Isolated Mammillary Bodies Damage—An Atypical Presentation of Wernicke Syndrome
Abstract
:1. Introduction
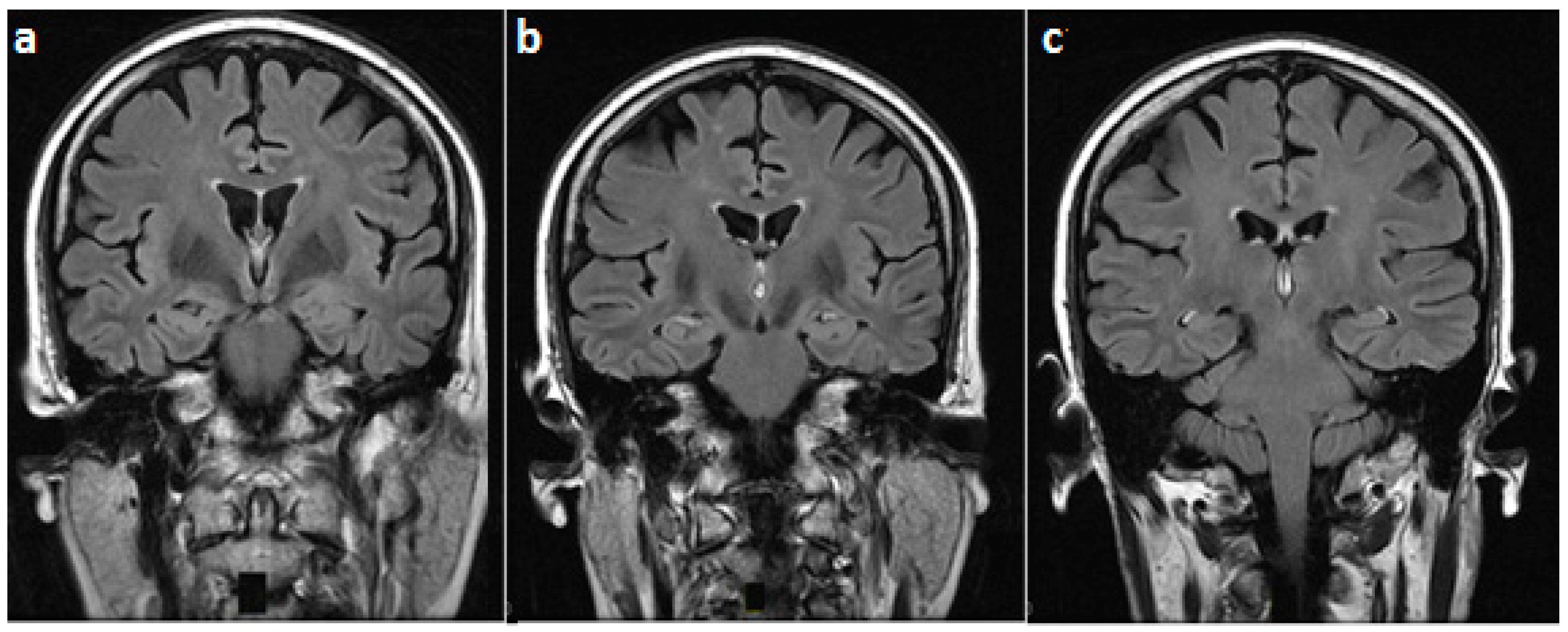
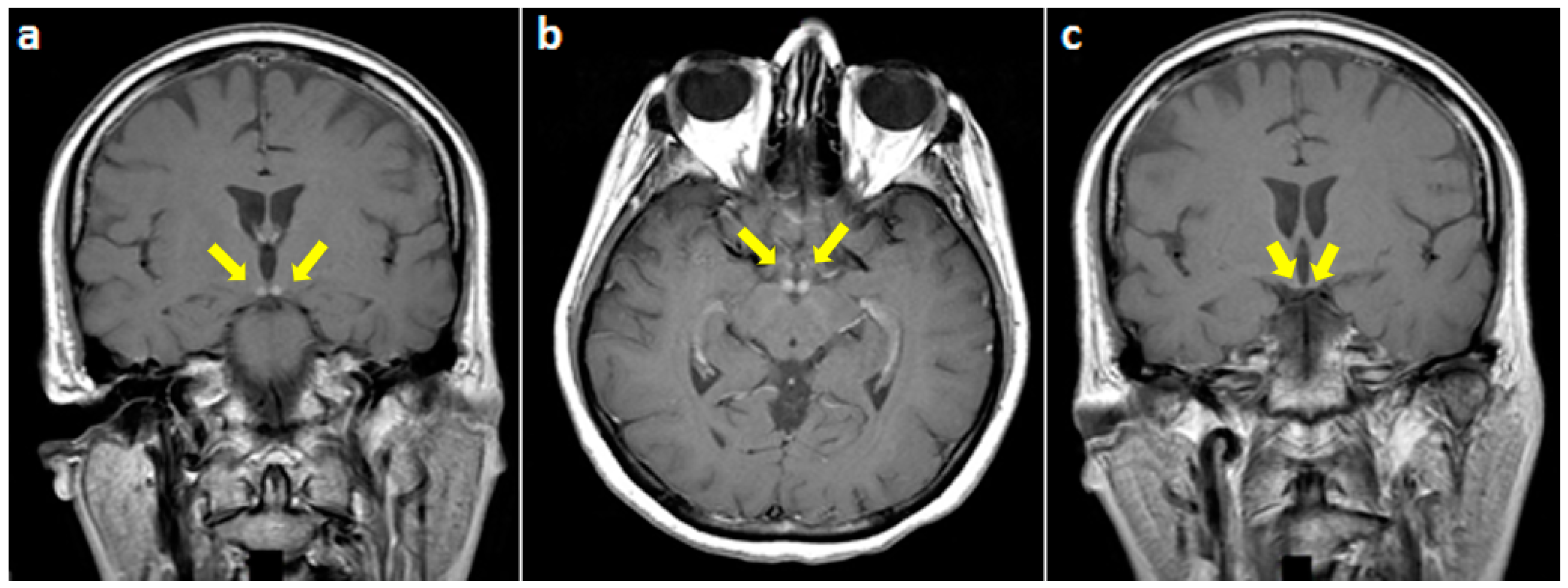
2. Case Presentation
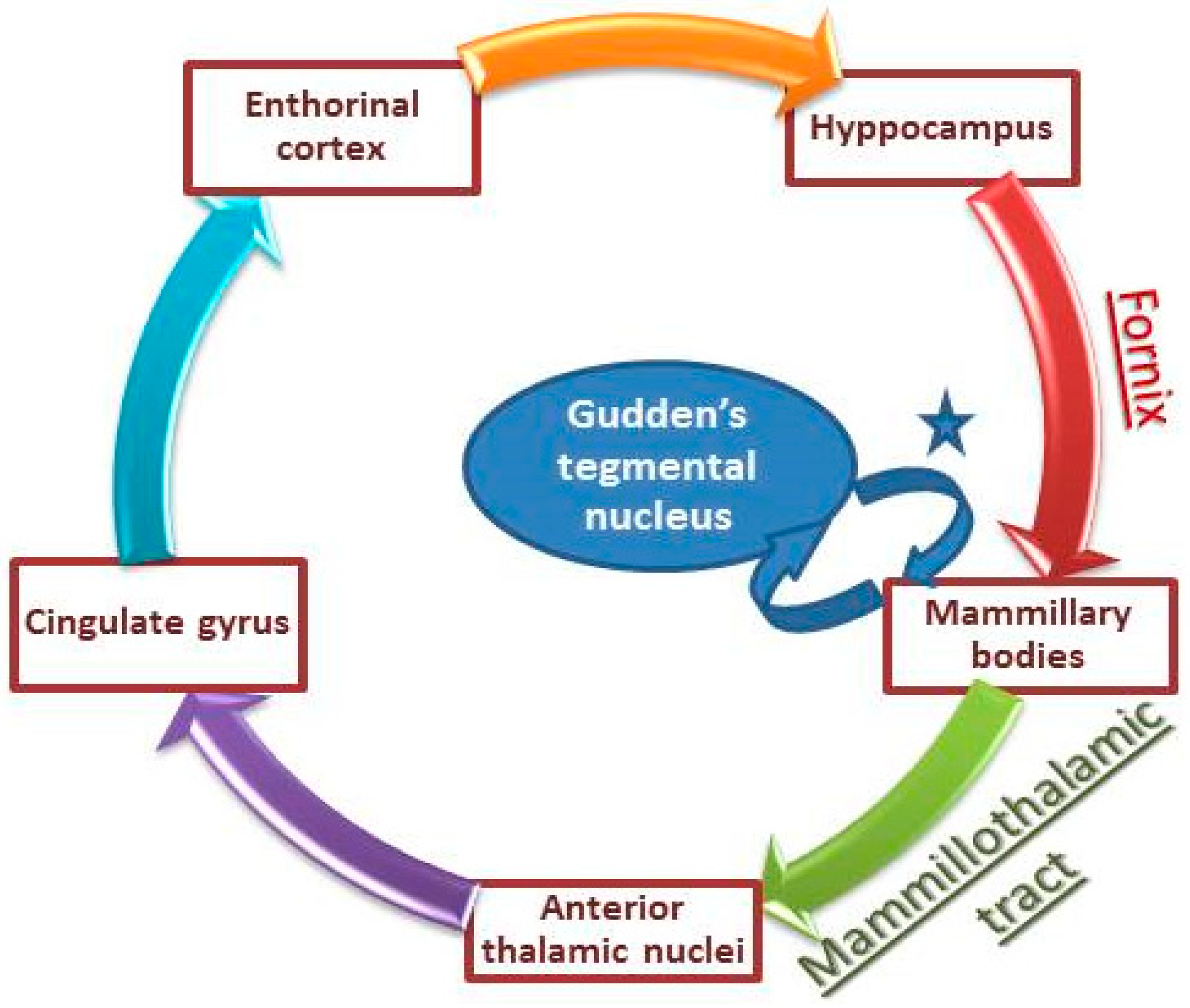
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Victor, M. The Wernicke-Korsakoff syndrome. In Handbook of Clinical Neurology; Vinken, P.J., Bruyn, G.W., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1976; Volume 28, Part II, pp. 243–270. [Google Scholar]
- Osiezagha, K.; Ali, S.; Freeman, C.; Barker, N.C.; Jabeen, S.; Maitra, S.; Olagbemiro, Y.; Richie, W.; Bailey, R.K. Thiamine deficiency and delirium. Innov. Clin. Neurosci. 2013, 10, 26–32. [Google Scholar] [PubMed]
- Shah, I.A.; Asimi, R.P.; Kawoos, Y.; Wani, M.; Saleem, T.; Baba, W.N. Nonalcoholic Wernicke’s Encephalopathy: A Retrospective Study from a Tertiary Care Center in Northern India. J. Neurosci. Rural Pract. 2017, 8, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Danet, L.; Barbeau, E.J.; Eustache, P.; Planton, M.; Raposo, N.; Sibon, I.; Albucher, J.F.; Bonneville, F.; Peran, P.; Pariente, J. Thalamic amnesia after infarct: The role of the mammillothalamic tract and mediodorsal nucleus. Neurology 2015, 85, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Male, S.; Zand, R. Isolated Mammillary Body Infarct Causing Global Amnesia: A Case Report. J. Stroke Cerebrovasc. Dis. 2017, 26, e50–e52. [Google Scholar] [CrossRef] [PubMed]
- Amuluru, K.; Filippi, C.G.; Lignelli, A. Acute amnesia due to isolated mammillary body infarct. J. Stroke Cerebrovasc. Dis. 2015, 24, e303–e305.6. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.; Gallucci, M.; Capellades, J.; Regnicolo, L.; Tumiati, B.; Giadás, T.C.; Bottari, W.; Mandrioli, J.; Bertolini, M. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. Am. J. Neuroradiol. 2007, 28, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Fei, G.Q.; Zhong, C.; Jin, L.; Wang, J.; Zhang, Y.; Zheng, X.; Zhang, Y.; Hong, Z. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am. J. Neuroradiol. 2008, 29, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bonucchi, J.; Hassan, I.; Policeni, B.; Kaboli, P. Thyrotoxicosis associated Wernicke’s encephalopathy. J. Gen. Intern. Med. 2008, 23, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Santos Andrade, C.; Tavares Lucato, L.; da Graça Morais Martin, M.; Joaquina Marques-Dias, M.; Antonio Pezzi Portela, L.; Scarabôtolo Gattás, G.; da Costa Leite, C. Non-alcoholic Wernicke’s encephalopathy: Broadening the clinicoradiological spectrum. Br. J. Radiol. 2010, 83, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.; Santa Cruz, D.; Bertolini, M.; Rovira, A.; Gallucci, M.; Carollo, C.; Pipitone, N. MR imaging findings in 56 patients with Wernicke encephalopathy: Nonalcoholics may differ from alcoholics. Am. J. Neuroradiol. 2009, 30, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.; Motti, L. Atypical Wernicke’s encephalopathy showing lesions in the cranial nerve nuclei and cerebellum. J. Neuroimaging 2008, 18, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Lee, H.K.; Lee, J.H.; Choi, C.G.; Suh, D.C. Wernicke’s encephalopathy: Atypical manifestation at MR imaging. AJNR Am. J. Neuroradiol. 2001, 22, 1480–1482. [Google Scholar] [PubMed]
- Weidauer, S.; Nichtweiss, M.; Lanfermann, H.; Zanella, F.E. Wernicke encephalopathy: MR findings and clinical presentation. Eur. Radiol. 2003, 13, 1001–1009. [Google Scholar] [PubMed]
- Manzo, G.; De Gennaro, A.; Cozzolino, A.; Serino, A.; Fenza, G.; Manto, A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: A review. Biomed. Res. Int. 2014, 2014, 503596. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, S.A.; Abboud, H.; Chalah, M.A.; Sabbagh, C.; Ayache, S.S. Isolated Mammillary Bodies Damage—An Atypical Presentation of Wernicke Syndrome. Behav. Sci. 2018, 8, 104. https://doi.org/10.3390/bs8110104
Abbas SA, Abboud H, Chalah MA, Sabbagh C, Ayache SS. Isolated Mammillary Bodies Damage—An Atypical Presentation of Wernicke Syndrome. Behavioral Sciences. 2018; 8(11):104. https://doi.org/10.3390/bs8110104
Chicago/Turabian StyleAbbas, Samar A., Halim Abboud, Moussa A. Chalah, Chadi Sabbagh, and Samar S. Ayache. 2018. "Isolated Mammillary Bodies Damage—An Atypical Presentation of Wernicke Syndrome" Behavioral Sciences 8, no. 11: 104. https://doi.org/10.3390/bs8110104
APA StyleAbbas, S. A., Abboud, H., Chalah, M. A., Sabbagh, C., & Ayache, S. S. (2018). Isolated Mammillary Bodies Damage—An Atypical Presentation of Wernicke Syndrome. Behavioral Sciences, 8(11), 104. https://doi.org/10.3390/bs8110104






