Effectiveness and Characterization of Severely Energy-Restricted Diets in People with Class III Obesity: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Publication Eligibility Criteria
2.3. Data Extraction
2.4. Data Cleaning
2.5. Data Analysis
2.5.1. Primary Outcomes
2.5.2. Secondary Outcomes
2.5.3. Meta-Analysis and Meta-Regression
- = change between baseline and end weight, low end of the range of the sample.
- = change between baseline and end weight, high end of the range of the sample.
- = change between baseline and end weight median.
- SDb = standard deviation of baseline weight mean.
- SDf = standard deviation of end weight mean.
- Corr = Correlation coefficient between the ‘baseline’ standard deviation and the ‘end’ standard deviation.
2.6. Risk of Bias and Quality Assessment
3. Results
3.1. Publication Selection
3.2. Risk of Bias and Quality Assessment
3.3. Characteristics of Publications Included in the Meta-Analysis
3.4. Participants
3.4.1. Participant Data for the Meta-Analysis of Interventions with 4-Week Duration
3.4.2. Participant Data for the Meta-Analysis of Interventions ≥6 Weeks in Duration
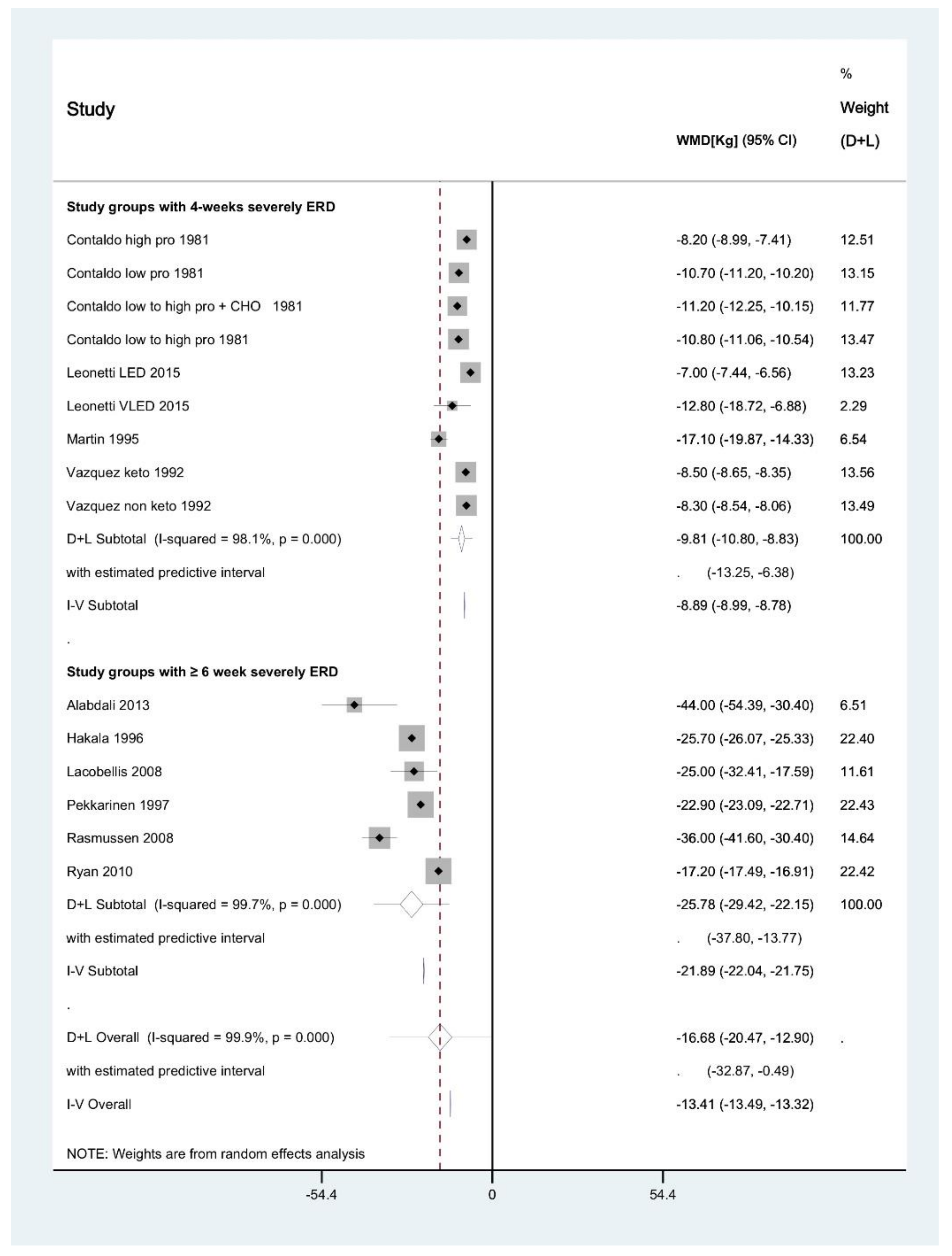
3.5. Meta-Analysis of Interventions with 4-Week Duration
3.6. Meta-Analysis of Interventions ≥6 Weeks in Duration
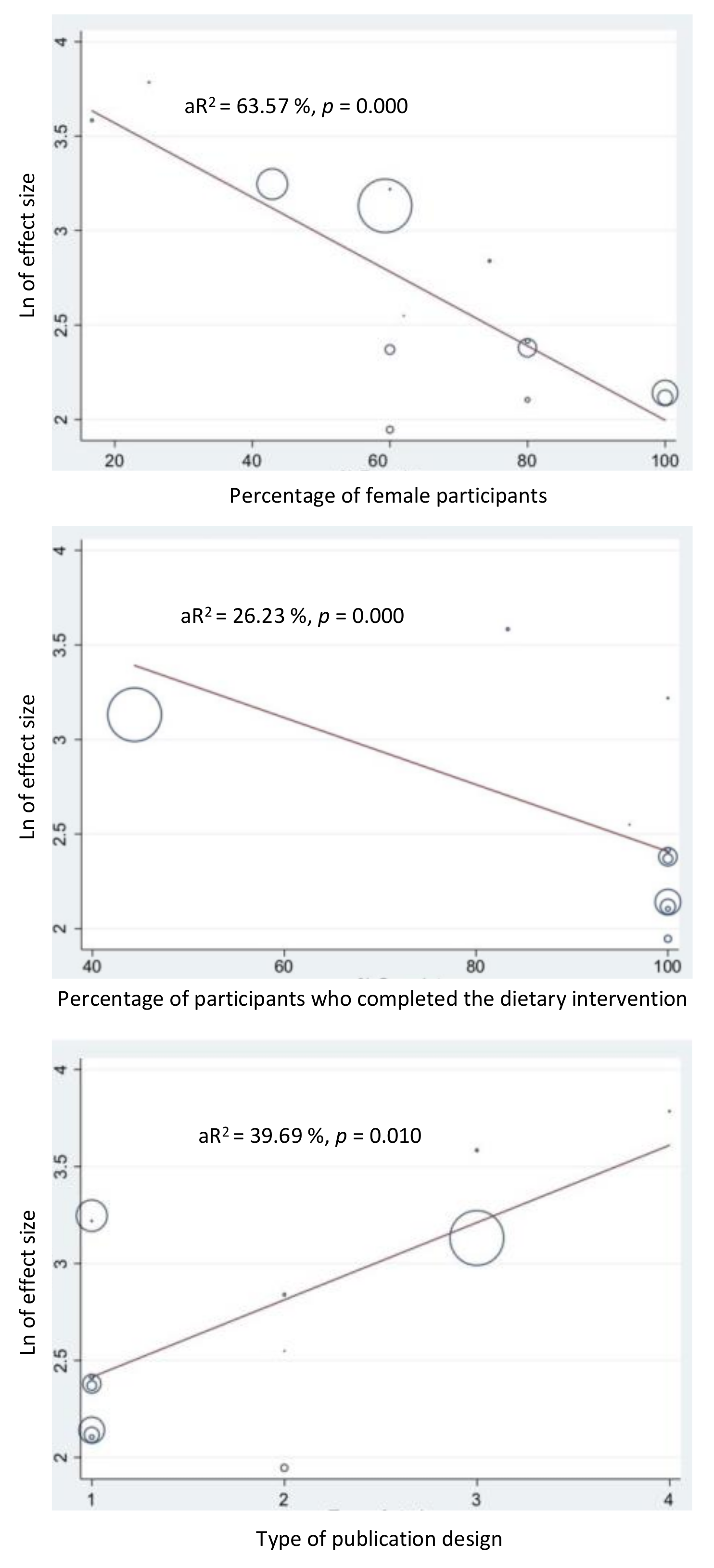
3.7. Meta-Regression of Factors Affecting Heterogeneity
3.8. Publication Not Included in Meta-Analysis
3.9. Severely Energy-Restricted Dietary Protocols
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Sturn, R. Increases in Clinically Severe Obesity in the United State, 1986–2000. Arch. Intern. Med. 2003, 163, 2146–2148. [Google Scholar] [CrossRef] [PubMed]
- Palmo, A. Severe obesity: Introductory outlines and the conventional non-surgical therapy. eSPEN J. 2013, 8, e216–e227. [Google Scholar] [CrossRef]
- Rennie, K.L.; Jebb, S.A. Prevalence of obesity in Great Britain. Obes. Rev. 2005, 6, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Helmert, U.; Strube, H. The development of obesity in Germany in the period from 1985 until 2000. Gesundheitswesen 2004, 66, 409. [Google Scholar] [CrossRef]
- Walls, H.L.; Wolfe, R.; Haby, M.M.; Magliano, D.J.; De Courten, M.; Reid, C.M.; McNeil, J.J.; Shaw, J.; Peeters, A. Trends in BMI of urban Australian adults, 1980–2000. Public Health Nutr. 2010, 13, 631–638. [Google Scholar] [CrossRef]
- Flegal, K.M.; Panagiotou, O.A.; Graubard, B.I. Estimating population attributable fractions to quantify the health burden of obesity. Annal. Epidemiol. 2015, 25, 201. [Google Scholar] [CrossRef]
- Williamson, D.F.; Thompson, T.J.; Thun, M.; Flanders, D.; Pamuk, E.; Byers, T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000, 23, 1499–1504. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- World Health Organization. Obesity—Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Allison, D.B.; Zannolli, R.; Faith, M.S.; Heo, M.; Pietrobelli, A.; Vanltallie, T.B.; Pi-Sunyer, F.X.; Heymsfield, S.B. Weight loss increases and fat loss decreases all-cause mortality rate: Results from two independent cohort studies. Int. J. Obes. 1999, 23, 603. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Ghanavati, M.; Lamuchi-Deli, N.; Payami, S.A.; Alavi-Rad, S.; Boustaninejad, M.; Afrisham, R.; Abbasnezhad, A.; Alipour, M. Rapid Weight Loss vs. Slow Weight Loss: Which is More Effective on Body Composition and Metabolic Risk Factors? Int. J. Endocrinol. Metab. 2017, 15, e13249. [Google Scholar] [CrossRef] [PubMed]
- Deibert, P.; König, D.; Vitolins, M.Z.; Landmann, U.; Frey, I.; Zahradnik, H.P.; Berg, A. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre-versus postmenopausal women. Nutr. J. 2007, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J. Updated review on the benefits of weight loss. Int. J. Obes. 2002, 26, S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; Kannel, W.B.; Silbershatz, H.; D’Agostino, R.B. Clustering of Metabolic Factors and Coronary Heart Disease. Arch. Intern. Med. 1999, 159, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Azagury, D.E.; Morton, J.M. Bariatric Surgery. Endocrinol. Metab. Clin. N. Am. 2016, 45, 647–656. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Morton, S.C.; Maglione, M.A.; Suttorp, M.; Tu, W.; Li, Z.; Maggard, M.; Mojica, W.A.; Shugarman, L.; Solomon, V.; et al. Pharmacological and Surgical Treatment of Obesity. Evidence Report/ Technology Assessment (Summary); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004. [Google Scholar]
- Velazquez, A.; Apovian, C.M. Updates on obesity pharmacotherapy. Annal. N. Y. Acad. Sci. 2018, 1411, 106–119. [Google Scholar] [CrossRef]
- Saunders, K.H.; Shukla, A.P.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Pharmacotherapy for Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 521–538. [Google Scholar] [CrossRef]
- Markovic, T.; Franklin, J. Non-surgical considerations in the management of the super obese patient. Obes. Res. Clin. Pract. 2012, 6, 12. [Google Scholar] [CrossRef]
- Martin, M.; Beekley, A.; Kjorstad, R.; Sebesta, J. Socioeconomic disparities in eligibility and access to bariatric surgery: A national population-based analysis. Surg. Obes. Relat. Dis. 2010, 6, 8–15. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Saleh, F.; Sharma, A.M.; Anvari, S.; Gmora, S.; Anvari, M.; Hong, D. Geographic and socioeconomic factors affecting delivery of bariatric surgery across high- and low-utilization healthcare systems. Br. J. Surg. 2017, 104, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Weight Loss Surgery in Australia 2014–2015: Australian Hospital Statistics Canberra ACT; Australian Government: Canberra, Australia, 2017. [Google Scholar]
- Gadde, K.M.; Apolzan, J.W.; Berthoud, H.-R. Pharmacotherapy for Patients with Obesity. Clin. Chem. 2018, 64, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Report of the Nineteenth Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses; Joint FAO/WHO Food standards Program: Rome, Italy, 1995. [Google Scholar]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; van Mierlo, C.A.J.; van der Knaap, H.C.M.; Heo, M.; Frier, H.I. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int. J. Obes. 2003, 27, 537–549. [Google Scholar] [CrossRef]
- Leslie, W.S.; Taylor, R.; Harris, L.; Lean, M.E.J. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: Systematic review and meta-analysis. Int. J. Obes. 2017, 41, 96–101. [Google Scholar] [CrossRef]
- Tsai, A.G. The Evolution of Very-Low-Calorie Diets: An update and Meta-analysis. Obesity 2006, 14, 1283–1293. [Google Scholar] [CrossRef]
- Parretti, H.M.; Jebb, S.A.; Johns, D.J.; Lewis, A.L.; Christian-Brown, A.M.; Aveyard, P. Clinical effectiveness of very-low-energy diets in the management of weight loss: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 225–234. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia ACT Canberra; Australian Government: Canberra, Australia, 2013. [Google Scholar]
- National Clinical Guideline Centre. Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults; National Clinical Guideline Centre: London, UK, 2010. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. 2), S102–S138. [Google Scholar] [CrossRef]
- Raynor, H.A.; Champagne, C.M. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J. Acad. Nutr. Diet. 2016, 116, 129–147. [Google Scholar] [CrossRef]
- Astbury, N.M.; Piernas, C.; Hartmann-Boyce, J.; Lapworth, S.; Aveyard, P.; Jebb, S.A. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes. Rev. 2019, 20, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.M. Very-low-calorie diets and sustained weight loss. Obes. Res. 2001, 9, 295S–301S. [Google Scholar] [CrossRef] [PubMed]
- Spahlholz, J.; Baer, N.; König, H.H.; Riedel-Heller, S.G.; Luck-Sikorski, C. Obesity and discrimination—A systematic review and meta-analysis of observational studies. Obes. Rev. 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hensrud, D.D.; Klein, S. Extreme Obesity: A New Medical Crisis in the United States. Mayo Clin. Proc. 2006, 81, S5–S10. [Google Scholar] [CrossRef]
- Okoro, C.A.; Hootman, J.M.; Strine, T.W.; Balluz, L.S.; Mokdad, A.H. Disability, Arthritis, and Body Weight among Adults 45 Years and Older. Obesity 2004, 12, 854–861. [Google Scholar] [CrossRef]
- Deitel, M. Overlooked Problems in Morbidly Obese Patients. Obes. Surg. 2001, 11, 541. [Google Scholar] [CrossRef]
- Brown, K.L.; LaRose, J.G.; Mezuk, B. The relationship between body mass index, binge eating disorder and suicidality. BMC Psychiatry 2018, 18, 196. [Google Scholar] [CrossRef]
- Mustelin, L.; Bulik, C.M.; Kaprio, J.; Keski-Rahkonen, A. Prevalence and correlates of binge eating disorder related features in the community. Appetite 2017, 109, 165–171. [Google Scholar] [CrossRef]
- Jackson, S.E.; Beeken, R.J.; Wardle, J. Obesity, perceived weight discrimination, and psychological well-being in older adults in England. Obesity 2015, 23, 1105–1111. [Google Scholar] [CrossRef]
- Magallares, A.; Bolaños-Rios, P.; Ruiz-Prieto, I.; Benito de Valle, P.; Irles, J.A.; Jáuregui-Lobera, I. The Mediational Effect of Weight Self-Stigma in the Relationship between Blatant and Subtle Discrimination and Depression and Anxiety. Span. J. Psychol. 2017, 20, E4. [Google Scholar] [CrossRef]
- Kostro, K.; Lerman, J.B.; Attia, E. The current status of suicide and self-injury in eating disorders: A narrative review. J. Eat. Disord. 2014, 2, 19. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ford, E.S.; Dhingra, S.; Li, C.; Strine, T.W.; Mokdad, A.H. Depression and anxiety among US adults: Associations with body mass index. Int. J. Obes. 2009, 33, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. An Overview of Factors Associated with Adherence to Lifestyle Modification Programs for Weight Management in Adults. Int. J. Environ. Res. Public Health 2017, 14, 922. [Google Scholar] [CrossRef]
- Susin, N.; de Melo Boff, R.; Ludwig, M.W.; Feoli, A.M.; da Silva, A.G.; Macagnan, F.E.; da Silva Oliveira, M. Predictors of adherence in a prevention program for patients with metabolic syndrome. J. Health Psychol. 2016, 21, 2156–2167. [Google Scholar] [CrossRef]
- Aggarwal, B.; Liao, M.; Allegrante, J.P.; Mosca, L. Low social support level is associated with non-adherence to diet at 1 year in the Family Intervention Trial for Heart Health (FIT Heart). J. Nutr. Educ. Behav. 2010, 42, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.W.; Snook, J. Disability Discrimination and Obesity: The Big Questions? Curr. Obes. Rep. 2015, 4, 504–509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Härkönen, J.; Räsänen, P.; Näsi, M. Obesity, Unemployment, and Earnings. Nord. J. Work. Life Stud. 2011, 1, 23–38. [Google Scholar] [CrossRef]
- Ahnis, A.; Riedl, A.; Figura, A.; Steinhagen-Thiessen, E.; Liebl, M.E.; Klapp, B.F. Psychological and sociodemographic predictors of premature discontinuation of a 1-year multimodal outpatient weight-reduction program: An attrition analysis. Patient Prefer. Adherence 2012, 6, 165–177. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Franklin, J.; Markovic, T.P.; Byrne, N.M.; Manson, E.; Caterson, I.D.; Sainsbury, A. Fast versus slow weight loss: Development process and rationale behind the dietary interventions for the TEMPO Diet Trial. Obes. Sci. Pract. 2016, 2, 162–173. [Google Scholar] [CrossRef]
- Phelan, S.; Wing, R.R.; Brannen, A.; McHugh, A.; Hagobian, T.A.; Schaffner, A.; Jelalian, E.; Hart, C.N.; Scholl, T.O.; Munoz-Christian, K.; et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am. J. Clin. Nutr. 2018, 107, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, F.; Di Biase, G.; Fischetti, A.; Mancini, M. Evaluation of the safety of very-low-calorie diets in the treatment of severely obese patients in a metabolic ward. Int. J. Obes. 1981, 5, 221–226. [Google Scholar] [PubMed]
- Vazquez, J.A.; Adibi, S.A. Protein sparing during treatment of obesity: Ketogenic versus nonketogenic very low calorie diet. Metab. Clin. Exp. 1992, 41, 406–414. [Google Scholar] [CrossRef]
- Pekkarinen, T.; Mustajoki, P. Comparison of behavior therapy with and without very-low-energy diet in the treatment of morbid obesity. A 5-year outcome. Arch. Intern. Med. 1997, 157, 1581–1585. [Google Scholar] [CrossRef]
- Leonetti, F.; Campanile, F.C.; Coccia, F.; Capoccia, D.; Alessandroni, L.; Puzziello, A.; Coluzzi, I.; Silecchia, G. Very Low-Carbohydrate Ketogenic Diet Before Bariatric Surgery: Prospective Evaluation of a Sequential Diet. Obes. Surg. 2015, 25, 64–71. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.G., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 7 December 2019).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Hakala, K.; Mustajoki, P.; Aittomaki, J.; Sovijarvi, A. Improved gas exchange during exercise after weight loss in morbid obesity. Clin. Physiol. 1996, 16, 229–238. [Google Scholar] [CrossRef]
- Dersimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Alabdali, F.; Rueda-Clausen, C.F.; Robbins, S.; Sharma, A.M. Efficacy and safety of long-term low-calorie diet in severely obese patients non-eligible for surgery. Clin. Obes. 2013, 3, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.F.; Tan, T.L.; Holmes, P.A.; Becker, D.A.; Horn, J.; Bixler, E.O. Can morbidly obese patients safely lose weight preoperatively? Am. J. Surg. 1995, 169, 245–253. [Google Scholar] [CrossRef]
- Rasmussen, M.H.; Wildschiodtz, G.; Juul, A.; Hilsted, J. Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss. Obesity 2008, 16, 1516–1521. [Google Scholar] [CrossRef]
- Ryan, D.H.; Johnson, W.D.; Myers, V.H.; Prather, T.L.; McGlone, M.M.; Rood, J.; Brantley, P.J.; Bray, G.A.; Gupta, A.K.; Broussard, A.P.; et al. Nonsurgical weight loss for extreme obesity in primary care settings: Results of the Louisiana Obese Subjects Study. Arch. Intern. Med. 2010, 170, 146–154. [Google Scholar] [CrossRef]
- Winkler, J.K.; Schultz, J.H.; Woehning, A.; Piel, D.; Gartner, L.; Hildebrand, M.; Roeder, E.; Nawroth, P.P.; Wolfrum, C.; Rudofsky, G. Effectiveness of a low-calorie weight loss program in moderately and severely obese patients. Obes. Facts 2013, 6, 469–480. [Google Scholar] [CrossRef]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998, 68, 899. [CrossRef]
- National Institute for Health and Care Excellence. Weight Management: Lifestyle Services for Overweight or Obese Adults; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Atukorala, I.; Makovey, J.; Lawler, L.; Messier, S.P.; Bennell, K.; Hunter, D.J. Is There a Dose-Response Relationship Between Weight Loss and Symptom Improvement in Persons With Knee Osteoarthritis?: Weight Loss and Symptomatic Knee OA Improvement. Arthritis Care Res. 2016, 68, 1106–1114. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Rothberg, A.E.; McEwen, L.N.; Kraftson, A.T.; Ajluni, N.; Fowler, C.E.; Nay, C.K.; Miller, N.M.; Burant, C.F.; Herman, W.H. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res. Care 2017, 5, e000341. [Google Scholar] [CrossRef]
- Poobalan, A.; Aucott, L.; Smith, W.C.; Avenell, A.; Jung, R.; Broom, J.; Grant, A.M. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes—a systematic review. Obes. Rev. 2004, 5, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Wadden, T.A.; Butryn, M.L.; Wilson, C. Lifestyle Modification for the Management of Obesity. Gastroenterology 2007, 132, 2226–2238. [Google Scholar] [CrossRef]
- Koutroumanidou, E.; Pagonopoulou, O. Combination of Very Low Energy Diets and Pharmacotherapy in the Treatment of Obesity: Meta-Analysis of Published Data. Diabetes Metab. Res. Rev. 2014, 30, 65–74. [Google Scholar] [CrossRef]
- Maston, G.; Franklin, J.; Sainsbury, A.; Manson, E.; Gibson, A.A.; Markovic, T.P. Attitudes and approaches to the use of meal replacement products among health professionals working in the management of obesity. In Proceedings of the 25th European Congress on Obesity, Vienna, Austria, 23–26 May 2018; Metabolism and Obesity Services: Sydney, Australia, 2018. [Google Scholar]
- Cheskin, L.J.; Mitchell, A.M.; Jhaveri, A.D.; Mitola, A.H.; Davis, L.M.; Lewis, R.A.; Yep, M.A.; Lycan, T.W. Efficacy of Meal Replacements Versus a Standard Food-Based Diet for Weight Loss in Type 2 Diabetes A Controlled Clinical Trial. Diabetes Educ. 2008, 34, 118–127. [Google Scholar] [CrossRef]
- Ashley, J.M.; Herzog, H.; Clodfelter, S.; Bovee, V.; Schrage, J.; Pritsos, C. Nutrient adequacy during weight loss interventions: A randomized study in women comparing the dietary intake in a meal replacement group with a traditional food group. Nutr. J. 2007, 6, 12. [Google Scholar] [CrossRef]
- Wright, G.; Dawson, B.; Jalleh, G.; Law, S. Impact of Compliance on Weight Loss and Health Profile in a Very Low Energy Diet Program. Aust. Fam. Physician 2010, 39, 49–52. [Google Scholar]
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and Real-World Settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef]
- Wing, R.R.; Hill, J.O. Successful weight loss maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef]

| Publication First Author and Year of Publication (Reference) | Bias Due to Confounding | Bias in Selection of Participants for the Intervention | Bias in Classification of Interventions | Bias Due to Deviations from Intended interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Contaldo 1981 [58] | M | M | L | L | L | M | L | M |
| Leonetti 2015 [61] | M | M | M | L | M | M | L | M |
| Martin 1995 [70] | M | M | M | L | L | M | M | M |
| Vazquez 1992 [59] | M | M | M | L | L | M | L | M |
| Hakala 1996 [64] | M | M | M | L | L | M | L | M |
| Iacobellis 2008 [69] | M | M | L | L | L | M | L | M |
| Rasmussen 2008 [71] | M | M | M | L | M | M | L | M |
| Pekkarinen 1997 [60] | M | M | L | L | M | M | L | M |
| Alabdali 2013 [68] | H | H | H | M | L | M | M | H |
| Ryan 2010 [72] | L | L | L | L | L | L | L | L |
| Winkler 2013 [73] * | M | L | L | L | M | M | L | M |
| Publication First Author and Year of Publication (Reference) Comparison Arm Publication Type | Mean Age of Participants at Baseline (Years) | Number of Participants at Recruitment (Sex Breakdown at Recruitment) | Mean Baseline Weight (kg) ± SD, unless Stated Otherwise | Mean Baseline BMI (kg/m2) ± SD, unless Stated Otherwise | VLED or LED and Daily Prescription for Energy, Carbohydrate, Fat and Protein Basis of Diet (TMR or FB) Adjunct Therapy | Intervention Duration (Weeks) | Reporting Time from Start of Intervention (Weeks) | Number of Completers (%) | Mean Weight Loss (kg) ± SD, unless Stated Otherwise | Mean Weight Loss (% of Initial Weight) ± SD, unless Stated Otherwise | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contaldo 1981 [58] Low protein Prospective case series | 41 ± 2 | 10 (6 F, 4 M) | NR | 44.7 ± 2.6 | VLED 330 kJ (80 kcal), 0 g CHO, 0 g Fat, 17 g Pro TMR (with allowance for unlimited tea consumption) No adjunct therapy, inpatient setting | 4 | 4 | 10 (100%) | 10.7 ± 0.8 | 9.6 ± NR | ||
| Contaldo 1981 [58] High protein Prospective case series | 43 ± 6 | 5 (4 F, 1 M) | NR | 48.8 ± 2.6 | VLED 740 kJ (180 kcal), 0 g CHO, 2 g Fat, 40 g Pro TMR (with allowance for unlimited tea consumption) No adjunct therapy, inpatient setting | 4 | 4 | 5 (100%) | 8.2 ± 0.9 | 7.0 ± NR | ||
| Contaldo 1981 [58] Low to high protein Prospective case series | 42 ± 5 | 5 (4 F, 1 M) | NR | 49.0 ± 2.1 | 2 weeks VLED 330 kJ (80 kcal), 0 g CHO, 0 g Fat, 17 g Pro 2 weeks VLED 740 kJ (180 kcal), 0 g CHO, 2 g Fat, 40 g ProTMR (with allowance for unlimited black tea consumption) No adjunct therapy, inpatient setting | 4 | 4 | 5 (100%) | 10.8 ± 0.3 | 8.3 ± NR | ||
| Contaldo 1981 [58] Low to high protein + CHO Prospective case series | 37 ± 2 | 5 (4 F, 1 M) | NR | 43.1 ± 4.4 | 2 weeks VLED 330 kJ (80 kcal), 0 g CHO, 0 g Fat, 17 g Pro 2 weeks VLED 740 kJ (180 kcal), 25.5 g CHO, 0 g Fat, 17 g Pro TMR (with allowance for unlimited black tea consumption) No adjunct therapy, inpatient setting | 4 | 4 | 5 (100%) | 11.2 ± 1.2 | 10.5 ± NR | ||
| Leonetti 2015 [61] VLED Prospective cohort | 48 ± 11 | 50 (31 F, 19 M) | 150.4 ± 26.3 | 53.5 ± 8.4 | Energy ramping protocol: i. 10 days VLED 2450 kJ (586 kcal), 15 g CHO, 24 g Fat, 80 g Pro (i) TMR (with supplemental ketone powder and low starchy vegetables) ii. 10 days VLED 3337 kJ (798 kcal) 55 g CHO, 30 g Fat, 80 g Pro iii. 10 days LED 4631 kJ (1106 kcal), 14.5 g CHO, 33 g Fat, 60 g Pro (ii & iii) FB (Unlimited daily allowance vegetables) No adjunct therapy | 4 | 4 | 48 (96%) | 12.8 ± 21.4 | 8.5 ± NR | ||
| Leonetti 2015 [61] LED Prospective cohort | 48 ± 11 | 30 (18 F, 12 M) | 153.2 ± 32.4 | 53.5 ± 8.4 | LED 5000 kJ (1200 kcal), 115 g CHO, 42 g Fat, 90 g Pro FB No adjunct therapy | 4 | 30 | 30 (100%) | 7.0 ± 1.2 | 4.6 ± NR | ||
| Martin 1995 [70] Prospective cohort | 40 ± 8 | 47 (35 F, 12 M) | 161.2 ± 31.0 | 58.4 ± 11.6 | VLED 1730 kJ (414 kcal), 0 g CHO, 0 g Fat, 70 g Pro TMR Adjunct behavioural therapy for 4 weeks | 4 | 4 | 47 (100%) | 17.1 ± 9.7 | 10.6 ± NR | ||
| Vazquez 1992 [59] Ketogenic VLED Prospective case series | 45 ± 4 | 8 (8 F) | NR | 47.0 ± 2.0 | VLED (ketogenic) 2448 kJ (594 kcal), 10 g CHO, 38 g Fat, 52 g Pro TMR (with allowance for unlimited black tea and coffee, and 750 mL daily allowance of diet soft drink) No adjunct therapy, inpatient setting | 4 | 4 | 8 (100%) | 8.5 ± 0.3 | NR | ||
| Vazquez 1992 [59] Non-ketogenic VLED Prospective case series | 43 ± 5 | 8 (8 F) | NR | 49.0 ± 4.0 | VLED (non-ketogenic) 2430 kJ (590 kcal), 76 g CHO, 10 g Fat, 50 g Pro TMR (with allowance for unlimited black tea and coffee, and 750 mL daily allowance of diet soft drink) No adjunct therapy, inpatient setting | 4 | 4 | 8 (100%) | 8.3 ± 0.5 | NR | ||
| Hakala 1996 [64] Prospective case series | 44 ± 8 | 7 (3 F, 4 M) | 140.4 ± 22.5 | 46.6 ± 6.3 | VLED 2100 kJ (502 kcal), 47% CHO, 1.8% Fat, 35% Pro TMR Adjunct behavioural therapy for 6 weeks | 6 | 16 | 7 (100%) | 25.7 ± 0.5 | 18.0 ± NR | ||
| Iacobellis 2008 [69] Prospective case series | 35 ± 10 | 20 (12 F, 8 M) | 154.0 ± NR | 45.0 ± 5.0 | LED 3700 kJ (885 kcal), macronutrient prescription NR TMR Adjunct behavioural therapy for 12 weeks | 12 | 24 | 20 (100%) | 25.0 ± 10.0 | 20.0 ± 2.8 | ||
| Rasmussen 2008 [71] Non-randomised controlled trial | 32 ± 2 | 6 (1 F, 5 M) | 126.0 ± 8.0 | 41.0 ± 1.0 | VLED 1600 kJ (383 kcal) macronutrient prescription NR TMR Adjunct behavioural therapy for 12 weeks | 12 | 12 | 5 (83%) | 36.0 ± 7.0 | 28.6 ± NR | ||
| Pekkarinen 1997 [60] Non-randomised controlled trial | 42 ± 9 | 25 (16 F, 11 M) | 131.2 ± 17.7 | 45.3 ± 4.0 | VLED 2100 kJ (502 kcal), 65 g CHO, 2 g Fat, 50 g Pro TMR (Unlimited daily allowance of low-starch vegetables) Adjunct behavioural therapy for 16 weeks | 7 | 7 | 12 (48%) | 22.9 ± 0.5 | 17.5 ± NR | ||
| Alabdali 2013 [68] Retrospective case series | 60 ± 8 | 8 (2 F, 6 M) | NR | 57.1 ± 8.8 | LED 3762 kJ (900 kcal), 67 g CHO, 30 g Fat, 90 g Pro TMR No adjunct therapy | 142 ± 43 | 112 | 8 (100%) | 44.0 ± 15.0 | 27.0 ± 13.0 | ||
| Ryan 2010 [72] Randomised controlled trial | 47 ± NR | 516 (326 F, 190 M) | 128.4 (median) | 45.6 ± 7.9 (median) | VLED 3720 kJ (890 kcal), 110 g CHO, 15 g Fat, 75 g Pro TMR (with 10 g daily allowance of oil) Adjunct behavioural therapy for 8 weeks, followed by pharmacotherapy for unknown length of time | 8 | 104 | 119 (39%) | 17.2 ± 1.6 | 8.3 ± 0.8 | ||
| Winkler 2013 [73] * Non-randomised controlled trial | 44 ± 1 | 115 (76 F, 39 M) | 136.3 ± 2.2 | 46.6 ± 0.5 | VLED 3344 kJ (800 kcal), 100 g CHO, 15 g Fat, 70 g Pro TMR (Unlimited daily allowance of low-starch vegetables) Adjunct behavioural therapy for 12 weeks | 12 | 52 | 115 (100%) | NR | 18.3 ± 0.9 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maston, G.; Gibson, A.A.; Kahlaee, H.R.; Franklin, J.; Manson, E.; Sainsbury, A.; Markovic, T.P. Effectiveness and Characterization of Severely Energy-Restricted Diets in People with Class III Obesity: Systematic Review and Meta-Analysis. Behav. Sci. 2019, 9, 144. https://doi.org/10.3390/bs9120144
Maston G, Gibson AA, Kahlaee HR, Franklin J, Manson E, Sainsbury A, Markovic TP. Effectiveness and Characterization of Severely Energy-Restricted Diets in People with Class III Obesity: Systematic Review and Meta-Analysis. Behavioral Sciences. 2019; 9(12):144. https://doi.org/10.3390/bs9120144
Chicago/Turabian StyleMaston, Gabrielle, Alice A. Gibson, H. Reza Kahlaee, Janet Franklin, Elisa Manson, Amanda Sainsbury, and Tania P. Markovic. 2019. "Effectiveness and Characterization of Severely Energy-Restricted Diets in People with Class III Obesity: Systematic Review and Meta-Analysis" Behavioral Sciences 9, no. 12: 144. https://doi.org/10.3390/bs9120144
APA StyleMaston, G., Gibson, A. A., Kahlaee, H. R., Franklin, J., Manson, E., Sainsbury, A., & Markovic, T. P. (2019). Effectiveness and Characterization of Severely Energy-Restricted Diets in People with Class III Obesity: Systematic Review and Meta-Analysis. Behavioral Sciences, 9(12), 144. https://doi.org/10.3390/bs9120144








