A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility
Abstract
1. Introduction
Immune & Endocrine Dysregulation in ASD & GJH
2. Methods
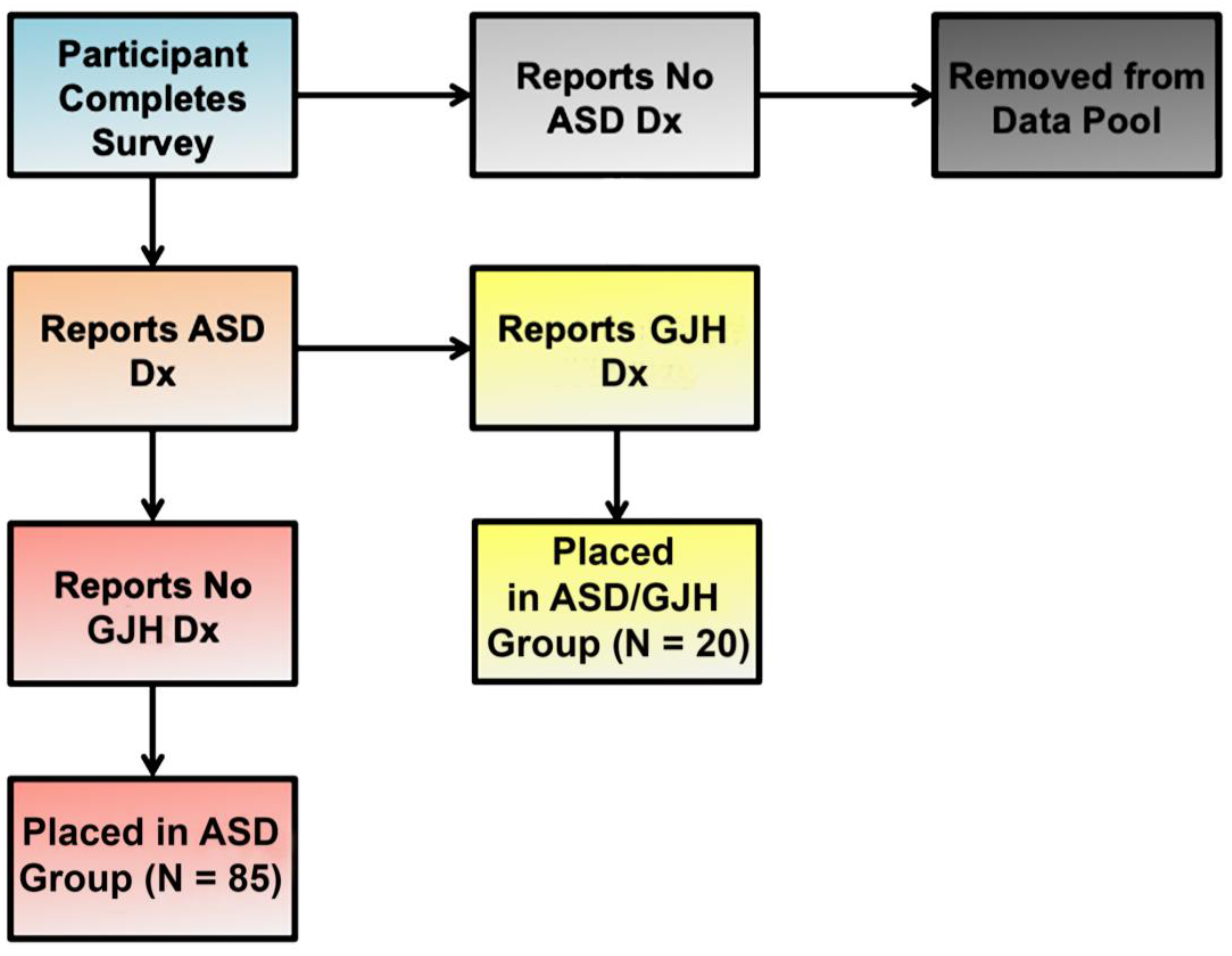
2.1. Study Population
2.2. Survey
2.3. Statistical Analyses
3. Results
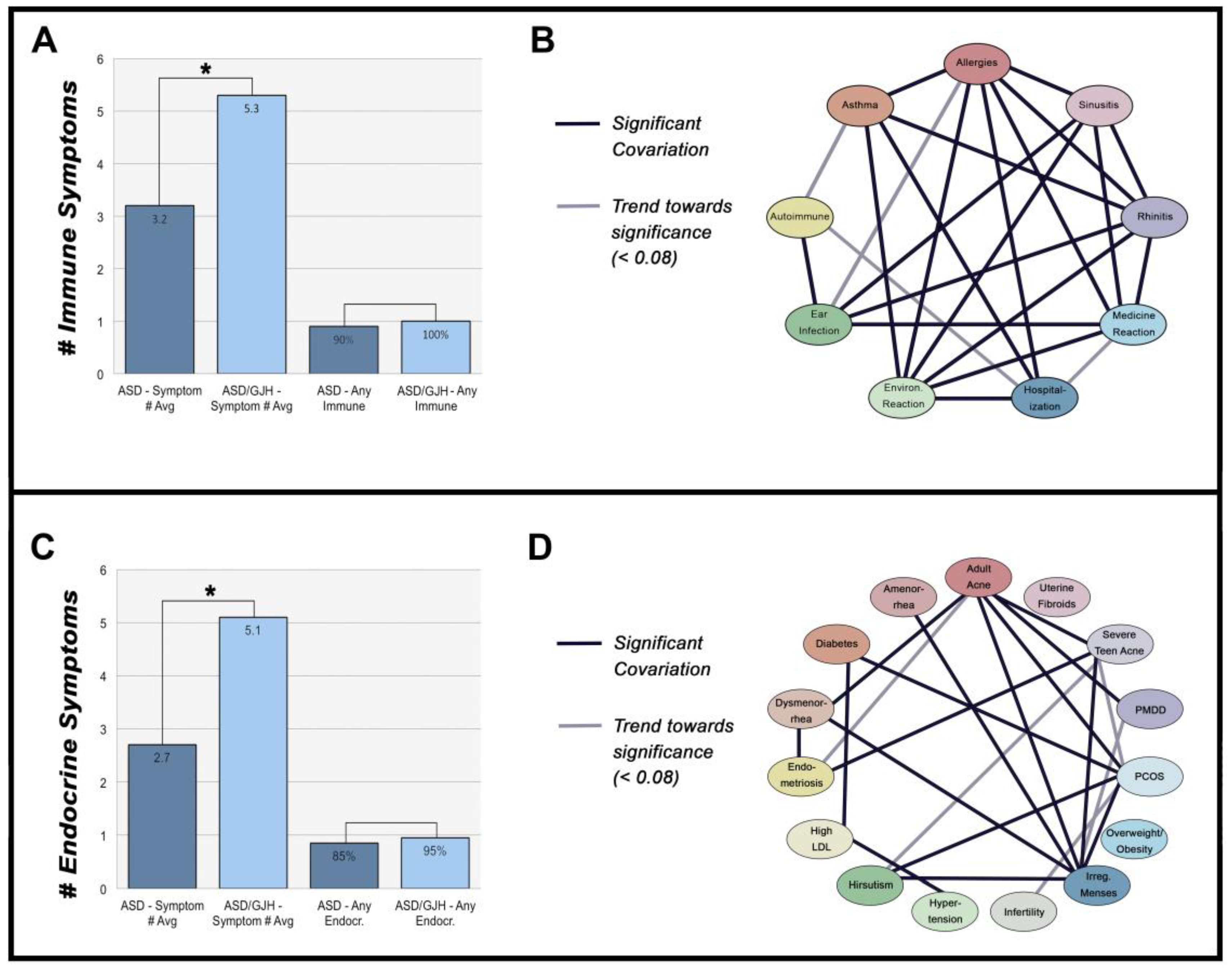
3.1. Immune-Mediated Disorders
3.2. Hormone Disorders
3.3. The Relationship between Immune- & Hormone-Mediated Symptoms
4. Discussion
4.1. Immune-Mediated Disorders in Association with Connective Tissue Disorders
4.2. The Effects of Estrogen on Collagen Production & the Immune System
4.3. Autism & Generalized Joint Hypermobility
… chronic aberrant elaboration of a particular set of mediators (drawn from amongst the mast cell’s repertoire of more than 200 such molecular signals) not only [influences] virtually every other system and organ in the body but also [influences] connective tissue development to yield the “hyperextensible” phenotype long associated with EDS Type III [(hypermobile type)].(p. 138)
4.4. The Etiology of Autism
4.5. Limitations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castori, M.; Tinkle, B.; Levy, J.; Grahame, R.; Malfait, F.; Hakim, A. A framework for the classification of joint hypermobility and related conditions. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, L.; Ursini, G.; Castori, M. Psychopathological manifestations of joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type: The link between connective tissue and psychological distress revised. Am. J. Med. Genet. C Semin. Med. Genet. 2015, 169, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lumley, M.A.; Jordan, M.; Rubenstein, R.; Tsipouras, P.; Evans, M.I. Psychosocial functioning in the Ehlers-Danlos syndrome. Am. J. Med. Genet. 1994, 53, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Velasco, C.; Pailhez, G.; Bulbena, A.; Baghdadli, A. Joint hypermobility and the heritable disorders of connective tissue; Clinical and empirical evidence of links with psychiatry. Gen. Hosp. Psychiatry 2015, 37, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Shetreat-Klein, M.; Shinnar, S.; Rapin, I. Abnormalities of joint mobility with autism spectrum disorders. Brain Dev. 2014, 36, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Eccles, J.A.; Iodice, V.; Dowell, N.G.; Owens, A.; Hughes, L.; Skipper, S.; Lycette, Y.; Humphries, K.; Harrison, N.A.; Mathias, C.J.; et al. Joint hypermobility and autonomic hyperactivity: Relevance to neurodevelopmental disorders. J. Neurol. Neurosurg. Psychiatry 2014, 85, e3. [Google Scholar] [CrossRef]
- Demas, G.E.; Adamo, S.A.; French, S.S. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: Implications for host defence. Funct. Ecol. 2011, 25, 29–39. [Google Scholar] [CrossRef]
- Bukovsky, A. Immune system involvement in the regulation of ovarian function an augmentation of cancer. Microsc. Res. Tech. 2006, 69, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Matelski, L.; Van de Water, J. Risk factors in autism: Thinking outside the brain. J. Autoimmun. 2016, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Careaga, M.; Rogers, S.; Handsen, R.L.; Amaral, D.G.; Van de Water, J.; Ashwood, P. Immune endophenotypes in children with autism spectrum disorder. Biol. Psychiatry 2017, 81, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Ingudomnukul, E.; Baron-Cohen, S.; Wheelwright, S.; Knickmeyer, R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm. Behav. 2007, 51, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jing, J.; Bowers, K.; Liu, B.; Bao, W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta-analysis. J. Autism Dev. Disord. 2014, 44, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Arver, S.; Widman, L.; Gardner, R.M.; Magnusson, C.; Dalman, C.; Kosidou, K. Maternal hirsutism and autism spectrum disorders in offspring. Autism Res. 2017, 10, 1544–1546. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Marotta, R.; Di Cello, A.; Russo, T.; Falbo, A.; Orio, F.; Tolino, A.; Zullo, F.; Esposito, R.; Sala, G.B.L. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: A longitudinal case-control study. Clin. Endocrinol. 2012, 77, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.; Vadas, P. A new disease cluster: Mast cell activation syndrome, postural orthostatic tachycardia syndrome, and Ehlers-Danlos syndrome. J. Allergy Clin. Immunol. 2015, 135, AB65. [Google Scholar] [CrossRef]
- Castori, M.; Camerota, F.; Celletti, C.; Grammatico, P.; Padua, L. Ehlers-Danlos syndrome hypermobility type and the excess of affected females: Possible mechanisms and perspectives. Am. J. Med. Genet. A 2010, 152, 2406–2408. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.J.; Mallett, V.T.; Frahm, J.D.; Richardson, D.A.; Evans, M.I. Gynecologic disorders in women with Ehlers-Danlos syndrome. J. Soc. Gynecol. Investig. 1995, 2, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.J.; Grahame, R. A simple questionnaire to detect hypermobility: An adjunct to the assessment of patients with diffuse musculoskeletal pain. Int. J. Clin. Pract. 2003, 57, 163–166. [Google Scholar] [PubMed]
- Lyall, K.; Van de Water, J.; Ashwood, P.; Hertz-Picciotto, I. Asthma and allergies in children with autism spectrum disorders: Results from the CHARGE study. Autism Res. 2015, 8, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Autism spectrum disorders and mastocytosis. Int. J. Immunopath Pharmacol. 2009, 22, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Comi, A.M.; Zimmerman, A.W.; Frye, V.H.; Law, P.A.; Peeden, J.N. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J. Child Neurol. 1999, 14, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Asthma and Allergy Foundation of America (AAFA). Allergy Facts and Figures. 2017. Available online: http://www.aafa.org/page/allergy-facts.aspx (accessed on 23 May 2017).
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; King, M.E.; Liu, X.; Moorman, J.E.; Zahran, H.S. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012, 94, 1–8. [Google Scholar]
- Cooper, G.S.; Bynum, M.L.; Somers, E.C. Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. J. Autoimmun. 2009, 33, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Teele, D.W.; Klein, J.O.; Rosner, B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study. J. Infect. Dis. 1989, 160, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Monasta, L.; Ronfani, L.; Marchetti, F.; Montico, M.; Vecchi Brumatti, L.; Bavcar, A.; Grasso, D.; Barbiero, C.; Tamburlini, G. Burden of disease caused by otitis media: Systematic review and global estimates. PLoS ONE 2012, 7, e36226. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.B.; Fu, Q.L.; Zhang, H.; Cheng, L.; Wang, Y.J.; Zhu, D.D.; Lv, W.; Liu, S.X.; Li, P.Z.; Ou, C.Q.; et al. Epidemiology of chronic rhinosinusitis: Results from a cross-sectional survey in seven Chinese cities. Allergy 2015, 70, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Thong, B.Y.; Tan, T.C. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 2011, 71, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Caress, S.M.; Steinemann, A.C. Prevalence of multiple chemical sensitivities: A population-based study in the southeastern United States. Am. J. Pub. Health 2004, 94, 746–747. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Downie–Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Murialdo, G.; Magri, F.; Tamagno, G.; Ameri, P.; Camera, A.; Colnaghi, S.; Perucca, P.; Ravera, G.; Galimberti, C.A. Seizure frequency and sex steroids in women with partial epilepsy on antiepileptic therapy. Epilepsia 2009, 50, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, I.; Cakmak, H.; Matalliotakis, M.; Kappou, D.; Arici, A. High rate of allergies among women with endometriosis. J. Obstet. Gyncaecol. 2012, 32, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Sinali, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Rep. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Collier, C.N.; Harper, J.C.; Cafardi, J.A.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Münster, K.; Helm, P.; Schmidt, L. Secondary amenorrhea: Prevalence and medical contact—A cross-sectional study from a Danish county. Br. J. Obstet. Gynaecol. 1992, 99, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.W.; Missmer, S.A. The epidemiology of endometriosis. Ann. Acad. Sci. 2002, 955, 11–22. [Google Scholar] [CrossRef]
- Upadhyay, U.D.; Waddell, E.N.; Young, S.; Kerker, B.D.; Berger, M.; Matte, T.; Angell, S.Y. Prevalence, awareness, treatment, and control of high LDL cholesterol in New York City, 2004. Prev. Chronic Dis. 2010, 7, A61. [Google Scholar] [PubMed]
- Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Rep. Update 2012, 18, 146–170. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Hypertension among Adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. 2015. Available online: https://www.cdc.gov/nchs/products/databriefs/db133.htm (accessed on 5 July 2017).
- Centers for Disease Control and Prevention (CDC). Infertility FAQs. 2017. Available online: https://www.cdc.gov/reproductivehealth/infertility/ (accessed on 5 July 2017).
- Kotagasti, T. Prevalence of different menstrual irregularities in women with abnormal uterine bleeding (AUB)—An observational study. Int. J. Curr. Res. Rev. 2015, 7, 66. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Obesity and Overweight. 2016. Available online: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm (accessed on 5 July 2017).
- Musmar, S.; Afaneh, A.; Mo’alla, H. Epidemiology of polycystic ovary syndrome: A cross sectional study of university students at An-Najah national university—Palestine. Rep. Biol. Endocrinol. 2013, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Halbreich, U.; Borenstein, J.; Pearlstein, T.; Kahn, L.S. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 2003, 28, 1–23. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Silverberg, N.B. Epidemiolgoy and extracutaneous comorbidities of severe acne in adolescence: A U.S. population-based study. Br. J. Dermatol. 2014, 170, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Bernuit, D.; Gerlinger, C.; Schaefers, M.; Geppert, K. Prevalence, symptoms and management of uterine fibroids: An international internet-based survey of 21,746 women. BMC Womens Health 2012, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Warren-Ulanch, J.; Arslanian, S. Treatment of PCOS in adolescence. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE, ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Cutolo, M.; Sulli, A.; Capellino, S.; Villaggio, B.; Montagna, P.; Seriolo, B.; Montagna, P.; Seriolo, B.; Straub, R.H. Sex hormones influence on the immune system: Basic and clinical aspects in autoimmunity. Lupus 2004, 13, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Alexeef, S.E.; Yau, V.; Qian, Y.; Davignon, M.; Lynch, F.; Crawford, P.; Davis, R.; Croen, L.A. Medical conditions in the first years of life associated with future diagnosis of ASD children. J. Autism Dev. Disord. 2017, 47, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Al-Ayadhi, L.Y. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J. Neuroimmunol. 2013, 261, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Atladóttir, H.O.; Pedersen, M.G.; Thorsen, P.; Mortensen, P.B.; Deleuran, B.; Eaton, W.W.; Parner, E.T. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009, 124, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Ashwood, P.; Van de Water, J.; Hertz-Picciotto, I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J. Autism Dev. Disord. 2014, 44, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Shulman, D.I.; Muhar, I.; Jorgensen, E.V.; Diamond, F.B.; Bercu, B.B.; Root, A.W. Autoimmune hyperthyroidism in prepubertal children and adolescents: Comparison of clinical and biochemical features at diagnosis and responses to medical therapy. Thyroid 1997, 7, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Felgentreff, K.; Siepe, M.; Kotthoff, S.; von Kodolitsch, Y.; Schachtrup, K.; Notarangelo, L.D.; Walter, J.E.; Ehl, S. Severe eczema and hyper-IgE in Loeys-Dietz syndrome—Contribution to new findings of immune dysfunction in connective tissue disorders. Clin. Immunol. 2014, 150, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Lam, S.T.; Tong, T.M.; Li, S.Y.; Lun, K.S.; Chan, D.H.C.; Fok, S.F.S.; Or, J.S.F.; Smith, D.K.; Yang, W.; et al. Identification of novel FBN1 and TGFBR2 mutations in 65 probands with Mardan syndrome or Marfan-like phenotypes. Am. J. Med. Genet. A 2009, 149, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Matt, P.; Schoenhoff, F.; Habashi, J.; Holm, T.; Van Erp, C.; Loch, D.; Carlson, O.D.; Griswold, B.F.; Fu, Q.; De Backer, J.; et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation 2009, 120, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.M.; Habashi, J.P.; Doyle, J.J.; Bedja, D.; Chen, Y.; van Erp, C.; Lindsay, M.E.; Kim, D.; Schoenhoff, F.; Cohn, R.D.; et al. Noncanonical TGF-beta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011, 332, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, V.; Warburton, D. Fibrillin controls TGF-beta activation. Nat. Genet. 2003, 33, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Frischmeyer-Guerrerio, P.A.; Guerrerio, A.l.; Oswald, G.; Chichester, K.; Myers, L.; Halusha, M.K.; Oliva-Hemker, M.; Wood, R.A.; Dietz, H.C. TGF-beta receptor mutations impose a strong predisposition for human allergic disease. Sci. Transl. Med. 2013, 5, 195ra94. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Enstrom, A.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.L.; Croen, L.A.; Ozonoff, S.; Pessah, I.N.; Van de Water, J. Decreased transforming growth factor beta1 in autism: A potential link between immune dysregulation and impairment in clinical behavioral outcomes. J. Neuroimmunol. 2008, 204, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.I.; et al. Decreased serum levels of transforming growth factor-β1 in patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Morlino, S.; Dordoni, C.; Celletti, C.; Camerota, F.; Ritelli, M.; Morrone, A.; Venturini, M.; Grammatico, P. Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome hypermobility type) in 82 Italian patients. Am. J. Med. Genet. A 2012, 158, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Muldowney, P.T.K. Living Life to the Fullest with Ehlers-Danlos Syndrome: Guide to Living a Better Quality Life While Having EDS; Outskirts Press: Denver, CO, USA, 2015. [Google Scholar]
- Talwar, R.M.; Wong, B.S.; Svoboda, K.; Harper, R.P. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J. Oral. Maxillofac. Surg. 2006, 64, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Henneman, D.H. Effect of estrogen on in vivo and in vitro collagen biosynthesis and maturation in old and young female guinea pigs. Endocrinology 1968, 83, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kongsgaard, M.; Holm, L.; Skovgaard, D.; Magnusson, S.P.; Qvortrup, K.; Larsen, J.O.; Aagaard, P.; Dahl, M.; Serup, A.; et al. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J. Appl. Physiol. 2009, 106, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L. Estrogen, a double-edged sword: Modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug. Targets Inflamm. Allergy 2004, 3, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zierau, O.; Zenclussen, A.C.; Jensen, F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front. Immunol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Sheppard, D.C. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef] [PubMed]
- Hawse, J.R.; Subramaniam, M.; Ingle, J.N.; Oursler, M.J.; Rajamannan, N.M.; Spelberg, T.C. Estrogen-TGF-beta cross-talk in bone and other cell types: Role of TIEG, Runx2, and other transcription factors. J. Cell. Biochem. 2012, 103, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B. Never Bet Against Occam; Sisters Media, LLC: Bethesda, MD, USA, 2016. [Google Scholar]
- Seneviratne, S.L.; Maitland, A.; Afrin, L. Mast cell disorders in Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 226–236. [Google Scholar] [CrossRef] [PubMed]
- De Wandele, I.; Rombaut, L.; Leybaert, L.; Van de Borne, P.; De Backer, T.; Malfait, F.; De Paepe, A.; Calders, P. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin. Arthritis Rheum. 2014, 44, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J. Clin. Investig. 1997, 99, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V.; Noctor, S.C.; Kriegstein, A.R. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur. J. Neurosci. 2011, 24, 3475–3488. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akaishi, T.; Matsuki, N.; Ohno, Y.; Nakazawa, K. β-estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007, 1150, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.M.; Chez, M.; Streif, E.M.; Keeling, R.M.; Golumbek, P.T.; Kwon, J.M.; Riviello, J.J.; Robinson, R.G.; Neuman, R.J.; Deuel, R.M.K. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol. Psychiatry 2006, 59, 354–363. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Ricci, I.; Sacco, R.; Liu, X.; D’Agruma, L.; Muscarella, L.A.; Guarnieri, V.; Militerni, R.; Bravaccio, C.; Elia, M.; et al. Paraoxonase gene variants are associated with autism in North America, but not in Italy: Possible regional specificity in gene-environment interactions. Mol. Psychiatry 2005, 10, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Cederlöf, M.; Larsson, H.; Lichtenstein, P.; Almqvist, C.; Serlachius, E.; Ludvigsson, J.F. Nationwide population-based cohort study of psychiatric disorders in individuals with Ehlers-Danlos syndrome or hypermobility syndrome and their siblings. BMC Psychiatry 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- National Organization for Rare Disorders (NORD). Ehlers Danlos Syndrome. 2017. Available online: https://rarediseases.org/rare-diseases/ehlers-danlos-syndrome/#affected-populations (accessed on 18 June 2017).
- Lyons, J.J.; Sun, G.; Stone, K.D.; Nelson, C.; Wisch, L.; O’Brien, M.; Jones, N.; Lindsley, A.; Komarow, H.D.; Bai, Y.; et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J. Allergy Clin. Immunol. 2014, 133, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Moneta, D.; Richichi, C.; Aliprandi, M.; Burrows, S.J.; Ravizza, T.; Perego, C.; De Simoni, M.G. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 2002, 43, 30–35. [Google Scholar] [CrossRef] [PubMed]
| Generalized Joint Hypermobility-Related Diagnoses | Description |
|---|---|
| Hypermobile Ehlers-Danlos Syndrome (hEDS)—Formerly known as EDS, Hypermobile Type, or EDS Type III. |
|
| Classical Ehlers-Danlos Syndrome (cEDS)—Also known as EDS Type I. |
|
| Generalized Hypermobility Spectrum Disorder (G-HSD) - Formerly known as “non-benign” JHS. |
|
| * Joint Hypermobility Syndrome (JHS)—Divided into “benign” and “non-benign” forms. Diagnosis now in disuse as of 2017. |
|
| Hypermobility Spectrum Disorders (HSD) | Composed of:
|
| Asymptomatic Joint Hypermobility |
|
| Marfan Syndrome (MFS) |
|
| Loeys-Dietz Syndrome (LDS) |
|
| Immune Symptomology | ASD (N = 85) | ASD/EDS (N = 20) | General Prevalence |
|---|---|---|---|
| Allergies | 45% | 60% | 30% in adults [23] |
| Asthma | 33% | 60% | 8.4% in general population [24] |
| Autoimmunity | 13% | 45% * | 7.6–9.4% in general population [25] |
| Chronic Ear Infections | 40% | 65% | 83% with ≥1 incidents between 0–3 years of age [26] 11% with ≥1 incidents of all ages [27] |
| Chronic Rhinitis | 38% | 60% | 8% in adults [28] |
| Chronic Sinusitis | 46% | 60% | 8% in adults [28] |
| Severe Reaction to Medications | 35% | 65% | 10–15% of hospitalized patients [29] |
| Severe Reaction to Environmental Chemicals | 39% | 65% | 13–16% in adults [30] |
| Endocrine Symptomology | ASD (N = 85) | ASD/EDS (N = 20) | General Prevalence |
|---|---|---|---|
| Adult Acne | 21% | 35% | 35% in women ages 30–39 [36] |
| Amenorrhea | 39% | 45% | 4.6% in women ages 15–44 [37] |
| Diabetes/Insulin Resistance | 6% | 10% | 7.9% in adults [38] |
| Dysmenorrhea | 28% | 85% * | 2–29% in adult women [39] |
| Endometriosis | 5% | 30% * | 4% in women [40] |
| High LDL Cholesterol | 14% | 30% | 28% in adults [41] |
| Hirsutism | 19% | 30% | 10% in adult women [42] |
| Hypertension | 14% | 20% | 29.1% in adults [43] |
| Infertility | 8% | 15% | 6% [44] |
| Irregular Menstruation | 27% | 55% | 18.2% in adult women [45] |
| Overweight/Obesity | 36% | 45% | 70.7% aged 20+ years [46] |
| Polycystic Ovary Syndrome (PCOS) | 8% | 25% | 7.3% in adult women [47] |
| Premenstrual Dysphoric Disorder (PMDD) | 21% | 30% | 3–8% of premenopausal women [48] |
| Severe Teen Acne | 14% | 45% * | 12.1% in males and females aged 17 [49] |
| Uterine Fibroids | 9% | 5% | 4.5–9.8% in adult women aged 40–49 [50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, E.L.; Sharp, J.L.; Edelson, S.M.; Kelly, D.P.; Casanova, M.F. A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility. Behav. Sci. 2018, 8, 35. https://doi.org/10.3390/bs8030035
Casanova EL, Sharp JL, Edelson SM, Kelly DP, Casanova MF. A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility. Behavioral Sciences. 2018; 8(3):35. https://doi.org/10.3390/bs8030035
Chicago/Turabian StyleCasanova, Emily L., Julia L. Sharp, Stephen M. Edelson, Desmond P. Kelly, and Manuel F. Casanova. 2018. "A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility" Behavioral Sciences 8, no. 3: 35. https://doi.org/10.3390/bs8030035
APA StyleCasanova, E. L., Sharp, J. L., Edelson, S. M., Kelly, D. P., & Casanova, M. F. (2018). A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility. Behavioral Sciences, 8(3), 35. https://doi.org/10.3390/bs8030035







