Subjective Assessment of Sleep in Infantile Autism: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
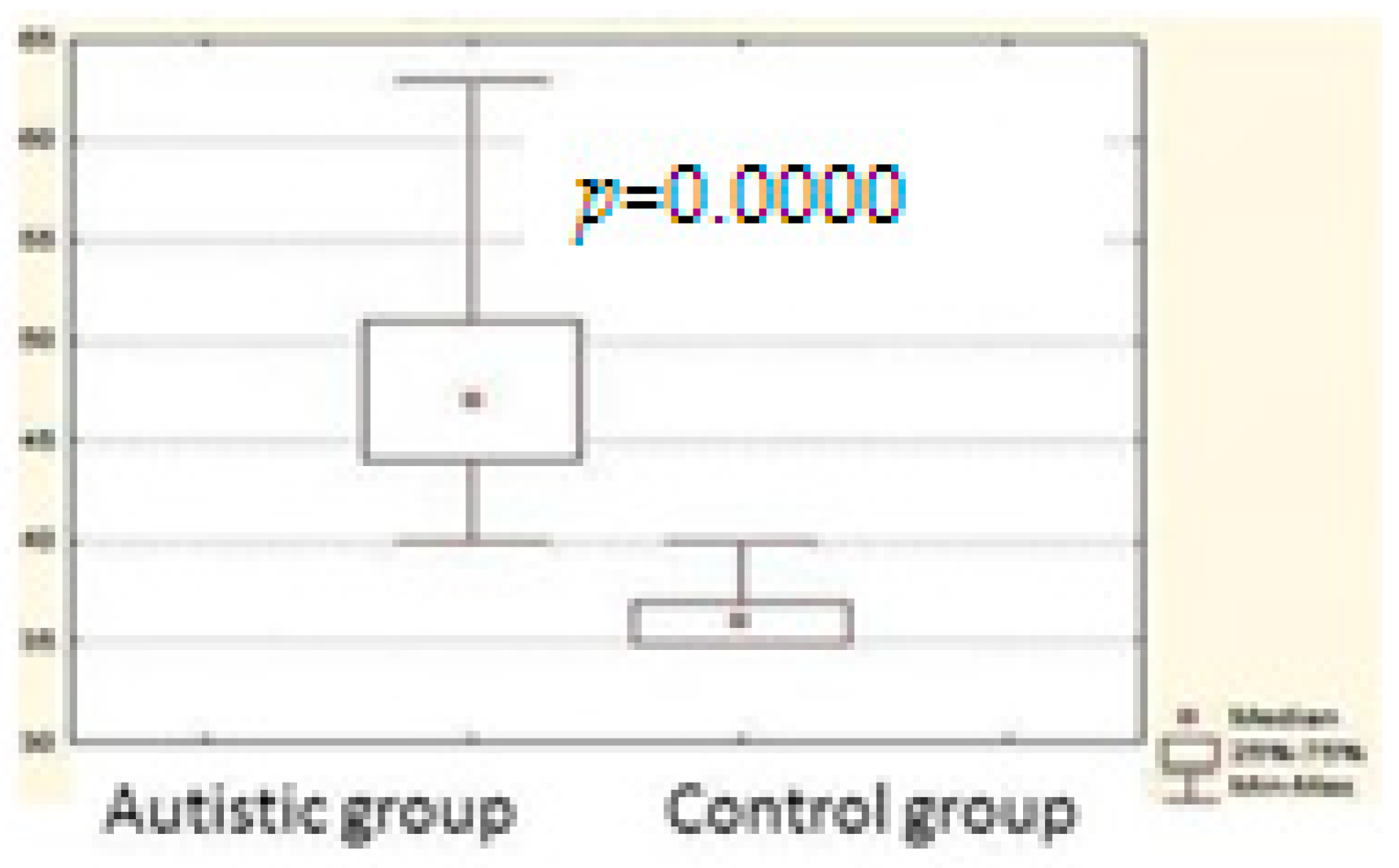
3. Results
4. Discussion
5. Limitation of the Study
Author Contributions
Funding
Conflicts of Interest
References
- Valdizán, J.R. Sleep: Functions and Pathology; Viguera Editres: Barcelona, Spain, 1999. [Google Scholar]
- Brown, D.W. Autism, Asperger’s syndrome and the Crick-Mitchisontheory of the biological function of REM sleep. Med. Hypotheses 1996, 47, 399–403. [Google Scholar] [CrossRef]
- Tuchman, R.F. Pervasive developmental disorders. Neurological perspective. Rev. Neurol. 1996, 24, 1446–1450. [Google Scholar]
- Wiggs, L.; Stores, G. Severe sleep disturbance and daytime challenging behavior in children with severe learning disabilities. Intellect. Disabil. Res. 1996, 40, 518–528. [Google Scholar] [CrossRef]
- Patzold, L.M.; Richdale, A.L.; Tonge, B.J. An investigation into sleep characteristics of children with autism and Asperger’s Disorder. J. Paediatr. Child Health 1998, 34, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J. Brief report: Sleep in parents of children with autism spectrum disorders. J. Pediatrpsychol. 2008, 33, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Souders, M.C.; Zavodny, S.; Eriksen, W.; Sinko, R.; Connell, J.; Kerns, C.; Schaaf, R.; Pinto-Martin, J. Sleep in children with autism spectrum disorder. Curr. Psychiatry Rep. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Halbower, A.C.; Mahone, E.M. Neuropsychological morbidity linked to childhood sleep-disordered breathing. Sleep Med. Rev. 2006, 10, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Christodulu, K.V.; Durand, V.M. Reducing bedtime disturbance and night waking using positive bedtime routines and sleep restriction. Focus Autism Other Dev. Disabil. 2004, 19, 130–139. [Google Scholar] [CrossRef]
- Plante, D.T.; Jensen, J.E.; Schoerning, L.; Winkelman, J.W. Reduced gamma-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychol. Pharmacol. 2012, 37, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Chou, T.C.; Scammell, T.E. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- McCauley, J.L.; Olson, I.M.; Delahanty, R. A linkage disequilibrium map of the 1-Mb 15q12 GABAA receptor subunit cluster and association to autism. Am. J. Med. Genet. 2004, 131, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Levitt, P.; Eagleson, K.L.; Powell, E.M. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004, 27, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef]
- Bourgeron, T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb. Perspect. Med. 2007, 72, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zee, P.C.; Attarian, H.; Videnovic, A. Circadian rhythm abnormalities. Continuum Lifelong Learn. Neurol. 2013, 19, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.E.; Adkins, K.W.; Calcutt, M.W.; Carter, M.D.; Goodpaster, R.L.; Wang, L.; Shi, Y.; Burgess, H.J.; Hachey, D.L.; Malow, B.A. Melatonin in children with autism spectrum disorders: Endogenous and pharmacokinetic profiles in relation to sleep. J. Autism Dev. Disord. 2014. [Google Scholar] [CrossRef]
- Deliens, G.; Leproult, R.; Schmitz, R.; Destrebecqz, A.; Peigneux, P. Sleep disturbances in autism spectrum disorders. Rev. J. Autism Dev. Disord. 2015, 2, 343–356. [Google Scholar] [CrossRef]
- Valdizán, J.R.; Abril, B.; Méndez, M.; Sans, O. Night polysomnogram in childhood autism without epilepsy. Rev. Neurol. 2002, 34, 1101–1105. [Google Scholar]
- Chervin, R.D.; Hedger, K.; Dilon, J.E.; Pituch, K.J. Pediatric Sleep Questionnaire (PSQ): Validity and reliability of scales-disordered brightening, snoring, sleepiness and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Bruni, O.; Octavian, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sep Disturbance Scale fos Children (SDSC). Cosmotion and validation of an instrument to assess sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–256. [Google Scholar] [CrossRef]
- Douglass, A.; Bornstein, R.; Nino-Murcia, G.; Keenan, S.; Miles, L.; Zarcone, V.; Guilleminault, C.; Dement, W.C. The sleep disorders questionnaire I. Creation and multivariate structure of SDQ. Sleep 1994, 17, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Petroski, G.F. Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Richdale, A.L.; Baglin, C.L. Self-report and caregiver-report of sleep and psychopathology in children with high-functioning autism spectrum disorder: A pilot study. Dev. Neurorehabil. 2015, 18, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.I.; Polido, J.C.; Mailloux, Z.; Coleman, G.G.; Cermak, S.A. Oral care and sensory sensitivities in children with autism spectrum disorders. Spec. Care Dent. 2011, 31, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D. Clinical Pharmacology of Other Drugs use as Hypnotics. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth T Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Honomichl, R.D.; Goodlin-Jones, B.L.; Burnham, M.; Gaylor, E.; Anders, T.F. Sleep patterns of children with pervasive developmental disorders. J. Autism Dev. Disord. 2002, 32, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.A.; Mulick, J.A. Parental report of sleep problems in children with autism. J. Autism Dev. Disord. 2000, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Arlington, V.A., Ed.; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar]
- Kotagal, S.; Broomall, E. Sleep in children with autism spectrum disorder. Pediatr. Neurol. 2012, 47, 242–251. [Google Scholar] [CrossRef]
- Amos, P. Rhythm and timing in autism: Learning to dance. Front. Integr. Neurosci. 2013, 7, 27. [Google Scholar] [CrossRef]
- Leu, R.M.; Beyderman, L.; Botzolakis, E.J.; Surdyka, K.; Wang, L.; Malow, B.A. Relation of melatonin to sleep architecture in children with autism. J. Autism Dev. Disord. 2011, 41, 427–433. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The pathophysiology of insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Feige, B.; Baglioni, C.; Spiegelhalder, K.; Hirscher, V.; Nissen, C.; Riemann, D. The microstructure of sleep in primary insomnia: An overview and extension. Int. J. Psychophysiol. 2013, 89, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Healey, E.S.; Kales, A.; Monroe, L.J.; Bixler, E.O.; Chamberlin, K.; Soldatos, C.R. Onset of insomnia: Role of life-stress events. Psychosom. Med. 1981, 43, 439–451. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders-Third Edition (ICSD-3); AASM: Darien, CT, USA, 2014. [Google Scholar]
- Souders, M.C.; Mason, T.B.A.; Valladares, O.; Bucan, M.; Levy, S.E.; Mandell, D.S.; Pinto-Martin, J. Sleep behaviors and sleep quality in children with autism spectrum disorder. Sleep 2009, 32, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.E.; Vehkalahti, K.; Vanhala, R.; von Wendt, L.; Nieminen-von Wendt, T.; Aronen, E.T. Sleep in children with Asperger syndrome. J. Autism Dev. Disord. 2008, 38, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Richdale, A.L. Annotation: Sleep problems and autism: Comparison with other disabilities, causes, and intervention. Dev. Med. Child Neurol. 1999, 41, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Sleep-wake patterns in autistic children. Jpn. J. Child Adolesc. Psychiatry 1984, 25, 205–217. (In Japanese) [Google Scholar]
- Richdale, A.L.; Prior, M.R. The sleep/wake rhythm in children with autism. Eur. Child Adolesc. Psychiatry 1995, 4, 175–186. [Google Scholar] [CrossRef]
- Allik, H.; Larsson, J.O.; Smedje, A. Sleep patterns of school-age children with Asperger syndrome or highfunctioning autism. J. Autism Dev. Disord. 2006, 36, 585–595. [Google Scholar] [CrossRef]
- Schwichtenberg, A.J.; Iosif, A.M.; Goodlin-Jones, B.; Tang, K.; Anders, T. Daytime sleep patterns in preschool children with autism, developmental delay, and typical development. Am. J. Intellect. Dev. Disabil. 2011, 116, 142–152. [Google Scholar] [CrossRef]
- Goldman, S.E.; McGrew, S.; Johnson, K.P.; Richdale, A.L.; Clemons, T.; Malow, B.A. Sleep is associated with problem behaviors in children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2011, 5, 1223–1229. [Google Scholar] [CrossRef]
- Ahmed, S.; Hasani, S.; Koone, M.; Thirumuruganathan, S.; Diaz-Abad, M.; Mitchell, R.; Isaiah, A.; Das, G. An Empirical Study of Questionnaires for the Diagnosis of Pediatric Obstructive Sleep Apnea. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, Hawaii, 17–21 July 2018; Volume 2018, pp. 4097–4100. [Google Scholar] [CrossRef]
| N | Age Years u(SD) | Sex | Schooling of Parents | ||||
|---|---|---|---|---|---|---|---|
| F | M | Low | Medium | Higher | |||
| Autistic group | 21 | 5.23 (1.99) | 5 (23%) | 16 (76%) | 2 (9%) | 6 (29%) | 13 (62%) |
| Control | 21 | 5.23 (1.99) | 12 (57%) | 9 (42%) | 0 | 7 (33%) | 14 (67%) |
| Subscales | Items |
|---|---|
| 1. Bedtime resistance | 1,3,4,5,6,8 |
| 2. Sleep onset | 2 |
| 3. Sleep duration | 9,10,11 |
| 4. Anxiety prior to sleep | 5,7,8,21 |
| 5. Awakenings at night | 16,24,25 |
| 6. Parasomnias | 12,13,14,15,17,22,23 |
| 7. Respiratory sleep disorders | 18,19,20 |
| 8. Daytime drowsiness | 26,27,28,29,30,31,32,33 |
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| Total scale | 671.0000 | 232.0000 | 0.000000 * |
| Subscale 1 | 610.5000 | 292.5000 | 0.000063 * |
| Subscale 2 | 546.0000 | 357.0000 | 0.017444 * |
| Subscale 3 | 556.5000 | 346.5000 | 0.008258 * |
| Subscale 4 | 581.0000 | 322.0000 | 0.001123 * |
| Subscale 5 | 639.0000 | 264.0000 | 0.000002 * |
| Subscale 6 | 639.0000 | 264.0000 | 0.000002 * |
| Subscale 7 | 471.5000 | 431.5000 | 0.614884 |
| Subscale 8 | 585.0000 | 318.0000 | 0.000784 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso-Alfonso, M.; Morales-Chacón, L.M.; González-Naranjo, J.E. Subjective Assessment of Sleep in Infantile Autism: A Comparative Study. Behav. Sci. 2019, 9, 12. https://doi.org/10.3390/bs9020012
Alfonso-Alfonso M, Morales-Chacón LM, González-Naranjo JE. Subjective Assessment of Sleep in Infantile Autism: A Comparative Study. Behavioral Sciences. 2019; 9(2):12. https://doi.org/10.3390/bs9020012
Chicago/Turabian StyleAlfonso-Alfonso, Maydelin, Lilia María Morales-Chacón, and Justa Elizabeth González-Naranjo. 2019. "Subjective Assessment of Sleep in Infantile Autism: A Comparative Study" Behavioral Sciences 9, no. 2: 12. https://doi.org/10.3390/bs9020012
APA StyleAlfonso-Alfonso, M., Morales-Chacón, L. M., & González-Naranjo, J. E. (2019). Subjective Assessment of Sleep in Infantile Autism: A Comparative Study. Behavioral Sciences, 9(2), 12. https://doi.org/10.3390/bs9020012





