Moral Judgment: An Overlooked Deficient Domain in Multiple Sclerosis?
Abstract
1. Introduction
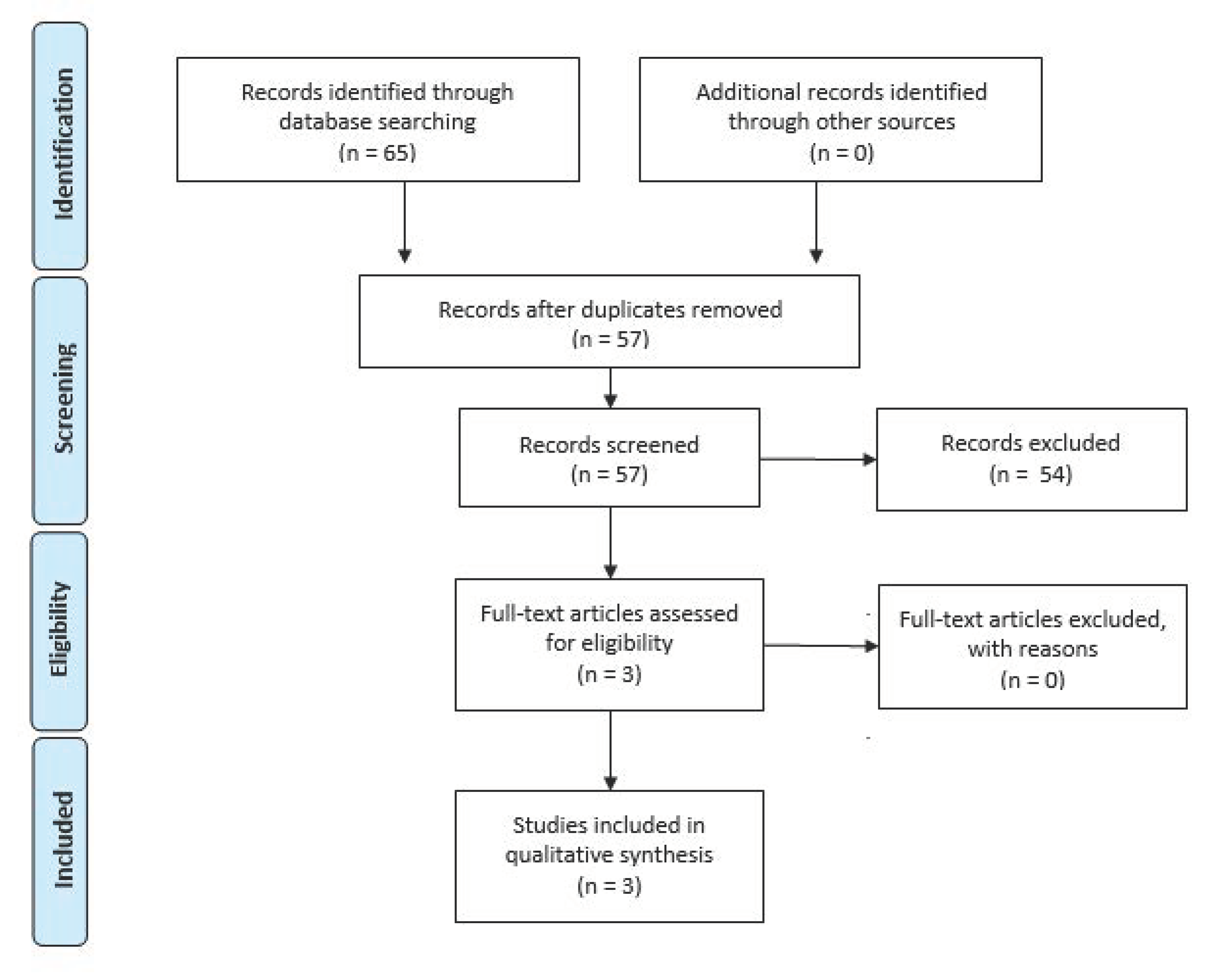
2. Study Selection
3. A Brief Overview of the Neurobiology of the Moral Brain
4. Exploration of Moral Judgment
5. Moral Cognition in Multiple Sclerosis Studies
6. Current Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Chalah, M.A.; Ayache, S.S. Is there a link between inflammation and fatigue in multiple sclerosis? J. Inflamm. Res. 2018, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Furlan, R.; Bernardi, G.; Martino, G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010, 17, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.M.; Stüve, O. Primary progressive multiple sclerosis—Why we are failing. Lancet 2016, 387, 1032–1034. [Google Scholar] [CrossRef]
- Chalah, M.A.; Ayache, S.S. Psychiatric event in multiple sclerosis: Could it be the tip of the iceberg? Rev. Bras. Psiquiatr. 2017, 39, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Créange, A.; Lefaucheur, J.P.; Ayache, S.S. Fatigue in Multiple Sclerosis: Neural Correlates and the Role of Non-Invasive Brain Stimulation. Front. Cell. Neurosci. 2015, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Ayache, S.S. Alexithymia in multiple sclerosis: A systematic review of literature. Neuropsychologia 2017, 104, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Leo, G.J.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Leo, G.J.; Ellington, L.; Nauertz, T.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991, 41, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; Cookfair, D.; Gavett, R.; Gunther, M.; Munschauer, F.; Garg, N.; Weinstock-Guttman, B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J. Int. Neuropsychol. Soc. 2006, 12, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Sanfilipo, M.P.; Benedict, R.H.; Weinstock-Guttman, B.; Bakshi, R. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 2006, 66, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Ayache, S.S. Deficits in Social Cognition: An Unveiled Signature of Multiple Sclerosis. J. Int. Neuropsychol. Soc. 2017, 23, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.C. Multiple Sclerosis: A Guide for Families; Demos Medical Publishing: New York, NY, USA, 2005. [Google Scholar]
- Moll, J.; Zahn, R.; de Oliveira-Souza, R.; Krueger, F.; Grafman, J. Opinion: The neural basis of human moral cognition. Nat. Rev. Neurosci. 2005, 6, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Group PRISMA. Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Rodrigues, P.; Gallardo-Pujol, D. How does morality work in the brain? A functional and structural perspective of moral behavior. Front. Integr. Neurosci. 2013, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Priori, A. Functional and clinical neuroanatomy of morality. Brain 2012, 135, 2006–2021. [Google Scholar] [CrossRef] [PubMed]
- Hutcherson, C.A.; Montaser-Kouhsari, L.; Woodward, J.; Rangel, A. Emotional and Utilitarian Appraisals of Moral Dilemmas Are Encoded in Separate Areas and Integrated in Ventromedial Prefrontal Cortex. J. Neurosci. 2015, 35, 12593–12605. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Ahn, M. A subjective utilitarian theory of moral judgment. J. Exp. Psychol. Gen. 2016, 145, 1359–1381. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Sommerville, R.B.; Nystrom, L.E.; Darley, J.M.; Cohen, J.D. An fMRI investigation of emotional engagement in moral judgment. Science 2001, 293, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Greene, J. Emotion and cognition in moral judgment: Evidence from neuroimaging. In Neurobiology of Human Values; Springer: Berlin, Heidelberg, Germany, 2005; Volume 66, pp. 57–66. [Google Scholar] [CrossRef]
- Greene, J.D.; Morelli, S.A.; Lowenberg, K.; Nystrom, L.E.; Cohen, J.D. Cognitive load selectively interferes with utilitarian moral judgment. Cognition 2008, 107, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Haidt, J. The emotional dog and its rational tail: A social intuitionist approach to moral judgment. Psychol. Rev. 2001, 108, 814–834. [Google Scholar] [CrossRef] [PubMed]
- De Neys, W.; Glumicic, T. Conflict monitoring in dual process theories of reasoning. Cognition 2008, 106, 1248–1299. [Google Scholar] [CrossRef] [PubMed]
- Cushman, F. Action, outcome, and value: A dual-system framework for morality. Pers. Soc. Psychol. Rev. 2013, 17, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Nystrom, L.E.; Engell, A.D.; Darley, J.M.; Cohen, J.D. The neural bases of cognitive conflict and control in moral judgment. Neuron 2004, 44, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, E.; Muccioli, M.; Làdavas, E.; di Pellegrino, G. Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2007, 2, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Young, L.; Adolphs, R.; Tranel, D.; Cushman, F.; Hauser, M.; Damasio, A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 2007, 446, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, J.J.; Feldmanhall, O.; Mende-Siedlecki, P. The neuroscience of moral cognition: From dual processes to dynamic systems. Curr. Opin. Psychol. 2015, 6, 167–172. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Marois, R. The roots of modern justice: Cognitive and neural foundations of social norms and their enforcement. Nat. Neurosci. 2012, 15, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Eres, R.; Louis, W.R.; Molenberghs, P. Common and distinct neural networks involved in fMRI studies investigating morality: An ALE meta-analysis. Soc. Neurosci. 2018, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.; Dungan, J.A.; Chakroff, A.; Young, L.L. Neural substrates for moral judgments of psychological versus physical harm. Soc. Cogn. Affect. Neurosci. 2018, 13, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Perera, M.; Reyes del Paso, G.A.; Pérez-García, M.; Verdejo-García, A. Heart rate correlates of utilitarian moral decision-making in alcoholism. Drug Alcohol Depend. 2013, 133, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kappes, A.; Rho, Y.; Van Bavel, J.J. At the heart of morality lies neuro-visceral integration: Lower cardiac vagal tone predicts utilitarian moral judgment. Soc. Cogn. Affect. Neurosci. 2016, 11, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Làdavas, E.; Mattioli, F.; Di Pellegrino, G. A psychophysiological investigation of moral judgment after ventromedial prefrontal damage. J. Cogn. Neurosci. 2010, 22, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.D.; McDonald, M.M.; Mott, M.L.; Asher, B. Virtual morality: Emotion and action in a simulated three-dimensional “trolley problem”. Emotion 2012, 12, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Cushman, F.; Gray, K.; Gaffey, A.; Mendes, W.B. Simulating murder: The aversion to harmful action. Emotion 2012, 12, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Fang, P.; Yang, X.; Ru, W.; Wang, B.; Gao, X.; Liu, J. The CAG polymorphism in androgen receptor (AR) gene impacts the moral permissibility of harmful behavior in females. Psychoneuroendocrinology 2017, 80, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, R.M.; Chaponis, J.; Siburian, R.; Gallagher, P.; Ransohoff, K.; Wikler, D.; Perlis, R.H.; Greene, J.D. Variation in the oxytocin receptor gene (OXTR) is associated with differences in moral judgment. Soc. Cogn. Affect. Neurosci. 2016, 11, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.T.; Montag, C.; Markett, S.; Felten, A.; Voigt, G.; Reuter, M. Ignorance is no excuse: Moral judgments are influenced by a genetic variation on the oxytocin receptor gene. Brain Cogn. 2012, 78, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Scheele, D.; Striepens, N.; Kendrick, K.M.; Schwering, C.; Noelle, J.; Wille, A.; Schläpfer, T.E.; Maier, W.; Hurlemann, R. Opposing effects of oxytocin on moral judgment in males and females. Hum. Brain Mapp. 2014, 35, 6067–6076. [Google Scholar] [CrossRef] [PubMed]
- Crockett, M.J.; Clark, L.; Hauser, M.D.; Robbins, T.W. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc. Natl. Acad. Sci. USA 2010, 107, 17433–17438. [Google Scholar] [CrossRef] [PubMed]
- Crockett, M.J.; Siegel, J.Z.; Kurth-Nelson, Z.; Ousdal, O.T.; Story, G.; Frieband, C.; Grosse-Rueskamp, J.M.; Dayan, P.; Dolan, R.J. Dissociable Effects of Serotonin and Dopamine on the Valuation of Harm in Moral Decision Making. Curr. Biol. 2015, 25, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.Z.; Crockett, M.J. How serotonin shapes moral judgment and behavior. Ann. N. Y. Acad. Sci. 2013, 1299, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Terbeck, S.; Kahane, G.; McTavish, S.; Savulescu, J.; Levy, N.; Hewstone, M.; Cowen, P.J. Beta adrenergic blockade reduces utilitarian judgement. Biol. Psychol. 2013, 92, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Arutyunova, K.R.; Alexandrov, Y.I.; Hauser, M.D. Sociocultural Influences on Moral Judgments: East-West, Male-Female, and Young-Old. Front. Psychol. 2016, 7, 1334. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.F.; Dookeeram, K.; Basdeo, V.; Francis, E.; Doman, M.; Mamed, D.; Maloo, S.; Degannes, J.; Dobo, L.; Ditshotlo, P.; et al. Stress alters personal moral decision making. Psychoneuroendocrinology 2012, 37, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.J. The trolley problem. Yale Law J. 1985, 94, 1395–1415. [Google Scholar] [CrossRef]
- Brink, D.O. Utilitarian morality and the personal point of view. J. Philos. 1986, 83, 417–438. [Google Scholar] [CrossRef]
- Foot, P. The problem of abortion and the doctrine of double effect. In Virtues and Vices; Blackwell: Oxford, UK, 1978. [Google Scholar]
- Thomson, J.J. Rights, Restitution, and Risk: Essays in Moral Theory; Harvard: Cambridge, MA, USA, 1986. [Google Scholar]
- Suter, R.S.; Hertwig, R. Time and moral judgment. Cognition 2011, 119, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Paxton, J.M.; Ungar, L.; Greene, J.D. Reflection and reasoning in moral judgment. Cogn. Sci. 2012, 36, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Michalska, K.J.; Kinzler, K.D. The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cereb. Cortex 2011, 22, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Batalla, I.; Contreras-Rodríguez, O.; Harrison, B.J.; Pera, V.; Hernández-Ribas, R.; Real, E.; Bosa, L.; Soriano-Mas, C.; Deus, J.; et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Soc. Cogn. Affect. Neurosci. 2011, 7, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lotto, L.; Manfrinati, A.; Sarlo, M. A new set of moral dilemmas: Norms for moral acceptability, decision times, and emotional salience. J. Behav. Decis. Mak. 2014, 27, 57–65. [Google Scholar] [CrossRef]
- Moll, J.; Eslinger, P.J.; Oliveira-Souza, R. Frontopolar and anterior temporal cortex activation in a moral judgment task: Preliminary functional MRI results in normal subjects. Arq. Neuropsiquiatr. 2001, 59, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Harenski, C.L.; Hamaan, S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage 2006, 30, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.A.; First, M.B.; Blacker, D. Handbook of Psychiatric Measures; American Psychiatric Publishing: Washington, DC, USA, 2008. [Google Scholar]
- Gleichgerrcht, E.; Tomashitis, B.; Sinay, V. The relationship between alexithymia, empathy and moral judgment in patients with multiple sclerosis. Eur. J. Neurol. 2015, 22, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Patil, I.; Young, L.; Sinay, V.; Gleichgerrcht, E. Elevated moral condemnation of third-party violations in multiple sclerosis patients. Soc. Neurosci. 2017, 12, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Realmuto, S.; Dodich, A.; Meli, R.; Canessa, N.; Ragonese, P.; Salemi, G.; Cerami, C. Moral Cognition and Multiple Sclerosis: A Neuropsychological Study. Arch. Clin. Neuropsychol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Koven, N.S. Specificity of meta-emotion effects on moral decision-making. Emotion 2011, 11, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Knobe, J. Theory of mind and moral cognition: Exploring the connections. Trends Cogn. Sci. 2005, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.E.; Grafman, J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu. Rev. Neurosci. 2010, 33, 299–324. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Dungan, J. Where in the brain is morality? Everywhere and maybe nowhere. Soc. Neurosci. 2011, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valdesolo, P.; DeSteno, D. Manipulations of emotional context shape moral judgment. Psychol. Sci 2006, 17, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; Anderson, E.; Shapira, J.S. An investigation of moral judgement in frontotemporal dementia. Cogn. Behav. Neurol. 2005, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kahane, G.; Shackel, N. Do abnormal responses show utilitarian bias? Nature 2008, 452, E5. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Torralva, T.; Rattazzi, A.; Marenco, V.; Roca, M.; Manes, F. Selective impairment of cognitive empathy for moral judgment in adults with high functioning autism. Soc. Cogn. Affect. Neurosci. 2013, 8, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Torralva, T.; Roca, M.; Pose, M.; Manes, F. The role of social cognition in moral judgment in frontotemporal dementia. Soc. Neurosci. 2011, 6, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Young, L. Low levels of empathic concern predict utilitarian moral judgment. PLoS ONE 2013, 8, e60418. [Google Scholar] [CrossRef] [PubMed]
- Charil, A.; Zijdenbos, A.P.; Taylor, J.; Boelman, C.; Worsley, K.J.; Evans, A.C.; Dagher, A. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: Application to 452 patient data sets. NeuroImage 2003, 19, 532–544. [Google Scholar] [CrossRef]
- Pagani, E.; Rocca, M.A.; Gallo, A.; Rovaris, M.; Martinelli, V.; Comi, G.; Filippi, M. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. Am. J. Neuroradiol. 2005, 26, 341–346. [Google Scholar] [PubMed]
- Sethi, V.; Yousry, T.A.; Muhlert, N.; Tozer, D.; Ron, M.; Golay, X.; Wheeler-Kingshott, C.; Miller, D.H.; Chard, D.T. Lobar distribution of cortical grey matter lesions in multiple sclerosis clinical subgroups. J. Neurol. Neurosurg. Psychiatry 2013, 84, e2. [Google Scholar] [CrossRef]
- Krause, M.; Wendt, J.; Dressel, A.; Berneiser, J.; Kessler, C.; Hamm, A.O.; Lotze, M. Prefrontal function associated with impaired emotion recognition in patients with multiple sclerosis. Behav. Brain Res. 2009, 205, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; Alves, C.; d’Almeida, O.C.; Afonso, A.; Félix-Morais, R.; Pereira, J.; Macário, C.; Sousa, L.; Castelo-Branco, M.; Santana, I.; et al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology 2017, 89, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Kauv, P.; Lefaucheur, J.P.; Hodel, J.; Créange, A.; Ayache, S.S. Theory of mind in multiple sclerosis: A neuropsychological and MRI study. Neurosci. Lett. 2017, 658, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mike, A.; Strammer, E.; Aradi, M.; Orsi, G.; Perlaki, G.; Hajnal, A.; Sandor, J.; Banati, M.; Illes, E.; Zaitsev, A.; et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: A structural MRI study. PLoS ONE 2013, 8, e82422. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; d’Almeida, O.C.; Afonso, A.; Freitas, S.; Macário, C.; Sousa, L.; Castelo-Branco, M.; Santana, I.; Cunha, L. Impairment of social cognition in multiple sclerosis: Amygdala atrophy is the main predictor. Mult. Scler. 2017, 23, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Cerasa, A.; Liguori, M.; Gioia, M.; Valentino, P.; Nisticò, R.; Nistico, R.; Quattrone, A.; Fera, F. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain 2009, 132, 3380–3391. [Google Scholar] [CrossRef] [PubMed]
- Jehna, M.; Neuper, C.; Ischebeck, A.; Loitfelder, M.; Ropele, S.; Langkammer, C.; Langkammer, C.; Ebner, F.; Fuchs, S.; Schmidt, R.; et al. The functional correlates of face perception and recognition of emotional facial expressions as evidenced by fMRI. Brain Res. 2011, 1393, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.H.; Saldias, A.; McCarrey, A.; Henry, J.D.; Scott, C.; Summers, F.; Whyte, M. Attentional lapses, emotional regulation and quality of life in multiple sclerosis. Br. J. Clin. Psychol. 2009, 48, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.H.; Henry, J.D.; Nouzova, E.; Cooper, C.; Radlak, B.; Summers, F. Difficulties with emotion regulation in multiple sclerosis: Links to executive function, mood, and quality of life. J. Clin. Exp. Neuropsychol. 2014, 36, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Tourbah, A.; Chaunu, M.-P.; Bakchine, S.; Montreuil, M. Social Cognition Abilities in Patients With Different Multiple Sclerosis Subtypes. J. Int. Neuropsychol. Soc. 2017, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Schirda, B.; Nicholas, J.A.; Prakash, R.S. Examining trait mindfulness, emotion dysregulation, and quality of life in multiple sclerosis. Health Psychol. 2015, 34, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Bechara, A.; Tranel, D.; Damasio, H.; Hauser, M.; Damasio, A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron 2010, 65, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Chahraoui, K.; Duchene, C.; Rollot, F.; Bonin, B.; Moreau, T. Longitudinal study of alexithymia and multiple sclerosis. Brain Behav. 2014, 4, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.J. Psychological aspects of multiple sclerosis. Clin. Neurol. Neurosurg. 2008, 110, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Luminet, O.; Rimé, B.; Bagby, R.M.; Taylor, G. A multimodal investigation of emotional responding in alexithymia. Cogn. Emot. 2004, 18, 741–766. [Google Scholar] [CrossRef]
- Demers, L.A.; Koven, N.S. The Relation of Alexithymic Traits to Affective Theory of Mind. Am. J. Psychol. 2015, 128, 31–42. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.P.; Stokes, M.; McDonald, E. Changes in quality of life and coping among people with multiple sclerosis over a 2 year period. Psychol. Health Med. 2009, 14, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, R.H.S.R.; Seaman, S.R.; Masterman, T.; Hensiek, A.E.; Sawcer, S.J.; Vukusic, S.; Achiti, I.; Confavreux, C.; Coustans, M.; Le Page, E.; et al. Multiple Sclerosis Severity Score: Using disability and disease duration to rate disease severity. Neurology 2005, 64, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Bagby, R.M.; Parker, J.D.; Taylor, G.J. The twenty-item Toronto Alexithymia Scale—I. Item selection and crossvalidation of the factor structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–126. [Google Scholar] [CrossRef]
- Goretti, B.; Niccolai, C.; Hakiki, B.; Sturchio, A.; Falautano, M.; Minacapelli, E.; Martinelli, V.; Incerti, C.; Nocentini, U.; Murgia, M.; et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): Normative values with gender, age and education corrections in the Italian population. BMC Neurol. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, S.; MacPherson, S.; Phillips, L.; Sacco, L.; Spinnler, H. How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol. Sci. 2003, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Una versione abbreviata del test di Stroop: Dati normativi nella popolazione italiana. Nuova Rivista di Neurologia 2002, 12, 111–115. [Google Scholar]
- Dodich, A.; Cerami, C.; Canessa, N.; Crespi, C.; Iannaccone, S.; Marcone, A.; Realmuto, S.; Lettieri, G.; Perani, D.; Cappa, S.F. A novel task assessing intention and emotion attribution: Italian standardization and normative data of the Story-based Empathy Task. Neurol. Sci. 2015, 36, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Dodich, A.; Cerami, C.; Canessa, N.; Crespi, C.; Marcone, A.; Arpone, M.; Realmuto, S.; Cappa, S.F. Emotion recognition from facial expressions: A normative study of the Ekman 60-Faces Test in the Italian population. Neurol. Sci. 2014, 35, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, M.; Auquier, P.; Fernandez, O.; Flachenecker, P.; Stecchi, S.; Constantinescu, C.; Idiman, E.; Boyko, A.; Beiske, A.G.; Vollmer, T.; et al. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Mult. Scler. 2008, 14, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Coyle, P.K.; Doscher, C.; Miller, A.; Cross, A.H.; Jandorf, L.; Halper, J.; Johnson, B.; Morgante, L.; Grimson, R. Fatigue therapy in multiple sclerosis: Results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 1995, 45, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Langdon, D.W. Cognition in multiple sclerosis. Curr. Opin. Neurol. 2011, 2011 24, 244–249. [Google Scholar] [CrossRef]
- Comi, G. Effects of disease modifying treatments on cognitive dysfunction in multiple sclerosis. Neurol. Sci. 2010, 31 (Suppl. 2), S261–S264. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Gender differences in brain functional connectivity density. Hum Brain Mapp. 2012, 33, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Lennon, R.; Eisenberg, N. Gender and age differences in empathy and sympathy. In Empathy and its Development; Eisenberg, N., Strayer, J., Eds.; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Graham, J.; Meindl, P.; Beall, E.; Johnson, K.M.; Zhang, L. Cultural differences in moral judgment and behavior, across and within societies. Curr. Opin. Psychol. 2016, 8, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.; Antley, A.; Davison, A.; Swapp, D.; Guger, C.; Barker, C.; Pistrang, N.; Sanchez-Vives, M.V. A virtual reprise of the Stanley Milgram obedience experiments. PLoS ONE 2006, 1, e39. [Google Scholar] [CrossRef]
- Yoder, K.J.; Decety, J. Spatiotemporal neural dynamics of moral judgment: A high-density ERP study. Neuropsychologia 2014, 60, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Montoya, E.R.; Terburg, D.; Bos, P.A.; Will, G.J.; Buskens, V.; Raub, W.; van Honk, J. Testosterone administration modulates moral judgments depending on second-to-fourth digit ratio. Psychoneuroendocrinology 2013, 38, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Decety, J.; Huang, P.C.; Chen, C.Y.; Cheng, Y. Testosterone administration in females modulates moral judgment and patterns of brain activation and functional connectivity. Hum. Brain Mapp. 2016, 37, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.M.; Leonard, A.M.; Weaver, K.; Dalton, J.A.; Mehta, M.A.; Kumari, V.; Williams, S.C.; Ettinger, U. A dose of ruthlessness: Interpersonal moral judgment is hardened by the anti-anxiety drug lorazepam. J. Exp. Psychol. Gen. 2013, 142, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Chalah, M.A. Transcranial direct current stimulation: A glimmer of hope for multiple sclerosis fatigue? J. Clin. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Chalah, M.A. Fatigue in multiple sclerosis—Insights into evaluation and management. Neurophysiol. Clin. 2017, 47, 139–171. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Chalah, M.A.; Mhalla, A.; Palm, U.; Ayache, S.S.; Mylius, V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol. Clin. 2017, 47, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, D.; Sack, A.T.; Roebroeck, A.; Russ, B.E.; Pascual-Leone, A. TMS affects moral judgment, showing the role of DLPFC and TPJ in cognitive and emotional processing. Front. Neurosci. 2014, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Knoch, D.; Pascual-Leone, A.; Meyer, K.; Treyer, V.; Fehr, E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 2006, 314, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lu, X.; Huang, D. tDCS Over DLPFC Leads to Less Utilitarian Response in Moral-Personal Judgment. Front. Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, M.; Heimrath, K.; Heinze, H.J.; Zaehle, T. Transcranial direct current stimulation of the left dorsolateral prefrontal cortex shifts preference of moral judgments. PLoS ONE 2015, 10, e0127061. [Google Scholar] [CrossRef] [PubMed]
- Choy, O.; Raine, A.; Hamilton, R.H. Stimulation of the Prefrontal Cortex Reduces Intentions to Commit Aggression: A Randomized, Double-Blind, Placebo-Controlled, Stratified, Parallel-Group Trial. J. Neurosci. 2018, 38, 6505–6512. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Vergari, M.; Pasqualetti, P.; Marceglia, S.; Mameli, F.; Ferrucci, R.; Mrakic-Sposta, S.; Zago, S.; Sartori, G.; Pravettoni, G.; et al. Brain switches utilitarian behavior: Does gender make the difference? PLoS ONE 2010, 5, e8865. [Google Scholar] [CrossRef] [PubMed]
- Sellaro, R.; Güroǧlu, B.; Nitsche, M.A.; van den Wildenberg, W.P.; Massaro, V.; Durieux, J.; Hommel, B.; Colzato, L.S. Increasing the role of belief information in moral judgments by stimulating the right temporoparietal junction. Neuropsychologia 2015, 77, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Chen, S.; Huang, D.; Zheng, H.; Jia, Y.; Luo, J. Modulation of Neural Activity in the Temporoparietal Junction with Transcranial Direct Current Stimulation Changes the Role of Beliefs in Moral Judgment. Front. Hum. Neurosci. 2015, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Leloup, L.; Miletich, D.D.; Andriet, G.; Vandermeeren, Y.; Samson, D. Cathodal Transcranial Direct Current Stimulation on the Right Temporo-Parietal Junction Modulates the Use of Mitigating Circumstances during Moral Judgments. Front. Hum. Neurosci. 2016, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Oizumi, R. Electric stimulation of the right temporo-parietal junction induces a task-specific effect in deceptive behaviors. Neurosci. Res. 2018, 128, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Camprodon, J.A.; Hauser, M.; Pascual-Leone, A.; Saxe, R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc. Natl. Acad. Sci. USA 2010, 107, 6753–6758. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Booth, J.; Lawrence, M.; Byrne, S.; Mair, F.; Mercer, S. Mindfulness based interventions in multiple sclerosis—A systematic review. BMC Neurol. 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Buckingham, R.; Schwartz, R.S.; Hodgkinson, S.; Beran, R.G.; Cordato, D.J. The Effect of Biofeedback as a Psychological Intervention in Multiple Sclerosis: A Randomized Controlled Study. Int. J. MS Care 2015, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Ayache, S.S. Cognitive behavioral therapies and multiple sclerosis fatigue: A review of literature. J. Clin. Neurosci. 2018, 52, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Presidents’ Council on Bioethics. Beyond Therapy: Biotechnology and the Pursuit of Happiness; Government Printing Office: Washington, DC, USA, 2003. [Google Scholar]
| Study | Gleichgerrcht et al., 2015 [61] | Patil et al., 2017 [62] | Realmuto et al., 2018 [63] |
|---|---|---|---|
| Patients’ demographic and clinical data | 38 RR MS patients | 38 consecutive RR MS patients | 45 RR MS patients |
| 87.30% females | 86.80% females | 68.89% females | |
| Mean age: 42.3 ± 11.3 years | Mean age: 42.3 ± 11.3 years | Mean age: 34.22 ± 7.65 years | |
| All receiving immunomodulatory drugs | All receiving immunomodulatory drugs | Immunomodulatory treatment: details N/A | |
| Mean education level: 15.4 ± 2.8 years | Mean education level: 15.4 ± 2.8 years | Mean education level: 13.49 ± 2.46 years | |
| Mean EDSS score [94]: 1.66 ± 1.6 | Mean EDSS score [94]: 1.66 ± 1.6 | Mean EDSS score [94]: 2.06 ± 1.46 | |
| Mean disease duration: 1.6 ± 8.7 years | Mean disease duration: 10.60 ± 8.7 years | Mean disease duration: 9.72 ± 6.22 years | |
| Mean number of relapses: 3.4 ± 1.92 | Mean number of relapses: 3.4 ± 1.92 | Mean number of relapses: details N/A | |
| Mean MSSS score [95]: 2.35 ± 2.4 | Mean MSSS score [95]: 2.35 ± 2.4 | Mean MSSS score [95]: 2.85 ± 2.59 | |
| Healthy control group | 38 age-, gender-, and education-matched healthy controls | 38 age-, gender-, and education-matched healthy controls | 45 age-, gender-, and education-matched healthy controls |
| Assessment tool for moral judgement | Moral dilemma task: a series of eight vignettes from Greene et al.’s battery [21,27] presenting situations measuring moral permissibility, emotional reactivity, and moral relativity | Moral intent task: 24 unique stories adapted from Young et al. 2010 [74] | Moral dilemmas including instrumental and incidental conditions [57] |
| Other measures | Alexithymia: TAS [96] Empathy: IRI [97] | Alexithymia: TAS [96] Empathy: IRI [97] | Non-social cognition evaluation: BICAMS [98], Cognitive Estimation task [99], and Stroop test [100] Social cognition evaluation: Ekman-60 Faces test, RMET, and Story-based Empathy task [101,102]. Quality of life: MuSIQoL [103] Fatigue: FSS [104] Depression and anxiety: HADS [105] |
| Group comparison | Patients exhibited reduced moral permissibility, increased moral relativity, increased emotional reactivity, low empathy, and high alexithymia rating compared to healthy controls | Compared to healthy controls, patients had comparable levels of moral judgement but exhibited reduced moral permissibility, increased moral relativity, increased emotional reactivity, low empathy and high alexithymia ratings | No significant group differences in the levels of moral judgment (rate of yes/no response in dilemmas resolution; attribution of emotional valence to moral actions) but had lower moral permissibility and emotional arousal (for the instrumental dilemmas 13.33% of patients had poor moral judgement performance) 77.6% of patients had non-social cognitive deficits (i.e., executive domains) 24% of patients had social cognitive deficits |
| Correlation analysis | Significant positive correlation between moral reactivity and MSSS scores Significant positive correlation between moral permissibility, empathy, and alexithymia scores | No significant correlation between moral judgement and empathy or alexithymia measures Tendency toward negative correlation between appropriateness of intentional harm and alexithymia (did not survive statistical corrections) Significant negative correlation between appropriateness of intentional harm and empathy measures, perspective taking, and empathic concern (did not survive statistical corrections) | Significant correlations between the attribution of emotional valence and mentalizing (did not survive statistical corrections) No other correlations between moral judgment and clinical, basic cognition, or social cognition measures |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayache, S.S.; Chalah, M.A. Moral Judgment: An Overlooked Deficient Domain in Multiple Sclerosis? Behav. Sci. 2018, 8, 105. https://doi.org/10.3390/bs8110105
Ayache SS, Chalah MA. Moral Judgment: An Overlooked Deficient Domain in Multiple Sclerosis? Behavioral Sciences. 2018; 8(11):105. https://doi.org/10.3390/bs8110105
Chicago/Turabian StyleAyache, Samar S., and Moussa A. Chalah. 2018. "Moral Judgment: An Overlooked Deficient Domain in Multiple Sclerosis?" Behavioral Sciences 8, no. 11: 105. https://doi.org/10.3390/bs8110105
APA StyleAyache, S. S., & Chalah, M. A. (2018). Moral Judgment: An Overlooked Deficient Domain in Multiple Sclerosis? Behavioral Sciences, 8(11), 105. https://doi.org/10.3390/bs8110105






