Removal of Cadmium and Lead from Synthetic Wastewater Using Galdieria sulphuraria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strain
2.2. Cultural Media
2.3. Synthetic Wastewater Solution Preparation
2.4. Exposure of G. sulphuraria CCMEE 5587.1 to Cd and Pb Added Media
2.5. Metal and Nutrient Removal Experiment
2.6. Measurement of Biomass Density
2.7. Measurements of Nutrients
2.8. Measurement of Cd and Pb Concentration
2.9. Statistical Analysis and Graph Plotting
3. Results
3.1. Growth of G. sulphuraria CCMEE 5587.1 in Different Concentrations of Cd and Pb
3.2. Growth Rate
3.3. Bioremoval of Cd and Pb
3.4. Nutrient Removal
4. Discussions
| Algal Strain | Initial Pb Concentration (mg L−1) | pH | Sorption Capacity (mg g−1) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| Green Algae | |||||
| Chlamydomonas reinhardtii | 0.10 | 6.0 | 0.042 | 8.00 | [36] |
| Chlorella vulgaris | 1.95–4.83 | 6.2 | - | 71.81–98.70 | [37] |
| Oedogonium westii | 0.10–0.80 | 5.0 | 0.261 | 61.00–96.00 | [34] |
| Ulva lactuca | 1.00 | 7.8 | 0.145 | 87.00 | [32] |
| Dunaliella salina AL-1 | 5.00–10.00 | - | - | 95.00 | [40] |
| Scenedesmus sp. | 0.05–10.00 | - | 13.60 | 70.00 | [41] |
| Pseudochlorococcum typicum | 10.00 | 7.0 | 5.11 | 70.06 | [33] |
| Red Algae | |||||
| Galdieria sulphuraria | 0.10 | 2.4 | - | 0.00 | [35] |
| Galdieria sulphuraria IPPAS P-513 | 5.00 | 2.7 | - | 84.00 | [30] |
| Galdieria maxima | 0.00–500.00 | 5.0 | 38.20 | - | [17] |
| Cyanidioschyzon merolae NIES 3377 | 0.00–500.00 | 5.0 | 214.00 | - | [17] |
| Cyanidium caldariumNIES 551 | 0.00–500.00 | 5.0 | 298.80 | - | [17] |
| Galdieria sulphuraria CCMEE 5587.1 | 5.00 | 2.5 | 0.00 | 0.00 | Existing Study |
| 2.50 | 0.5 | 25.10 | |||
| 1.25 | 0.225 | 23.47 | |||
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019, 103, 3297–3316. [Google Scholar] [CrossRef]
- Plöhn, M.; Escudero-Oñate, C.; Funk, C. Biosorption of Cd(II) by Nordic microalgae: Tolerance, kinetics and equilibrium studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2016, 29, 211–221. [Google Scholar] [CrossRef]
- Purushanahalli Shivagangaiah, C.; Sanyal, D.; Dasgupta, S.; Banik, A. Phycoremediation and photosynthetic toxicity assessment of lead by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa. Physiol. Plant. 2021, 173, 246–258. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Perera, I.A.; Megharaj, M. Acid-tolerant microalgae can withstand higher concentrations of invasive cadmium and produce sustainable biomass and biodiesel at pH 3.5. Bioresour. Technol. 2019, 281, 469–473. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Gaur, J. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Agrawal, K.; Shah, M.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Afkar, E.; Ababna, H.; Fathi, A. Toxicological response of the green alga Chlorella vulgaris, to some heavy metals. Am. J. Environ. Sci. 2010, 6, 230. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pan, S.; Rahman, A.; Tan, M.; Kharel, H.L.; Agrawal, S.; Nawaz, T. Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment. Water 2022, 14, 2389. [Google Scholar] [CrossRef]
- Cho, Y.-L.; Lee, Y.-C.; Hsu, L.-C.; Wang, C.-C.; Chen, P.-C.; Liu, S.-L.; Teah, H.-Y.; Liu, Y.-T.; Tzou, Y.-M. Molecular mechanisms for Pb removal by Cyanidiales: A potential biomaterial applied in thermo-acidic conditions. Chem. Eng. J. 2020, 401, 125828. [Google Scholar] [CrossRef]
- Kharel, H.L.; Shrestha, I.; Tan, M.; Nikookar, M.; Saraei, N.; Selvaratnam, T. Cyanidiales-Based Bioremediation of Heavy Metals. BioTech 2023, 12, 29. [Google Scholar] [CrossRef]
- Oesterhelt, C.; Schmälzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Reddy, H.; Kanapathipillai, N.; Nirmalakhandan, N.; Deng, S.; Lammers, P. Algal biofuels from urban wastewaters: Maximizing biomass yield using nutrients recycled from hydrothermal processing of biomass. Bioresour. Technol. 2015, 182, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Dixon, K.L.; Nawaz, T.; Rahman, A.; Selvaratnam, T. Evaluation of Galdieria sulphuraria for nitrogen removal and biomass production from raw landfill leachate. Algal Res. 2021, 54, 102183. [Google Scholar] [CrossRef]
- Rahman, A.; Pan, S.; Houston, C.; Selvaratnam, T. Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water 2021, 13, 1183. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Křivčík, J.; Mikulášek, P. Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem. Eng. J. 2014, 256, 324–334. [Google Scholar] [CrossRef]
- Kumar, M.; Kushwaha, A.; Goswami, L.; Singh, A.K.; Sikandar, M. A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater. Clean. Eng. Technol. 2021, 5, 100288. [Google Scholar] [CrossRef]
- Cui, M.; Jang, M.; Cho, S.-H.; Khim, J.; Cannon, F.S. A continuous pilot-scale system using coal-mine drainage sludge to treat acid mine drainage contaminated with high concentrations of Pb, Zn, and other heavy metals. J. Hazard. Mater. 2012, 215, 122–128. [Google Scholar] [CrossRef]
- Palma, H.; Killoran, E.; Sheehan, M.; Berner, F.; Heimann, K. Assessment of microalga biofilms for simultaneous remediation and biofuel generation in mine tailings water. Bioresour. Technol. 2017, 234, 327–335. [Google Scholar] [CrossRef]
- Toplin, J.A.; Norris, T.B.; Lehr, C.R.; McDermott, T.R.; Castenholz, R.W. Biogeographic and phylogenetic diversity of thermoacidophilic cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 2008, 74, 2822–2833. [Google Scholar] [CrossRef]
- Isachsen, I. Cadmium Tolerance in the Thermo-Acidophilic Red Alga C. merolae, Possible Mechanisms and Implications for Bioremediation; Arizona State University: Tempe, AZ, USA, 2022. [Google Scholar]
- Ostroumov, S.; Tropin, I.; Kiryushin, A. Removal of cadmium and other toxic metals from water: Thermophiles and new biotechnologies. Russ. J. Gen. Chem. 2018, 88, 2962–2966. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Xing, G.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef]
- Henriques, B.; Teixeira, A.; Figueira, P.; Reis, A.T.; Almeida, J.; Vale, C.; Pereira, E. Simultaneous removal of trace elements from contaminated waters by living Ulva lactuca. Sci. Total Environ. 2019, 652, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal. Behav. 2012, 7, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, I.; Khan, S.; Waqas, M.; Asma, M.; Nawab, J.; Gul, N.; Raiz, A.; Li, G. Heavy metal uptake capacity of fresh water algae (Oedogonium westti) from aqueous solution: A mesocosm research. Int. J. Phytoremediation 2016, 18, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ostroumov, S.; Shestakova, T.; Tropin, I. Biosorption of copper by biomass of extremophilic algae. Russ. J. Gen. Chem. 2015, 85, 2961–2964. [Google Scholar] [CrossRef]
- Flouty, R.; Estephane, G. Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: A comparative study. J. Environ. Manag. 2012, 111, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Regaldo, L.M.; Gervasio, S.G.; Troiani, H.E.; Gagneten, A.M. Bioaccumulation and Toxicity of Copper and Lead in Chlorella vulgaris. J. Algal Biomass Utln. 2013, 4, 59–66. [Google Scholar]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- USEPA, Effluent Guidelines Database. Available online: https://www.epa.gov/eg/effluent-guidelines-database (accessed on 20 September 2023).
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Pham, T.-L.; Dao, T.-S.; Bui, H.N.; Pham, T.K.N.; Ngo, T.T.H.; Bui, H.M. Lipid production combined with removal and bioaccumulation of Pb by Scenedesmus sp. Green Alga. Pol. J. Environ. Stud. 2020, 29, 1785–1791. [Google Scholar] [CrossRef]
- Kumar, J.N.; Oommen, C. Removal of heavy metals by biosorption using freshwater alga Spirogyra hyalina. J. Environ. Biol. 2012, 33, 27. [Google Scholar]
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2011, 60, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.M.; Fonseca, S.C.; Castro, P.M.; Malcata, F.X. Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J. Appl. Phycol. 2011, 23, 97–103. [Google Scholar] [CrossRef]
- Alharbi, R.M. Toxicity and bioaccumulation of lead, cadmium and zinc in Chroococcus minutus and Chlorococcum aegyptiacum. Int. J. Pharm. Res. Allied Sci. 2017, 6, 290–300. [Google Scholar]
- Pinto, E.; Sigaud-kutner, T.C.; Leitao, M.A.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae 1. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Napan, K.; Teng, L.; Quinn, J.C.; Wood, B.D. Impact of heavy metals from flue gas integration with microalgae production. Algal Res. 2015, 8, 83–88. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Moreno-Sánchez, R. Cd2+ transport and storage in the chloroplast of Euglena gracilis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2005, 1706, 88–97. [Google Scholar] [CrossRef]
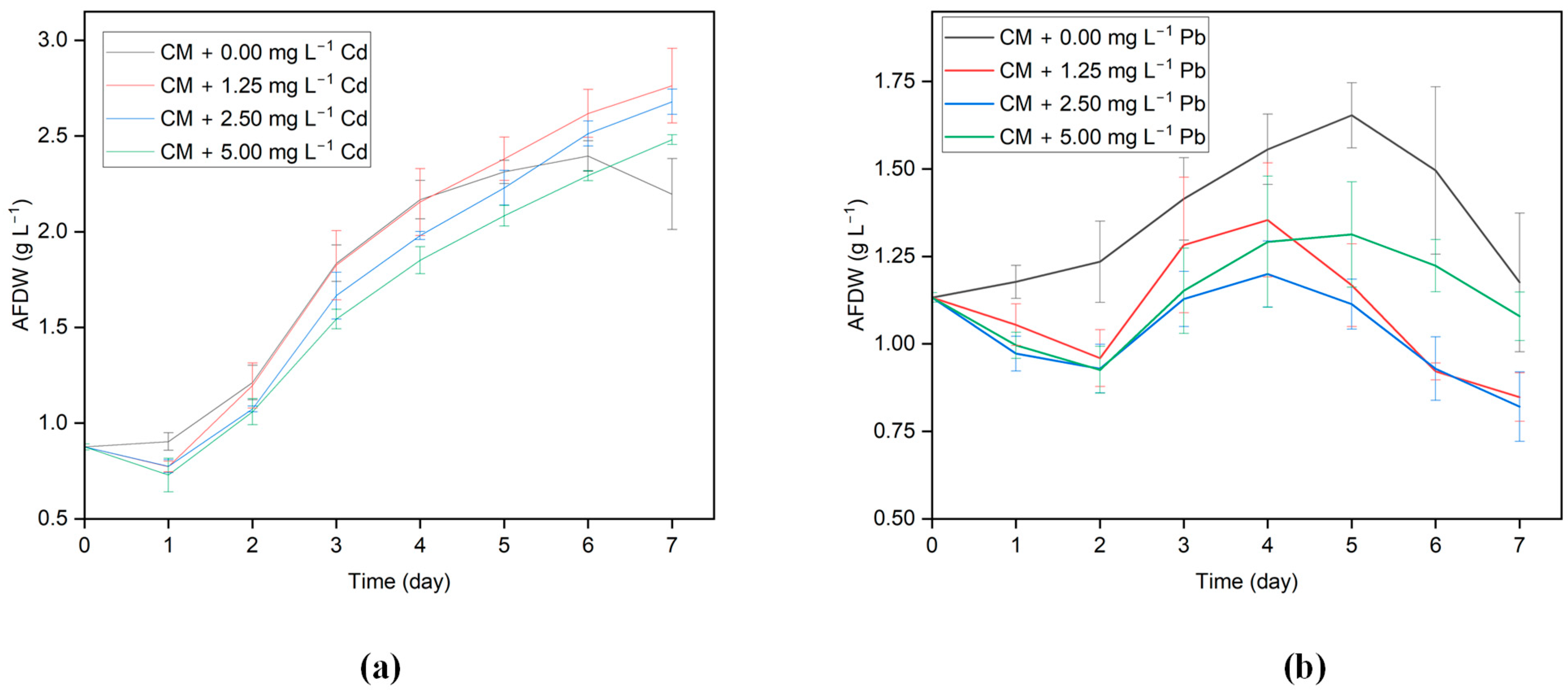

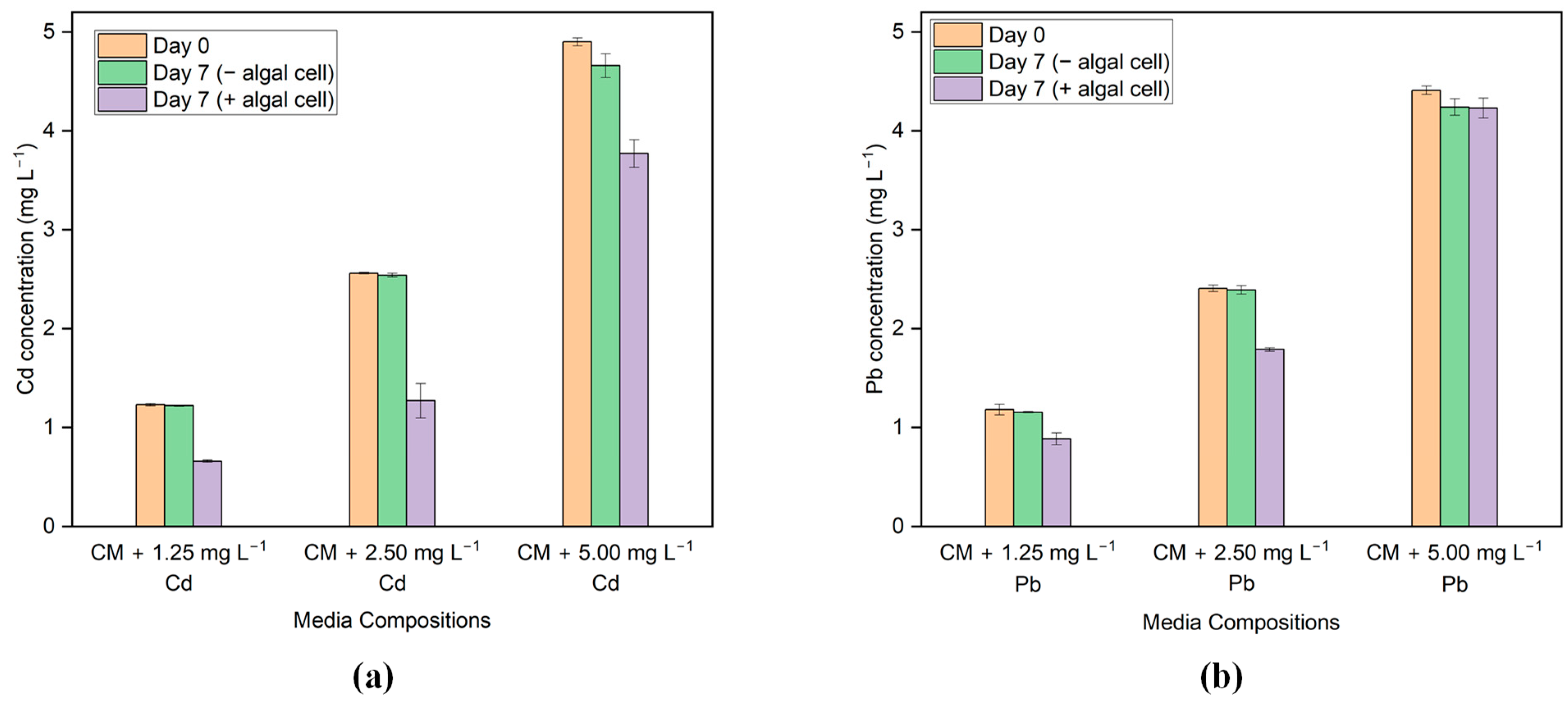
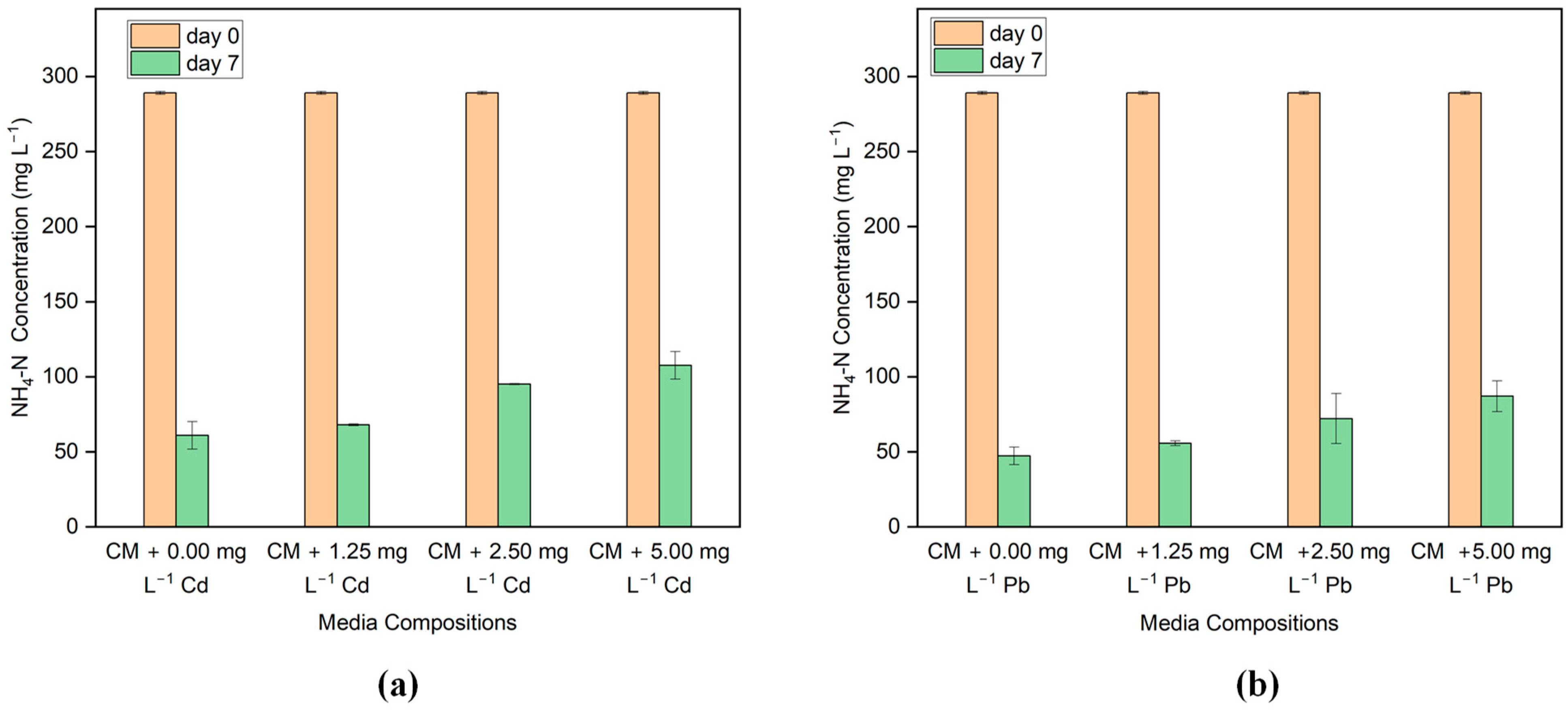
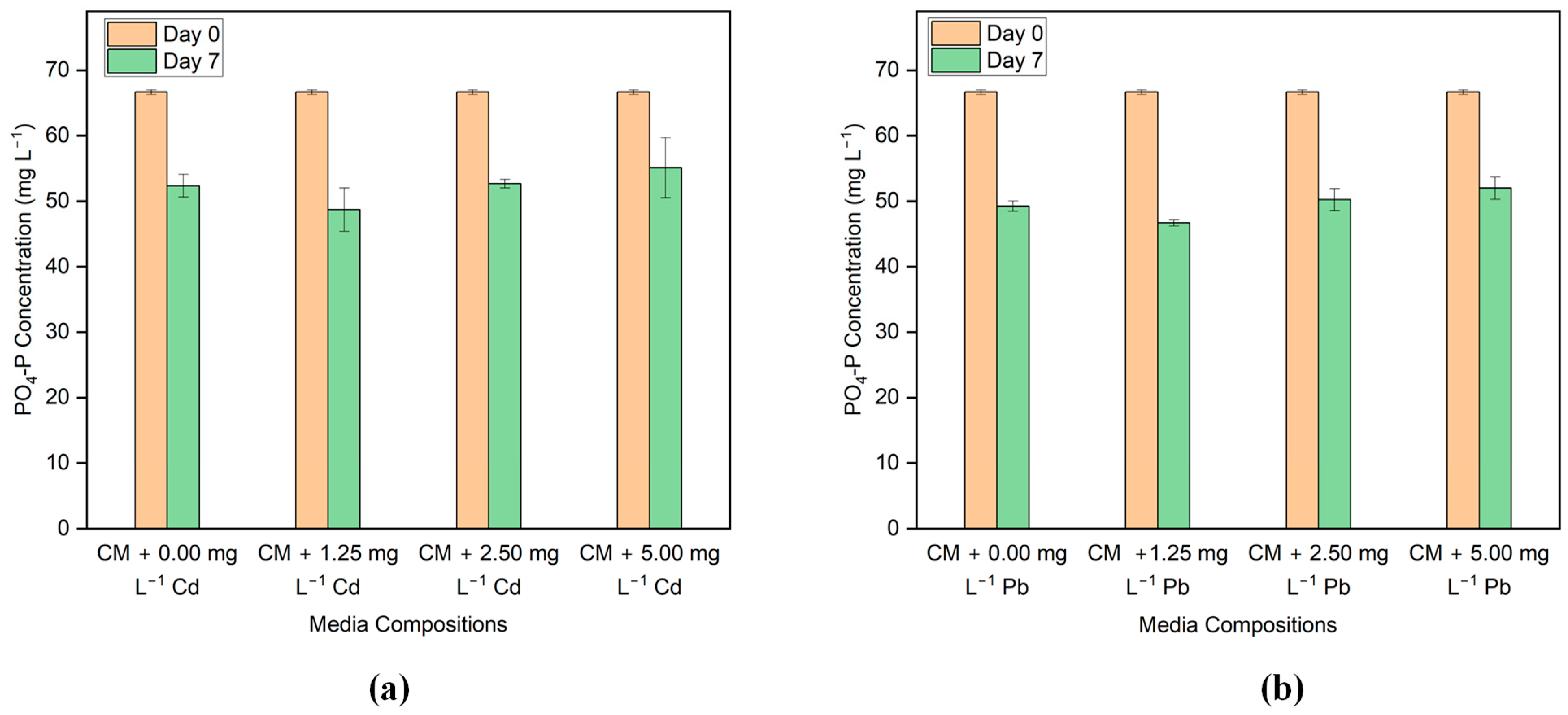
| Algal Strain | Initial Cd Concentration (mg L−1) | pH | Sorption Capacity (mg g−1) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| Green Algae | |||||
| Chlorella vulgaris | 2.50 | 7.2 | 49.00 | 72.00 | [3] |
| Chlorella vulgaris | 100.00 | - | 16.34 | 95.20 | [4] |
| Coelastrella sp. | 2.50 | 7.2 | 65.00 | 82.00 | [3] |
| Oedogonium westii | 0.50–2.00 | 5.0 | 0.974 | 55.00–95.00 | [34] |
| Ulva lactuca | 0.20 | 7.8 | 0.018 | 56.00 | [32] |
| Desmodesmus sp. MAS1 | 1.00–2.00 | 3.5 | 0.37–0.77 | 60.00 | [6] |
| Heterochlorella sp. MAS3 | 1.00–2.00 | 3.5 | 0.16–0.36 | 80.00 | [6] |
| Chlorella minutissima UTEX2341 | 67.48 | 6.0 | 35.36 | 74.34 | [31] |
| Pseudochlorococcum typicum | 10.00 | 7.0 | 6.26 | 86.24 | [33] |
| Red Algae | |||||
| Cyanidioschyzon merolae 10D | 1.00 | 1.75 | - | 31.55 | [29] |
| Cyanidioschyzon merolae MS1 | 5.00 | 1.75 | - | 1.16 | [29] |
| Galdieria sulphuraria IPPAS P-513 | 5.00 | 2.7 | - | 24.00 | [30] |
| Galdieria sulphuraria CCMEE 5587.1 | 5.00 | 2.5 | 1.02 | 19.09 | Existing Study |
| 2.50 | 1.45 | 49.80 | |||
| 1.25 | 0.64 | 45.90 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharel, H.L.; Shrestha, I.; Tan, M.; Selvaratnam, T. Removal of Cadmium and Lead from Synthetic Wastewater Using Galdieria sulphuraria. Environments 2023, 10, 174. https://doi.org/10.3390/environments10100174
Kharel HL, Shrestha I, Tan M, Selvaratnam T. Removal of Cadmium and Lead from Synthetic Wastewater Using Galdieria sulphuraria. Environments. 2023; 10(10):174. https://doi.org/10.3390/environments10100174
Chicago/Turabian StyleKharel, Hari Lal, Ina Shrestha, Melissa Tan, and Thinesh Selvaratnam. 2023. "Removal of Cadmium and Lead from Synthetic Wastewater Using Galdieria sulphuraria" Environments 10, no. 10: 174. https://doi.org/10.3390/environments10100174
APA StyleKharel, H. L., Shrestha, I., Tan, M., & Selvaratnam, T. (2023). Removal of Cadmium and Lead from Synthetic Wastewater Using Galdieria sulphuraria. Environments, 10(10), 174. https://doi.org/10.3390/environments10100174









