- ISPRA. Measurement Procedure for the Determination of the Chemical Oxygen Demand (COD) by Cuvette Test: Method 5135 (in Italian); Higher Institute for Environmental Protection and Research: Rome, Italy, 2014. [Google Scholar]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(Electro)Catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied Photoelectrocatalysis on the Degradation of Organic Pollutants in Wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Zertal, A.; Molnár-Gábor, D.; Malouki, M.A.; Sehili, T.; Boule, P. Photocatalytic Transformation of 4-Chloro-2-Methylphenoxyacetic Acid (MCPA) on Several Kinds of TiO2. Appl. Catal. B 2004, 49, 83–89. [Google Scholar] [CrossRef]
- Chow, H.; Ingelsson, M.; Roberts, E.P.L.; Pham, A.L.-T. How Does Periodic Polarity Reversal Affect the Faradaic Efficiency and Electrode Fouling during Iron Electrocoagulation? Water Res. 2021, 203, 117497. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.R.S.; Roy, A.; Gadgil, A.J.; van Genuchten, C.M. Long-Term Electrode Behavior during Treatment of Arsenic Contaminated Groundwater by a Pilot-Scale Iron Electrocoagulation System. Water Res. 2020, 175, 115668. [Google Scholar] [CrossRef]


| Parameter [u.m.] | Value |
|---|---|
| COD [mg L−1] | 75–80 |
| UV254 [A.U.] | 0.40–0.50 |
| pH [−] | 7.5–7.9 |
| ECon [mS cm−1] | 2500–2900 |
| TSS [mg L−1] | 15–25 |
| SOUR [mgO2 gVSS−1 h−1] | 2–2.5 |
| Color | Light yellow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Carnevale Miino, M.; Caccamo, F.M.; Abbà, A.; Bestetti, M.; Franz, S. Impact of Polarization Reversal during Photoelectrocatalytic Treatment of WWTP Effluents. Environments 2023, 10, 38. https://doi.org/10.3390/environments10030038
Collivignarelli MC, Carnevale Miino M, Caccamo FM, Abbà A, Bestetti M, Franz S. Impact of Polarization Reversal during Photoelectrocatalytic Treatment of WWTP Effluents. Environments. 2023; 10(3):38. https://doi.org/10.3390/environments10030038
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Marco Carnevale Miino, Francesca Maria Caccamo, Alessandro Abbà, Massimiliano Bestetti, and Silvia Franz. 2023. "Impact of Polarization Reversal during Photoelectrocatalytic Treatment of WWTP Effluents" Environments 10, no. 3: 38. https://doi.org/10.3390/environments10030038
APA StyleCollivignarelli, M. C., Carnevale Miino, M., Caccamo, F. M., Abbà, A., Bestetti, M., & Franz, S. (2023). Impact of Polarization Reversal during Photoelectrocatalytic Treatment of WWTP Effluents. Environments, 10(3), 38. https://doi.org/10.3390/environments10030038











