Contribution of Stormwater Outfalls to Microplastic Pollution in a Subtropical Estuary Using Data Collected with the Assistance of Citizen Scientists
Abstract
:1. Introduction
2. Materials and Methods
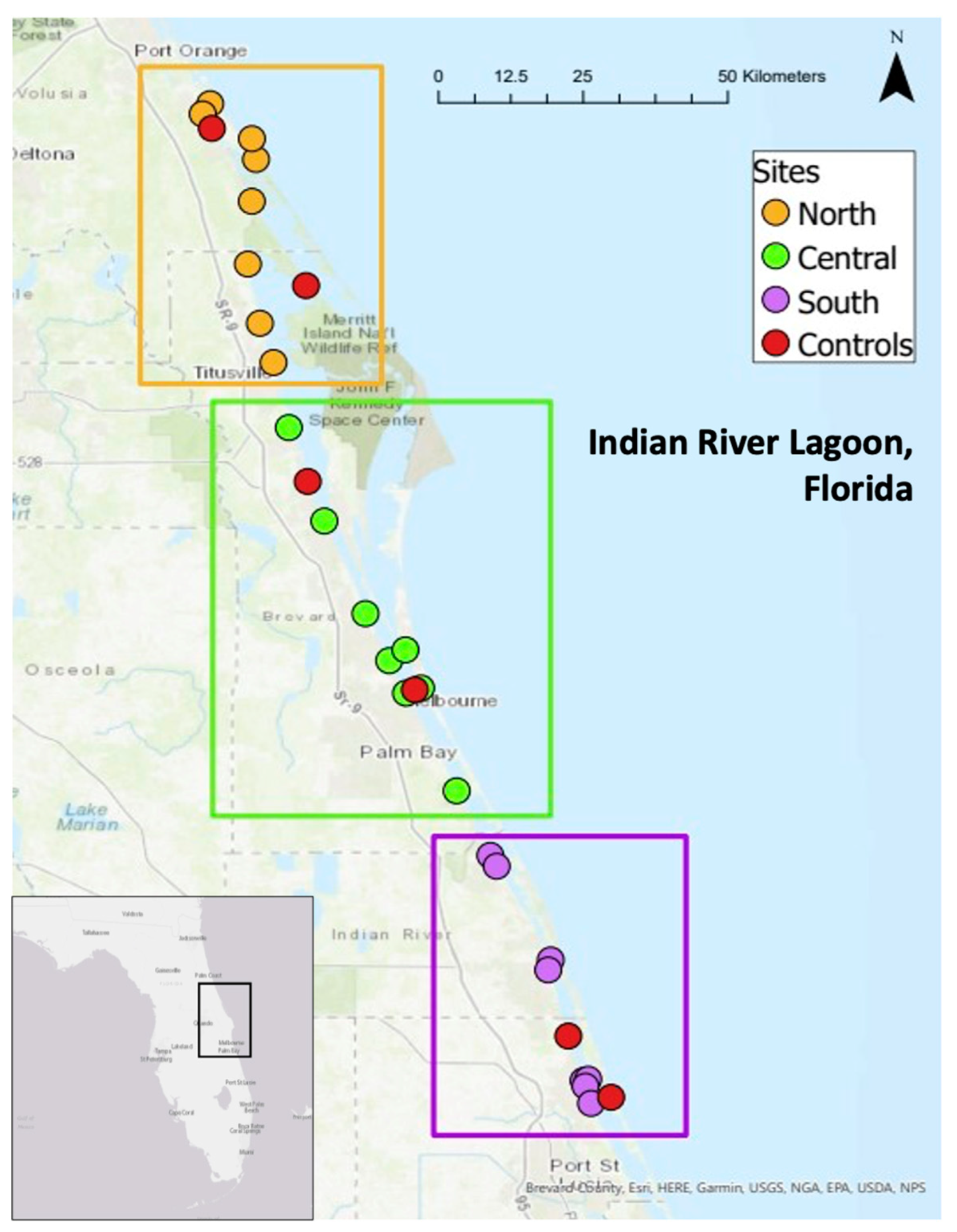
2.1. Study Location and Sampling Methods
2.2. Sample Collection and Processing
2.3. Fourier-Transform Infrared Spectroscopy
2.4. Statistical Methods
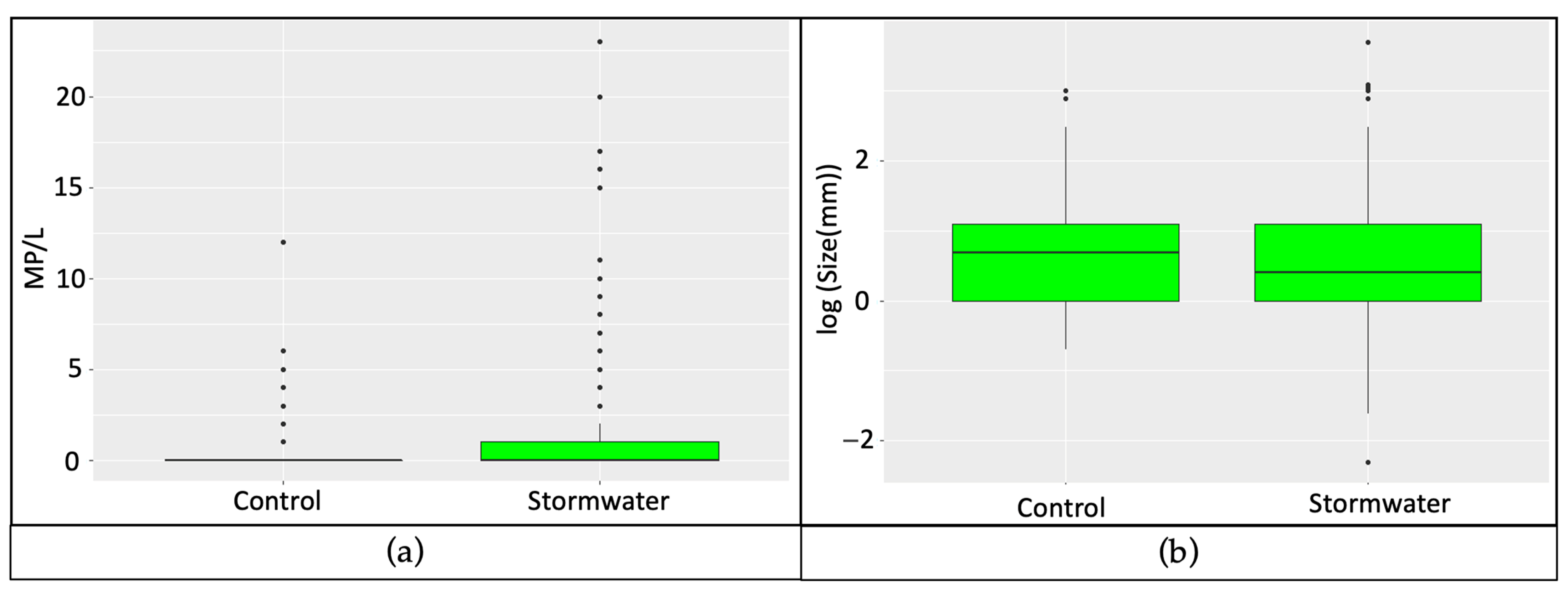
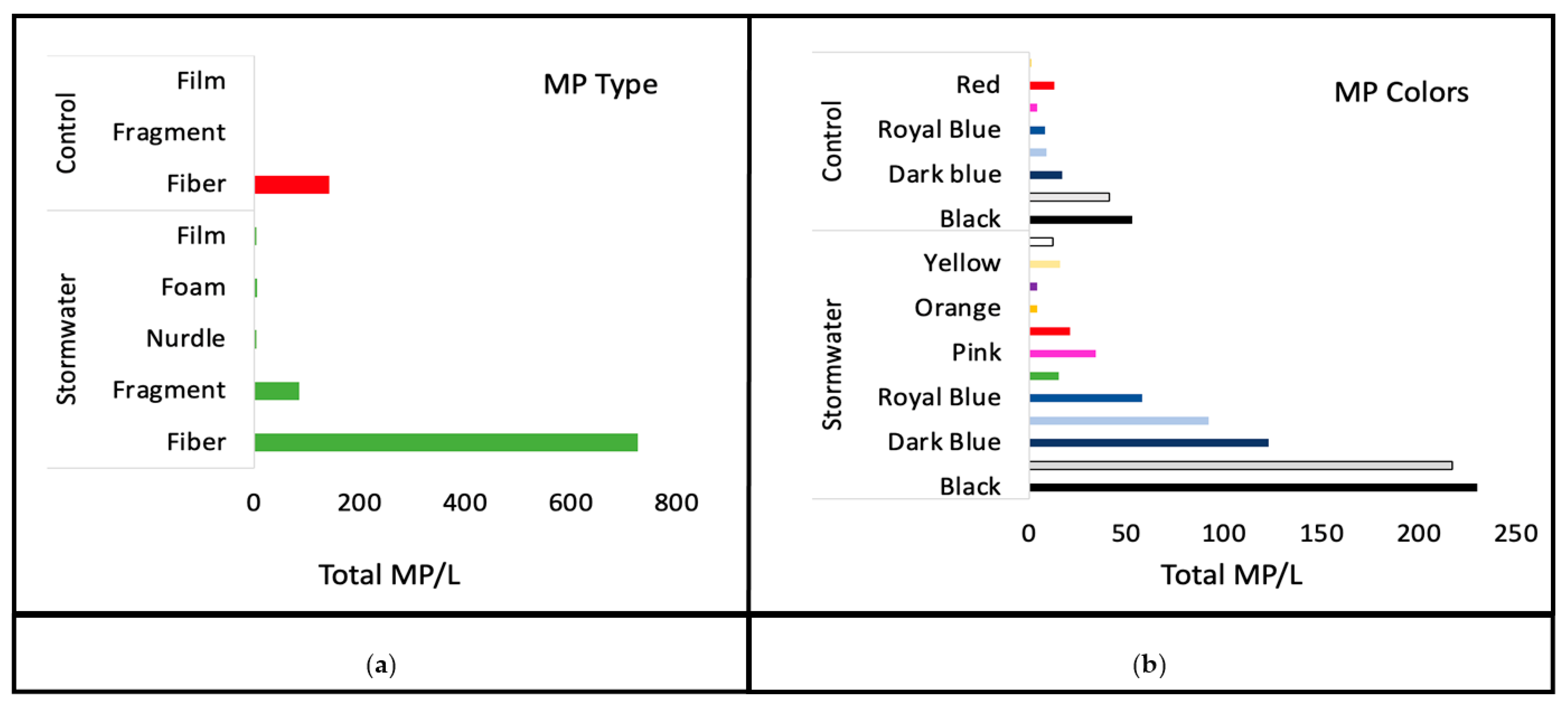
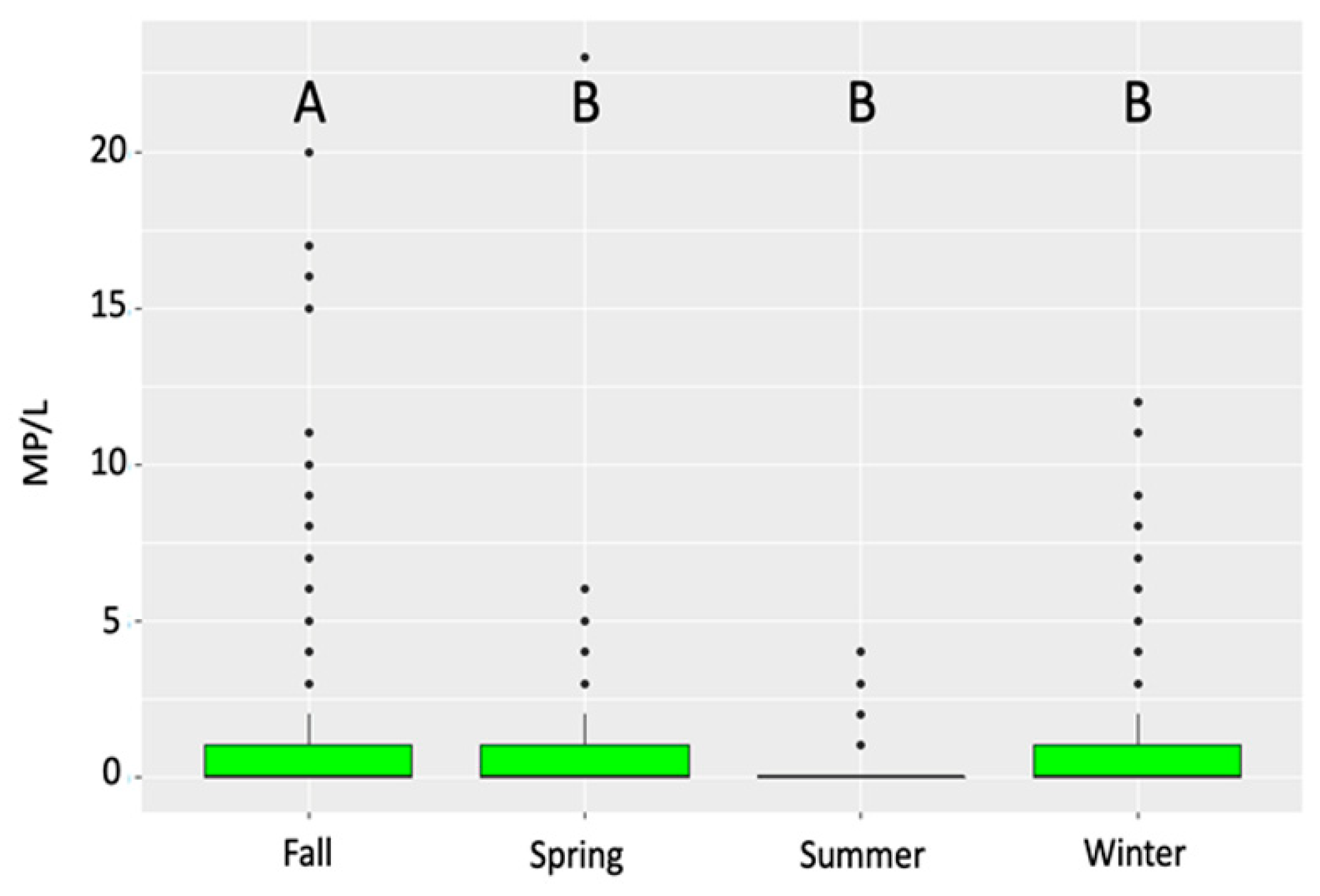
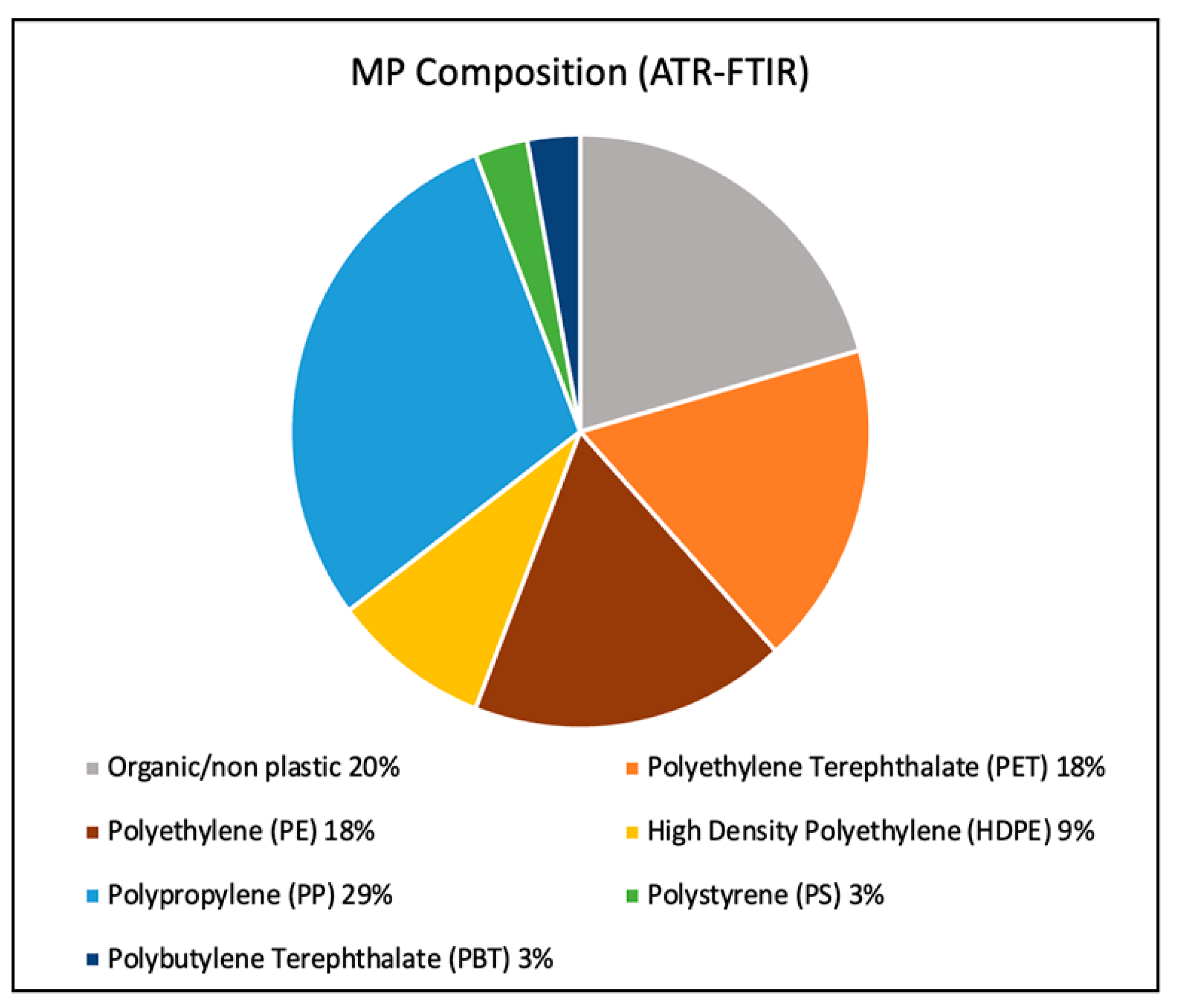
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Starr, N.; Siegler, T.R.; Jambeck, J.; Mallos, N.; Leonard, G.B. The United States’ Contribution of Plastic Waste to Land and Ocean. Sci. Adv. 2020, 6, eabd0288. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Machovsky-Capuska, G.E.; Andrades, R. Plastic Ingestion as an Evolutionary Trap: Toward a Holistic Understanding. Science 2021, 373, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Vinithkumar, N.V.; Dharani, G. First Evidence of Microplastics Bioaccumulation by Marine Organisms in the Port Blair Bay, Andaman Islands. Mar. Pollut. Bull. 2020, 155, 111163. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Chao, M.; He, X.; Lan, X.; Tian, C.; Feng, C.; Shen, Z. Microplastic Bioaccumulation in Estuary-Caught Fishery Resource. Environ. Pollut. 2022, 306, 119392. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.F.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Craig, C.; Fox, D.A.; Zhai, L.; Walters, L.J. In-Situ Microplastic Egestion Efficiency of the Eastern Oyster Crassostrea Virginica. Mar. Pollut. Bull. 2022, 178, 113653. [Google Scholar] [CrossRef]
- Herzke, D.; Anker-Nilssen, T.; Nøst, T.H.; Götsch, A.; Christensen-Dalsgaard, S.; Langset, M.; Fangel, K.; Koelmans, A.A. Negligible Impact of Ingested Microplastics on Tissue Concentrations of Persistent Organic Pollutants in Northern Fulmars off Coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. [Google Scholar] [CrossRef]
- Terepocki, A.K.; Brush, A.; Kleine, L.U.; Shugart, G.W.; Hodum, P.J. Size and Dynamics of Microplastic in Gastrointestinal Tracts of Northern Fulmars (Fulmarus Glacialis) and Sooty Shearwaters (Ardenna grisea). Mar. Pollut. Bull. 2017, 116, 143–150. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.A.; Zhai, L.; Walters, L.J. Microplastic Accumulation in the Gastrointestinal Tracts in Birds of Prey in Central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hinton, D.E.; Shi, H. Microplastics in Small Waterbodies and Tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018, 52, 8885–8893. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P.W. Microplastic Fibers in the Intertidal Ecosystem Surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Loseto, L.L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.N. Microplastics in Beluga Whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef]
- Lusher, A.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and Macroplastic Ingestion by a Deep Diving, Oceanic Cetacean: The True’s Beaked Whale Mesoplodon Mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.; Broderick, A.C.; Fuller, W.A.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.T.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.R.; Omeyer, L.C.M.; et al. Microplastic Ingestion Ubiquitous in Marine Turtles. Glob. Change Biol. 2018, 25, 744–752. [Google Scholar] [CrossRef]
- Caron, A.G.M.; Thomas, C.R.; Berry, K.L.E.; Motti, C.A.; Ariel, E.; Brodie, J. Ingestion of Microplastic Debris by Green Sea Turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a Sequential Extraction Protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Park, T.; Kim, M.-K.; Lee, S.H.; Lee, Y.H.; Kim, M.; Song, H.Y.; Park, J.; Zoh, K.-D. Occurrence and Characteristics of Microplastics in Fish of the Han River, South Korea: Factors Affecting Microplastic Abundance in Fish. Environ. Res. 2022, 206, 112647. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.A.; Frias, J.; Henriques, B.; Sobral, P.; Marques, J.C. Occurrence of Microplastics in Commercial Fish from a Natural Estuarine Environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Murphy, F.; Russell, M.; Ewins, C.; Quinn, B. The Uptake of Macroplastic & Microplastic by Demersal & Pelagic Fish in the Northeast Atlantic around Scotland. Mar. Pollut. Bull. 2017, 122, 353–359. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, N.; Wang, M. Effects of Microplastics on Marine Copepods. Ecotoxicol. Environ. Saf. 2021, 217, 112243. [Google Scholar] [CrossRef]
- Desforges, J.-P.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef]
- De Sa, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Bishop, K. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Kurchaba, N.; Cassone, B.J.; Northam, C.; Ardelli, B.F.; Lemoine, C. Effects of MP Polyethylene Microparticles on Microbiome and Inflammatory Response of Larval Zebrafish. Toxics 2020, 8, 55. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal Alterations in European Sea Bass Dicentrarchus Labrax (Linnaeus, 1758) Exposed to Microplastics: Preliminary Results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Huang, J.-N.; Zhang, Y.; Xu, L.; He, K.; Wen, B.; Yang, P.-W.; Ding, J.; Li, J.; Ma, H.; Gao, J.-Z.; et al. Microplastics: A Tissue-Specific Threat to Microbial Community and Biomarkers of Discus Fish (Symphysodon aequifasciatus). J. Hazard. Mater. 2021, 424, 127751. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Weixiang, L.; Li, X.; Tong, J.; Xiong, W.; Zhu, Z.; Gao, X.; Li, S.; Jia, M.; Yang, Z.; Liang, J. Effects of Environmental and Anthropogenic Factors on the Distribution and Abundance of Microplastics in Freshwater Ecosystems. Sci. Total Environ. 2023, 856, 159030. [Google Scholar]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic Abundance, Characteristics, and Removal in Wastewater Treatment Plants in a Coastal City of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.M. Microplastic Is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L. Characterisation of Plastic Microbeads in Facial Scrubs and Their Estimated Emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.W.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic Pollution Is Widely Detected in US Municipal Wastewater Treatment Plant Effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Kookana, R.S. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Do, T.; Park, Y.; Lim, B.; Kim, S.; Chae, M.-Y.; Chun, C.-H. Effect of the First-Flush Phenomenon on the Quantification of Microplastics in Rainwater. Mar. Pollut. Bull. 2023, 187, 114559. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.M.; Stewart, C.J.; Haberstroh, C.; Arias, M.E. Characteristics and Fate of Plastic Pollution in Urban Stormwater Ponds. Environ. Pollut. 2023, 320, 121052. [Google Scholar] [CrossRef]
- Boucher, J.; Faure, F.; Pompini, O.; Plummer, Z.; Wieser, O.; de Alencastro, L.F. (Micro) Plastic Fluxes and Stocks in Lake Geneva Basin. Trends Anal. Chem. 2019, 112, 66–74. [Google Scholar] [CrossRef]
- Grbic, J.; Helm, P.A.; Athey, S.N.; Rochman, C.M. Microplastics Entering Northwestern Lake Ontario Are Diverse and Linked to Urban Sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Q.; Zhao, X.; Li, L.; Nie, Y.-H.; Liu, H.; Ye, J. The Occurrence of Microplastics in Water Bodies in Urban Agglomerations: Impacts of Drainage System Overflow in Wet Weather, Catchment Land-Uses, and Environmental Management Practices. Water Res. 2020, 183, 116073. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic Rain in Protected Areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Roychand, R.; Pramanik, B.K. Identification of Micro-Plastics in Australian Road Dust. J. Environ. Chem. Eng. 2020, 8, 103647. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in Rainwater/Stormwater Environments: Influencing Factors, Sources, Transport, Fate, and Removal Techniques. TrAC Trends Anal. Chem. 2023, 165, 117147. [Google Scholar] [CrossRef]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Shah, K.N.; Gravina, R.J.; Al-Mamun, A.; Pramanik, B.K. Understanding the Fate and Control of Road Dust-Associated Microplastics in Stormwater. Chem. Eng. Res. Des. 2021, 152, 47–57. [Google Scholar] [CrossRef]
- Hitchcock, J.N. Storm Events as Key Moments of Microplastic Contamination in Aquatic Ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef]
- Walters, L.J.; Craig, C.; Dark, E.; Wayles, J.; Encomio, V.; Coldren, G.; Sailor-Tynes, T.; Fox, D.W.; Zhai, L. Quantifying Spatial and Temporal Trends of Microplastic Pollution in Surface Water and in the Eastern Oyster Crassostrea Virginica for a Dynamic Florida Estuary. Environments 2022, 9, 131. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended Microplastics in the Surface Water of the Yangtze Estuary System, China: First Observations on Occurrence, Distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Gray, A.D.; Wertz, H.; Leads, R.R.; Weinstein, J.E. Microplastic in Two South Carolina Estuaries: Occurrence, Distribution, and Composition. Mar. Pollut. Bull. 2018, 128, 223–233. [Google Scholar] [CrossRef]
- Fok, L.; Cheung, P.K. Hong Kong at the Pearl River Estuary: A Hotspot of Microplastic Pollution. Mar. Pollut. Bull. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Cheung, P.K.; Cheung, L.T.O.; Fok, L. Seasonal Variation in the Abundance of Marine Plastic Debris in the Estuary of a Subtropical Macro-Scale Drainage Basin in South China. Sci. Total Environ. 2016, 562, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.W.; Smigaj, M.; Tani, M. The Benefits and Negative Impacts of Citizen Science Applications to Water as Experienced by Participants and Communities. Wiley Interdiscip. Rev. Water 2020, 8, e1488. [Google Scholar] [CrossRef]
- Results—Microplastic Survey. Available online: https://microplasticsurvey.org/results (accessed on 8 June 2023).
- Indian River Lagoon National Estuary Program. 2030 Comprehensive Conservation and Management Plan. 2019. Available online: https://onelagoon.org/management-plan/ (accessed on 15 June 2023).
- Arthur, C.; Baker, J.A.; Bamford, H.A. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008; NOAA: Washington, DC, USA, 2009.
- Lapointe, B.E.; Herren, L.W.; Debortoli, D.; Vogel, M.A. Evidence of Sewage-Driven Eutrophication and Harmful Algal Blooms in Florida’s Indian River Lagoon. Harmful Algae 2015, 43, 82–102. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Herren, L.W.; Brewton, R.A.; Alderman, P. Nutrient Over-Enrichment and Light Limitation of Seagrass Communities in the Indian River Lagoon, an Urbanized Subtropical Estuary. Sci. Total Environ. 2020, 699, 134068. [Google Scholar] [CrossRef]
- Benson, G.W.; Donnelly, M.J.; Sacks, P.E.; Walters, L.J. Documenting Loss and Fragmentation of Intertidal Oyster (Crassostrea Virginica) Reefs in a Subtropical Estuary. Environments 2023, 10, 133. [Google Scholar] [CrossRef]
- Adams, D.; Edwards, D.; Schneider, J.; Searles, A. Range Expansion and Population Shifts of Estuarine Fishes in a Changing Subtropical Estuary. Mar. Ecol. Prog. Ser. 2023, SHIFT. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in Seawater: Sampling Strategies, Laboratory Methodologies, and Identification Techniques Applied to Port Environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef]
- Zhao, J.; Ran, W.; Jiang, T.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic Pollution in Sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640–641, 637–645. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. (n.d.). Available online: https://www.r-project.org/ (accessed on 23 June 2023).
- Posit|The Open-Source Data Science Company. Posit. 2 October 2023. Available online: http://www.rstudio.com/ (accessed on 24 June 2023).
- Craig, C. Correlations in Microplastic Abundance between Water, the Eastern Oyster, Crassostrea Virginica, and Their Biodeposits in a Dynamic Florida Estuary; University of Central Florida: Orlando, FL, USA, 2021. [Google Scholar]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and Their Possible Sources: The Example of Ofanto River in Southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Piñon-Colin, T.D.J.; Rodriguez-Jimenez, R.; Rogel-Hernández, E.; Álvarez-Andrade, A.; Wakida, F.T. Microplastics in Stormwater Runoff in a Semiarid Region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.H.; Bang, K.-H.; Ketchum, L.H.J.; Jk, C.; Yu, M.Y. First Flush Analysis of Urban Storm Runoff. Sci. Total Environ. 2002, 293, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.P.W.; Christiansen, K.S.; Bode, E.T.; Hoellein, T.J. A Watershed-Scale, Citizen Science Approach to Quantifying Microplastic Concentration in a Mixed Land-Use River. Water Res. 2018, 147, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Shruti; Pérez-Guevara, F.; Elizalde-Martínez; Kutralam-Muniasamy, G. Current Trends and Analytical Methods for Evaluation of Microplastics in Stormwater. Curr. Trends Anal. Methods Eval. Microplast. Stormwater 2021, 30, e00123. [Google Scholar] [CrossRef]
- Almroth, B.C.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying Shedding of Synthetic Fibers from Textiles; a Source of Microplastics Released into the Environment. Environ. Sci. Pollut. Res. 2017, 25, 1191–1199. [Google Scholar] [CrossRef]
- TextileExchange. Preferred Fiber & Materials Market Report 2021; TextileExchange: Lamesa, Texas, 2021. [Google Scholar]
- Zhao, Y.; Qiao, R.; Zhang, S.; Wang, G. Metabolomic Profiling Reveals the Intestinal Toxicity of Different Length of Microplastic Fibers on Zebrafish (Danio Rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef]
- Bhatt, V.; Chauhan, J.S. Microplastic in Freshwater Ecosystem: Bioaccumulation, Trophic Transfer, and Biomagnification. Environ. Sci. Pollut. Res. 2022, 30, 9389–9400. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Liu, H.; Zhang, J.; Wang, D. Migration Characteristics of Microplastics Based on Source-Sink Investigation in a Typical Urban Wetland. Water Res. 2022, 213, 118154. [Google Scholar] [CrossRef]
- Olesen, K.B.; Stephansen, D.A.; Van Alst, N.; Vollertsen, J. Microplastics in a Stormwater Pond. Water 2019, 11, 1466. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.R.; De Meester, S. Characteristics and Sinking Behavior of Typical Microplastics Including the Potential Effect of Biofouling: Implications for Remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef]
- Gago, J.M.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic Microfibers in the Marine Environment: A Review on Their Occurrence in Seawater and Sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Liu, F.; Vianello, A.; Vollertsen, J. Retention of Microplastics in Sediments of Urban and Highway Stormwater Retention Ponds. Environ. Pollut. 2019, 255 Pt 2, 113335. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.W.; Tsang, Y.F.; Leung, M.; Fang, J.K.H.; Chan, K.M. Microplastics from Effluents of Sewage Treatment Works and Stormwater Discharging into the Victoria Harbor, Hong Kong. Mar. Pollut. Bull. 2020, 157, 111181. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Goßmann, I.; Halbach, M.; Scholz-Böttcher, B.M. Car and Truck Tire Wear Particles in Complex Environmental Samples—A Quantitative Comparison with “Traditional” Microplastic Polymer Mass Loads. Sci. Total Environ. 2021, 773, 145667. [Google Scholar] [CrossRef]
- Wik, A.; Dave, G. Occurrence and Effects of Tire Wear Particles in the Environment—A Critical Review and an Initial Risk Assessment. Environ. Pollut. 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Drapper, D.; Hornbuckle, A.; Rintoul, L.; Kookana, R.S. Microplastic Pollution in a Stormwater Floating Treatment Wetland: Detection of Tyre Particles in Sediment. Sci. Total Environ. 2020, 713, 136356. [Google Scholar] [CrossRef] [PubMed]
- Tixier, G.; Lafont, M.; Grapentine, L.; Rochfort, Q.; Marsalek, J. Ecological Risk Assessment of Urban Stormwater Ponds: Literature Review and Proposal of a New Conceptual Approach Providing Ecological Quality Goals and the Associated Bioassessment Tools. Ecol. Indic. 2011, 11, 1497–1506. [Google Scholar] [CrossRef]
- Schwartz, D.K.; Sample, D.J.; Grizzard, T.J. Evaluating the Performance of a Retrofitted Stormwater Wet Pond for Treatment of Urban Runoff. Environ. Monit. Assess. 2017, 189, 256. [Google Scholar] [CrossRef] [PubMed]
- Viol, I.L.; Mocq, J.; Julliard, R.; Kerbiriou, C. The Contribution of Motorway Stormwater Retention Ponds to the Biodiversity of Aquatic Macroinvertebrates. Biol. Conserv. 2009, 142, 3163–3171. [Google Scholar] [CrossRef]
- Hamer, A.; Smith, P.J.; McDonnell, M.D. The Importance of Habitat Design and Aquatic Connectivity in Amphibian Use of Urban Stormwater Retention Ponds. Urban Ecosyst. 2011, 15, 451–471. [Google Scholar] [CrossRef]
- Birch, G.F.; Matthai, C.; Fazeli, M.S. Efficiency of a Retention/Detention Basin to Remove contaminants from Urban Stormwater. Urban Water J. 2006, 3, 69–77. [Google Scholar] [CrossRef]
- Yazdi, M.N.; Scott, D.T.; Sample, D.J.; Wang, X. Efficacy of a Retention Pond in Treating Stormwater Nutrients and Sediment. J. Clean. Prod. 2021, 290, 125787. [Google Scholar] [CrossRef]
- Stang, C.; Mohamed, B.A.; Li, L.Y. Microplastic Removal from Urban Stormwater: Current Treatments and Research Gaps. J. Environ. Manag. 2022, 317, 115510. [Google Scholar] [CrossRef]
- Steward, J.S.; VanArman, J.A. Indian River Lagoon Joint Reconnaissance Report; CM–137; St. Johns River Water Management District: Palatka, FL, USA, 1987. [Google Scholar]
- Jiang, M. Modeling Water Residence Time and Connectivity in the Northern Indian River Lagoon. Estuaries Coasts 2023, 46, 1170–1189. [Google Scholar] [CrossRef]
- Smith, N.P. Tidal and Nontidal Flushing of Florida’s Indian River Lagoon. Estuaries 1993, 16, 739. [Google Scholar] [CrossRef]
- Van Der Velde, T.; Milton, D.A.; Lawson, T.J.; Wilcox, C.; Lansdell, M.; Davis, G.; Perkins, G.C.; Hardesty, B.D. Comparison of Marine Debris Data Collected by Researchers and Citizen Scientists: Is Citizen Science Data Worth the Effort? Biol. Conserv. 2017, 208, 127–138. [Google Scholar] [CrossRef]
- Bosker, T.; Behrens, P.; Vijver, M.G. Determining Global Distribution of Microplastics by Combining Citizen Science and In-Depth Case Studies. Integr. Environ. Assess. Manag. 2017, 13, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.; Murphy, A.G. Plastic in Surface Waters of the Inside Passage and Beaches of the Salish Sea in Washington State. Mar. Pollut. Bull. 2015, 97, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A Large-Scale Investigation of Microplastic Contamination: Abundance and Characteristics of Microplastics in European Beach Sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Nel, H.; Smith, G.H.S.; Harmer, R.; Sykes, R.; Schneidewind, U.; Lynch, I.; Krause, S. Citizen Science Reveals Microplastic Hotspots within Tidal Estuaries and the Remote Scilly Islands, United Kingdom. Mar. Pollut. Bull. 2020, 161, 111776. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Thiel, M. Distribution and Abundance of Small Plastic Debris on Beaches in the SE Pacific (Chile): A Study Supported by a Citizen Science Project. Mar. Environ. Res. 2013, 87–88, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Guezou, A.; Medor, S.; Nickson, C.; Savage, G.; Alarcón-Ruales, D.; Galloway, T.S.; Muñoz-Pérez, J.P.; Nelms, S.E.; Porter, A.; et al. Microplastic Distribution and Composition on Two Galápagos Island Beaches, Ecuador: Verifying the Use of Citizen Science Derived Data in Long-Term Monitoring. Environ. Pollut. 2022, 311, 120011. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.A.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent Approaches and Advanced Wastewater Treatment Technologies for Mitigating Emerging Microplastics Contamination—A Critical Review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, S.J.; Craig, C.A.; Wayles, J.; Sailor-Tynes, T.; Dark, E.; Sweat, L.H.; Fox, D.W.; Zhai, L.; Walters, L.J. Contribution of Stormwater Outfalls to Microplastic Pollution in a Subtropical Estuary Using Data Collected with the Assistance of Citizen Scientists. Environments 2023, 10, 181. https://doi.org/10.3390/environments10100181
Busch SJ, Craig CA, Wayles J, Sailor-Tynes T, Dark E, Sweat LH, Fox DW, Zhai L, Walters LJ. Contribution of Stormwater Outfalls to Microplastic Pollution in a Subtropical Estuary Using Data Collected with the Assistance of Citizen Scientists. Environments. 2023; 10(10):181. https://doi.org/10.3390/environments10100181
Chicago/Turabian StyleBusch, Sidney J., Casey A. Craig, Jessy Wayles, Tess Sailor-Tynes, Emily Dark, L. Holly Sweat, David W. Fox, Lei Zhai, and Linda J. Walters. 2023. "Contribution of Stormwater Outfalls to Microplastic Pollution in a Subtropical Estuary Using Data Collected with the Assistance of Citizen Scientists" Environments 10, no. 10: 181. https://doi.org/10.3390/environments10100181





