Sophisticated Fowl: The Complex Behaviour and Cognitive Skills of Chickens and Red Junglefowl
Abstract
1. Introduction
2. The Sensory Abilities of Fowl
3. The Social Life of Fowl
4. The Personality of Fowl
5. Affective State in Fowl
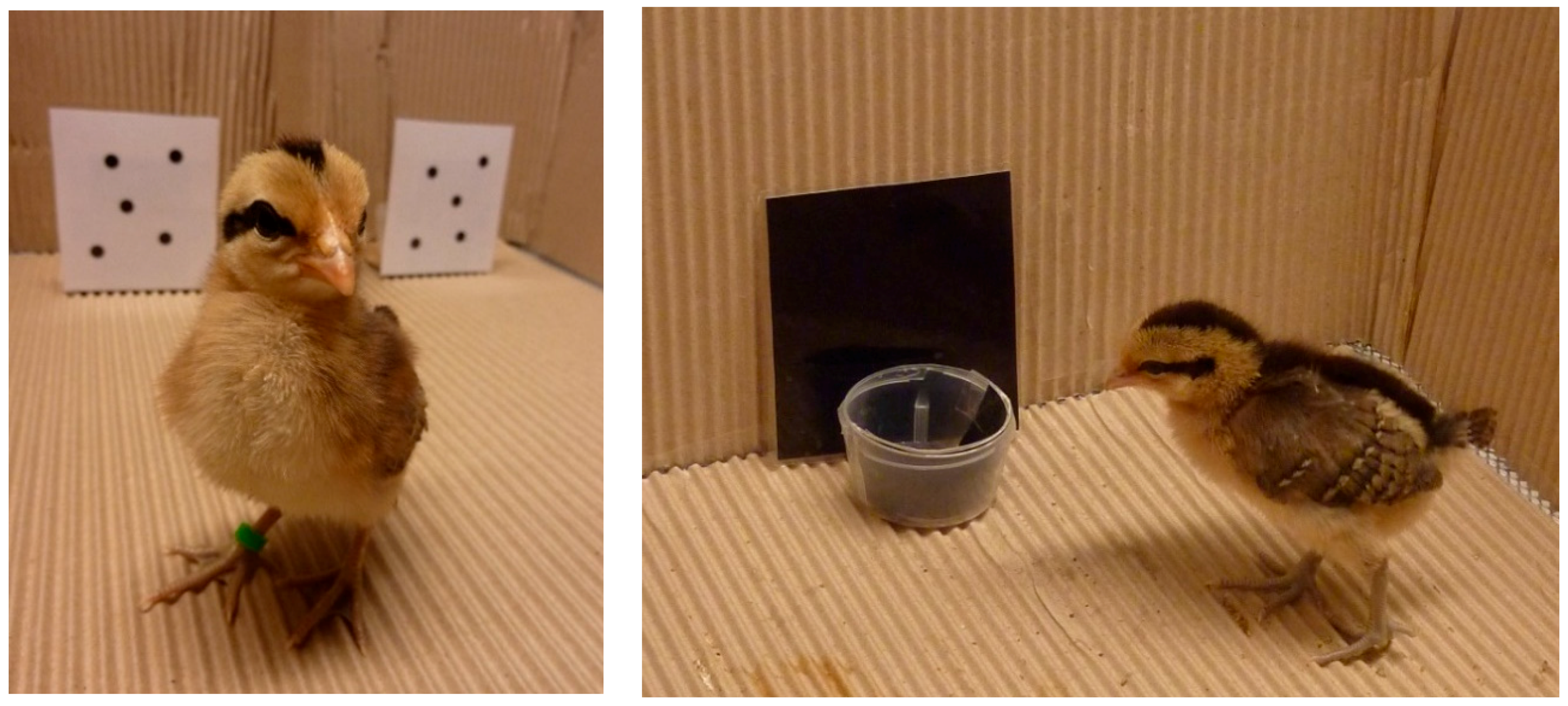
6. Cognitive Abilities in Fowl
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sawai, H.; Kim, H.L.; Kuno, K.; Suzuki, S.; Gotoh, H.; Takada, M.; Takahata, N.; Satta, Y.; Akishinonomiya, F. The origin and genetic variation of domestic chickens with special reference to Junglefowls Gallus g. gallus and G. varius. PLoS ONE 2010, 5, e10639. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Zhou, B. Did chickens go north? New evidence for domestication. J. Archaeol. Sci. 1988, 15, 515–533. [Google Scholar] [CrossRef]
- Lui, Y.; Wu, G.; Yao, Y.; Miao, Y.; Luikart, G.; Baig, M.; Beja-Pereira, A.; Zhao, L.; Palanichamy, M.G.; Zhang, Y. Multiple maternal origins of chickens: Out of the Asian jungles. Mol. Phylogenet. Evol. 2006, 38, 12–19. [Google Scholar] [CrossRef]
- Fumihito, A.; Mityake, T.; Sumi, S.I.; Takada, M.; Ohno, S.; Kondo, N. One subspecies of the red jungle fowl (Gallus gallus gallus) suffice as the matriarchic ancestor of all domestic chickens. Proc. Natl. Acad. Sci. USA 1994, 91, 12505–12509. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Larson, G.; Gunnarsson, U.; Bed’hom, B.; Tixier-Boichard, M.; Strömstedt, L.; Wright, D.; Jungerius, A.; Vereijken, A.; Randi, E.; et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008, 4, e1000010. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T. The Wood-gush legacy: A sociobiology perspective to fertility and welfare in chickens. Appl. Anim. Behav. Sci. 2016, 181, 12–18. [Google Scholar] [CrossRef]
- Nicol, C.J. The Behavioural Biology of Chickens, 2nd ed.; CABI: Oxford, UK, 2015; ISBN 9781780642499. [Google Scholar]
- Collias, N.E.; Collias, E.C. Social organization of a red junglefowl, Gallus gallus, population related to evolution theory. Anim. Behav. 1996, 51, 1337–1354. [Google Scholar] [CrossRef]
- Marino, L. Thinking chickens: A review of cognition, emotion, and behavior in the domestic chicken. Anim. Cogn. 2017, 20, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Shettleworth, S.J. Cognition, Evolution, and Behavior, 2nd ed.; Oxford University Press: Oxford, UK, 2010; ISBN 9780195319842. [Google Scholar]
- Stern, C.D. The chick: A great model system becomes even greater. Dev. Cell. 2005, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Hubbard, S.J.; Tickle, C.; Wilson, S.A. The chicken as a model for large-scale analysis of vertebrate gene function. Nat. Rev. Genet. 2003, 4, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Schjelderup-Ebbe, T. Beiträge zur Sozialpsychologie des haus huhns. Z. Psychol. Leipzig 1922, 88, 225–252. [Google Scholar]
- Cloutier, S.; Newberry, R.C. Recent social experience, body weight and initial patterns of attack predict the social status attained by unfamiliar hens in a new group. Behaviour 2000, 137, 705–726. [Google Scholar] [CrossRef]
- Hazel, S.J.; O’Dwyer, L.; Ryan, T. “Chickens are a lot smarter than I originally thought”: Changes in student attitudes to chickens following a chicken training class. Animals 2015, 5, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Dall, S.R.X.; Houston, A.I.; McNamara, J.M. The behavioural ecology of personality: Consistent individual differences from an adaptive perspective. Ecol. Lett. 2004, 7, 734–739. [Google Scholar] [CrossRef]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.; Munro, U.; Rogers, L.J.; Wiltschko, R.; Wiltschko, W. Chickens orient using the magnetic compass. Curr. Biol. 2005, 15, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Early experiential effects on laterality: Research on chicks has relevance to other species. Laterality 1997, 2, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain. Res. 2005, 162, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Andrew, R.J. Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav. Process. 1994, 33, 41–58. [Google Scholar] [CrossRef]
- Stamp Dawkins, M. How do hens view other hens? The use of lateral and binocular visual fields in social recognition. Behaviour 1995, 132, 591–606. [Google Scholar] [CrossRef]
- Stamp Dawkins, M.; Woodington, A. Distance and the presentation of visual stimuli to birds. Anim. Behav. 1997, 54, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Rubene, D.; Håstad, O.; Tauson, R.; Wall, H.; Ödeen, A. Presence of UV wavelengths improves the temporal resolution of the avian visual system. J. Exp. Biol. 2010, 213, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Lisney, T.J.; Rubene, D.; Rozsa, J.; Løvlie, H.; Håstad, O.; Ödeen, A. Behavioural assessment of flicker fusion frequency in chicken Gallus gallus domesticus. Vis. Res. 2011, 51, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.H.; White, R.P.; Wathes, C.M. Light intensity and social communication between hens. Br. Poult. Sci. 2009, 50, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.M. Avian olfaction: Then and now. J. Ornithol. 2007, 148, S191–S194. [Google Scholar] [CrossRef]
- Jones, R.B.; Roper, T.J. Olfaction in the domestic fowl: A critical review. Physiol. Behav. 1997, 62, 1009–1018. [Google Scholar] [CrossRef]
- Zidar, J.; Løvlie, H. Scent of the enemy: Behavioural responses to predator faecal odour in the fowl. Anim. Behav. 2012, 84, 547–554. [Google Scholar] [CrossRef]
- Jones, R.B.; Gentle, M.J. Olfaction and behavioral modification in domestic chicks (Gallus domesticus). Physiol. Behav. 1985, 34, 917–924. [Google Scholar] [CrossRef]
- Jones, T.A.; Jones, S.M.; Paggett, K.C. Emergence of Hearing in the Chicken Embryo. J. Neurophysiol. 2006, 96, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Romanini, C.E.; Exadaktylos, V.; Bahr, C.; Berckmans, D.; Bergoug, H.; Eterradossi, N.; Roulston, N.; Verhelst, R.; McGonnell, I.M.; et al. Embryonic development and the physiological factors that coordinate hatching in domestic chickens. Poult. Sci. 2013, 92, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Warchol, M.E.; Dallos, P. Neural response to very low-frequency sound in the avian cochlear nucleus. J. Comp. Physiol. A 1989, 166, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.S.; Salvi, R.J. Psychoacoustics of normal adult chickens: Thresholds and temporal integration. J. Acoust. Soc. Am. 1993, 94, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sluckin, W.; Salzen, E.A. Imprinting and perceptual learning. Q. J. Exp. Psychol. 1961, 13, 65–77. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Parker, M.O.; Davey, E.L.; Grist, H.; Owen, R.C.; Szladovits, B.; Demmers, T.G.M.; Wathes, C.M.; Abeyesinghe, S.M. Effect of low light and high noise on behavioural activity, physiological indicators of stress and production in laying hens. Br. Poult. Sci. 2011, 52, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Jendral, M.J.; Robinson, F.E. Beak trimming in chickens: Historical, economical, physiological and welfare implications, and alternatives for preventing feather pecking and cannibalistic activity. Avian Poult. Biol. Rev. 2004, 15, 9–23. [Google Scholar] [CrossRef]
- Freire, R.; Eastwood, M.A.; Joyce, M. Minor beak trimming in chickens leads to loss of mechanoreception and magnetoreception. J. Anim. Sci. 2014, 89, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Walcott, C.; Green, R.P. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science 1974, 184, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, W.; Freire, R.; Munro, U.; Ritz, T.; Rogers, L.; Thalau, P.; Wiltschko, R. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 2007, 210, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Løvlie, H.; Pizzari, T. Sex in the morning or in the evening? females adjust daily mating patterns to the intensity of sexual harassment. Am. Nat. 2007, 170, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- McBride, G.; Parer, I.P.; Foenander, F. The Social Organization and Behavior of the Feral Domestic Fowl. Anim. Behav. Monogr. 1969, 2, 125–181. [Google Scholar] [CrossRef]
- Guhl, A.M. Social behavior of the domestic fowl. Trans. Kans. Acad. Sci. 1968, 71, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Banks, E.M. Social organisation in red jungle fowl hens (Gallus gallus subsp.). Ecology 1956, 37, 239–248. [Google Scholar] [CrossRef]
- Ligon, J.D.; Thornhill, R.; Zuk, M.; Johnson, K. Male-male competition, ornamentation and the role of testosterone in sexual selection in red jungle fowl. Anim. Behav. 1990, 40, 367–373. [Google Scholar] [CrossRef]
- Favati, A.; Leimar, O.; Løvlie, H. Personality predicts social dominance in male domestic fowl. PLoS ONE 2014, 9, e103535. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T.; Froman, D.P.; Birkhead, T.R. Pre- and post-insemination episodes of sexual selection in the fowl. Heredity 2002, 89, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Collias, N.; Collias, E.; Jennrich, R.I. Dominant red junglefowl (Gallus gallus) hens in an unconfined flock rear the most young over their lifetime. Auk 1994, 111, 863–872. [Google Scholar] [CrossRef]
- Cornwallis, C.K.; Birkhead, T.R. Plasticity in reproductive phenotypes reveals status-specific correlations between behavioral, morphological, and physiological sexual traits. Evolution 2008, 63, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Favati, A.; Leimar, O.; Radesäter, T.; Løvlie, H. Social status and personality: Stability in social state can promote consistency of behavioural responses. Proc. Biol. Sci. 2014, 281, 20132531. [Google Scholar] [CrossRef] [PubMed]
- Guhl, A.M.; Allee, W.C. Some measurable effects of social organization in flocks of hens. Physiol. Biochem. Zool. 1977, 17, 320–347. [Google Scholar] [CrossRef]
- Wood-Gush, D.G.M. The Behaviour of the Domestic Fowl, 1st ed.; Heinemann: London, UK, 1971; ISBN 0435629204. [Google Scholar]
- McDonald, G.C.; Spurgin, L.G.; Fairfield, E.A.; Richardson, D.S.; Pizzari, T. Pre- and postcopulatory sexual selection favor aggressive, young males in polyandrous groups of red junglefowl. Evolution 2017, 71, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.W.; Doyle, P.; Bagshaw, E.; Richardson, D.S.; Wigby, S.; Pizzari, T. The contrasting role of male relatedness in different mechanisms of sexual selection in red junglefowl. Evolution 2016, 71, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T. Food, vigilance, and sperm: The role of male direct benefits in the evolution of female preference in a polygamous bird. Behav. Ecol. 2003, 14, 593–601. [Google Scholar] [CrossRef]
- Smith, L.C.; Taubert, J.; Weldon, K.; Evans, C.S. Individual recognition based on communication behaviour of male fowl. Behav. Process. 2016, 125, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Candland, D.K. Discriminability of facial regions used by the domestic chicken in maintaining the social dominance order. J. Comp. Physiol. Psychol. 1969, 69, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Johnson, J. The chicken challenge—What contemporary studies of fowl mean for science and ethics. Between Species 2012, 15, 75–101. [Google Scholar] [CrossRef]
- Løvlie, H.; Zidar, J.; Berneheim, C. A cry for help: Female distress calling during copulation is context-dependent. Anim. Behav. 2014, 92, 151–157. [Google Scholar] [CrossRef]
- Pizzari, T. Indirect partner choice through manipulation of male behaviour by female fowl, Gallus g. domesticus. Proc. Biol. Sci. 2001, 268, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T.; Cornwallis, C.K.; Løvlie, H.; Jakobsson, S.; Birkhead, T.R. Sophisticated sperm allocation in male fowl. Nature 2003, 426, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Etches, R.J. Reproduction in Poultry, 1st ed.; CABI Publishing: Wallingford, UK, 1996; ISBN 9780851987385. [Google Scholar]
- Ligon, J.D.; Zwartjes, P.W. Female red junglefowl choose to mate with multiple males. Anim. Behav. 1995, 49, 127–135. [Google Scholar] [CrossRef]
- Gillingham, M.A.F.; Richardson, D.S.; Løvlie, H.; Moynihan, A.; Worley, K.; Pizzari, T. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proc. Biol. Sci. 2009, 276, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T.; Løvlie, H.; Cornwallis, C.K. Sex-specific, counter-acting responses to inbreeding in a bird. Proc. Biol. Sci. 2004, 271, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T.; Birkhead, T.R. Female feral fowl eject sperm of subdominant males. Nature 2000, 405, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Løvlie, H.; Gillingham, M.A.F.; Worley, K.; Pizzari, T.; Richardson, D.S. Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proc. Biol. Sci. 2013, 280, 20131296. [Google Scholar] [CrossRef] [PubMed]
- Collias, N.E. The vocal repertoire of the red junglefowl: A spectrographic classification and the code of communication. Condor 1987, 89, 510–524. [Google Scholar] [CrossRef]
- Kruijt, J.P. Ontogeny of Social Behaviour in Burmese Red Junglefowl (Gallus gallus spadiceus) Bonnaterre. Behav. Suppl. 1964, 12, 1–201. [Google Scholar]
- Abeyesinghe, S.M.; McLeman, M.A.; Owen, R.C.; McMahon, C.E.; Wathes, C.M. Investigating social discrimination of group members by laying hens. Behav. Process. 2009, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.K.; Jensen, P.; Elgland, M.; Laur, K.; Fyrner, T.; Konradsson, P.; Laska, M. Red junglefowl have individual body odors. J. Exp. Biol. 2010, 213, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.; Johnson, J. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychol. Rev. 1991, 98, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Guhl, A.M.; Ortman, L.L. Visual patterns in the recognition of individuals among chickens. Condor 1953, 55, 287–298. [Google Scholar] [CrossRef]
- Salva, O.R.; Farroni, T.; Regolin, L.; Vallortigara, G.; Johnson, M.H. The evolution of social orienting: Evidence from chicks (Gallus gallus) and human newborns. PLoS ONE 2011, 6, e18802. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, J.J.; Johnson, M.H.; Horn, G.; Bateson, P. Long-lasting effects of IMHV lesions on social preferences in domestic fowl. Behav. Nurosci. 1989, 103, 438–441. [Google Scholar] [CrossRef]
- Rosher, C.; Favati, A.; Dean, R.; Løvlie, H. Relatedness and age reduce aggressive male interactions over mating. Behav. Ecol. 2017, 28, 760–766. [Google Scholar] [CrossRef]
- Leone, E.H.; Estévez, I. Economic and welfare benefits of environmental enrichment for broiler breeders. Poult. Sci. 2008, 87, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Estévez, I.; Mallapur, A.; Miller, C.; Christman, M.C. Short- and long-term movement patterns in complex confined environments in broiler chickens: The effects of distribution of cover panels and food resources. Poult. Sci. 2010, 89, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.C.D.O.; Miranda, K.O.D.S.; Filho, L.C.D.; Pereira, G.D.V.; Piedade, S.M.D.S.P.; Berno, P.R. Presence of roosters in an alternative egg production system aiming at animal welfare. Rev. Bras. Zootec. 2017, 46, 175–184. [Google Scholar] [CrossRef]
- Favati, A.; Zidar, J.; Thorpe, H.; Jensen, P.; Løvlie, H. The ontogeny of personality traits in the red junglefowl, Gallus gallus. Behav. Ecol. 2016, 27, 484–493. [Google Scholar] [CrossRef]
- Zidar, J.; Sorato, E.; Malmqvist, A.M.; Jansson, E.; Rosher, C.; Jensen, P.; Favati, A.; Løvlie, H. Early experience affects adult personality in the red junglefowl: A role for cognitive stimulation? Behav. Process. 2017, 134, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zidar, J.; Balogh, A.; Favati, A.; Jensen, P.; Leimar, O.; Løvlie, H. A comparison of animal personality and coping styles in red junglefowl. Anim. Behav. 2017, 130, 209–220. [Google Scholar] [CrossRef]
- Sorato, E.; Zidar, J.; Løvlie, H. Heritability of personality in the red junglefowl. Unpublished work.
- Johnsen, T.S.; Zuk, M. Parasites, morphology, and blood characters in male red jungle fowl during development. Condor 1998, 100, 749e752. [Google Scholar] [CrossRef]
- Favati, A.; Leimar, O.; Uden, E.; Løvlie, H. Personality remains: No effect of 3-week social status experience on personality in male fowl. Behav. Ecol. 2018. [Google Scholar] [CrossRef]
- Favati, A.; Løvlie, H.; Leimar, O. Individual aggression, but not winner-loser effects, predicts social rank in male domestic fowl. Behav. Ecol. 2017, 28, 874–882. [Google Scholar] [CrossRef]
- Fraisse, F.; Cockrem, J.F. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006, 47, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Weinberg, J.; Levine, S. Psychobiology of coping in animals: The effects of predictability. In Coping and Health, 1st ed.; Levine, S., Ursin, H., Eds.; Plenum Press: New York, NY, USA, 1980; Volume 12, pp. 39–59. ISBN 978-1-4684-1042-6. [Google Scholar]
- D’Eath, R.B.; Keeling, L.J. Social discrimination and aggression by laying hens in large groups: From peck orders to social tolerance. Appl. Anim. Behav. Sci. 2003, 84, 197–212. [Google Scholar] [CrossRef]
- Väisänen, J.; Lindqvist, C.; Jensen, P. Co-segregation of behaviour and production related traits in an F3 intercross between red junglefowl and White Leghorn laying hens. Livest. Prod. Sci. 2005, 94, 149–158. [Google Scholar] [CrossRef]
- De Haas, E.N.; Lee, C.; Rodenburg, T.B. Learning and judgment can be affected by predisposed fearfulness in laying hens. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B. Fear and adaptability in poultry: Insights, implications and imperatives. World’s Poult. Sci. J. 1996, 52, 131–174. [Google Scholar] [CrossRef]
- Fraser, D. Applying science to animal welfare standards. In Proceedings of the Global Conference on Animal Welfare: An OIE initiative, Paris, France, 23–25 February 2004; Office International des Epizooties: Paris, France, 2004. [Google Scholar]
- Brantsæter, M.; Tahamtani, F.M.; Nordgreen, J.; Sandberg, E.; Hansen, T.B.; Rodenburg, T.B.; Oppermann Moe, R.; Janczak, A.M. Access to litter during rearing and environmental enrichment during production reduce fearfulness in adult laying hens. Appl. Anim. Behav. Sci. 2017, 189, 49–56. [Google Scholar] [CrossRef]
- Zidar, J.; Campderrich, I.; Janson, E.; Wichman, A.; Winberg, S.; Keeling, L.; Løvlie, H. Environmental complexity buffers against stress-induced negative judgement bias in female chickens. Unpublished work.
- Oppermann Moe, R.; Nordgreen, J.; Janczak, A.M.; Spruijt, B.M.; Zanella, A.J.; Bakkend, M. Trace classical conditioning as an approach to the study of reward-related behaviour in laying hens: A methodological study. Appl. Anim. Behav. Sci. 2009, 121, 171–178. [Google Scholar] [CrossRef]
- Harding, E.J.; Paul, E.S.; Mendl, M. Cognitive bias and affective state. Nature 2004, 427, 312. [Google Scholar] [CrossRef] [PubMed]
- Salmeto, A.L.; Hymel, K.A.; Carpenter, E.C.; Brilot, B.O.; Bateson, M.; Sufka, K.J. Cognitive bias in the chick anxiety–depression model. Brain Res. 2011, 1373, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Deakin, A.; Browne, W.J.; Hodge, J.J.L.; Paul, E.S.; Mendl, M. A Screen-Peck Task for Investigating Cognitive Bias in Laying Hens. PLoS ONE 2016, 11, e0158222. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.S.; Solomon, R.L. An opponent-process theory of motivation: 111. Some affective dynamics of imprinting. Learn. Motiv. 1974, 5, 149–164. [Google Scholar] [CrossRef]
- Berns, P.V.; Bell, L.M. Tonic immobility in chicks during presentations and withdrawals of an imprinting stimulus. Anim. Learn. Behav. 1979, 7, 383–389. [Google Scholar] [CrossRef]
- Nakamori, T.; Maekawa, F.; Sato, K.; Tanaka, K.; Ohki-Hamazaki, H. Neural basis of imprinting behavior in chicks. Dev. Growth Differ. 2013, 55, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Regolin, L.; Vallortigara, G. Perception of partly occluded objects by young chicks. Percept. Psychophys. 1995, 57, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Regolin, L.; Rigoni, M.; Zanforlin, M. Delayed search for a concealed imprinted object in the domestic chick. Anim. Cogn. 1998, 1, 17–24. [Google Scholar] [CrossRef]
- Rose, S.P. God’s organism? The chick as a model system for memory studies. Learn. Mem. 2000, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nicol, C.J.; Pope, S.J. Effects of social learning on the acquisition of discriminatory pecking in hens. Bull. Psychon. Soc. 1992, 30, 293–296. [Google Scholar] [CrossRef]
- Nicol, C.J.; Pope, S.J. Social learning in small flocks of laying hens. Anim. Behav. 1994, 47, 1289–1296. [Google Scholar] [CrossRef]
- Nicol, C.J.; Pope, S.J. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Anim. Behav. 1999, 57, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Eklund, B.; Jensen, P. Domestication effects on behavioural synchronization and individual distances in chickens. Behav. Process. 2011, 86, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, S.; Newberry, R.C.; Honda, K.; Alldredge, R. Cannibalistic behaviour spread by social learning. Anim. Behav. 2002, 63, 1153–1162. [Google Scholar] [CrossRef]
- MacLean, E.L.; Merritt, D.J.; Brannon, E.M. Social complexity predicts transitive reasoning in prosimian primates. Anim. Behav. 2008, 76, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hogue, M.E.; Beaugrand, J.P.; Laguë, P.C. Coherent use of information by hens observing their former dominant defeating or being defeated by a stranger. Behav. Process. 1996, 38, 241–252. [Google Scholar] [CrossRef]
- Daisley, J.N.; Vallortigara, G.; Regolin, L. Logic in an assymetric (social) brain: Transitive inference in the young domestic chick. Soc. Neurosci. 2010, 5, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Karakashian, S.J.; Gyger, M.; Marler, P. Audience effects on alarm calling chickens (Gallus gallus). J. Comp. Psychol. 1988, 102, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Palleroni, A.; Hauser, M.; Marler, P. Do responses of galliform birds vary adaptively with predator size? Anim. Cogn. 2005, 8, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Taylor, A.; Evans, C. Tactical multimodal signalling in birds: Facultative variation in signal modality reveals sensitivity to social costs. Anim. Behav. 2011, 82, 521–527. [Google Scholar] [CrossRef]
- Vogeley, K.; Bussfeld, P.; Newen, A.; Herrmann, S.; Happe, F.; Falkai, P.; Maier, W.; Shah, N.J.; Fink, G.R.; Zilles, K. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage 2001, 14, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Nicol, C.J.; Pope, S.J. The maternal feeding display of domestic hens is sensitive to perceived chick error. Anim. Behav. 1996, 52, 767–774. [Google Scholar] [CrossRef]
- Edgar, J.L.; Paul, E.S.; Nicol, C.J. Protective mother hens: Cognitive influence on the avian maternal response. Anim. Behav. 2013, 86, 223–229. [Google Scholar] [CrossRef]
- Edgar, J.L.; Lowe, J.C.; Paul, E.S.; Nicol, C.J. Avian maternal response to chick distress. Proc. Biol. Sci. 2011, 278, 3129–3134. [Google Scholar] [CrossRef] [PubMed]
- Marler, P.; Dufty, A.; Pickert, R. Vocal communication in the domestic chicken: Does a sender communicate information about the quality of a food referent to a receiver? Anim. Behav. 1986, 34, 188–193. [Google Scholar] [CrossRef]
- Vannelli, R. Advanced Cognition. In Evolutionary Theory and Human Nature, 1st ed.; Springer: Boston, MA, USA, 2001; ISBN 978-0-7923-7473-2. [Google Scholar]
- Kokolakis, A.; Smith, C.L.; Evans, C.S. Aerial alarm calling by male fowl (Gallus gallus) reveals subtle new mechanisms of risk management. Anim. Behav. 2010, 79, 1373–1380. [Google Scholar] [CrossRef]
- Wilson, D.R.; Evans, S.C. Mating success increases alarm-calling effort in male fowl, Gallus gallus. Anim. Behav. 2008, 76, 2029–2035. [Google Scholar] [CrossRef]
- Gyger, M.; Marler, P. Food calling in the domestic fowl Gallus gallus: The role of external referents and deception. Anim. Behav. 1988, 36, 358–365. [Google Scholar] [CrossRef]
- Abeyesinghe, S.M.; Nicol, C.J.; Hartnell, S.J.; Wathes, C.M. Can domestic fowl, Gallus gallus domesticus, show self-control? Anim. Behav. 2005, 70, 1–11. [Google Scholar] [CrossRef]
- Tangney, J.P.; Baumeister, R.F.; Boone, A.L. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J. Pers. 2004, 72, 271–324. [Google Scholar] [CrossRef] [PubMed]
- Mischel, W.; Shoda, Y.; Rodriguez, M.I. Delay of gratification in children. Science 1989, 26, 933–938. [Google Scholar] [CrossRef]
- Regolin, L.; Rugani, R.; Pagni, P.; Vallortigara, G. Delayed Search for Social and Nonsocial Goals by Young Domestic Chicks, Gallus gallus domesticus. Anim. Behav. 2005, 70, 855–864. [Google Scholar] [CrossRef]
- Regolin, L.; Rose, S.P.R. Long-term memory for a spatial task in young chicks. Anim. Behav. 1999, 57, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Forkman, B. Domestic hens have declarative representations. Anim. Cogn. 2000, 3, 135–137. [Google Scholar] [CrossRef]
- Rugani, R.; Regolin, L.; Vallortigara, G. Discrimination of small numerosities in young chicks. J. Exp. Psychol. Anim. B 2008, 34, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Rugani, R.; Regolin, L.; Vallortigara, G. Imprinted numbers: Newborn chicks’ sensitivity to number vs. continuous extent of objects they have been reared with. Dev. Sci. 2010, 13, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Regolin, L.; Chiandetti, C.; Rugani, R. Rudiments of mind: Insights through the chick model on number and space cognition in animals. Comp. Cogn. Behav. Rev. 2010, 5, 78–99. [Google Scholar] [CrossRef]
- Regolin, L.; Rugani, R.; Stancher, G.; Vallortigara, G. Spontaneous discrimination of possible and impossible objects by newly hatched chicks. Biol. Lett. 2011, 7, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, L.; Polli, C. Representation of two geometric features of the environment in the domestic chick (Gallus gallus). Anim. Cogn. 2004, 7, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.E.; Haskell, M.; Appleby, M.C.; Waran, N.K. Perception of time duration by domestic hens. Appl. Anim. Behav. Sci. 2002, 71, 319–333. [Google Scholar] [CrossRef]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through animals’ left and right perceptual worlds. Brain. Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. Biol. Sci. 2004, 271, S420–S422. [Google Scholar] [CrossRef] [PubMed]
- Zidar, J.; Balogh, A.; Favati, A.; Jensen, P.; Leimar, O.; Sorato, E.; Løvlie, H. The relationship between learning speed and personality is age- and task-dependent in the red junglefowl. Unpublished work.
- Panigrahy, K.K.; Behera, K.; Mandal, A.K.; Sethy, K.; Panda, S. Effect of age and sex in determining cognitive ability in Vanaraja chickens. Br. Poult. Sci. 2017, 58, 605–609. [Google Scholar] [CrossRef] [PubMed]
- De Haas, E.N.; Lee, C.; Hernandez, C.E.; Naguib, M.; Rodenburg, T.B. Individual differences in personality in laying hens are related to learning a colour cue association. Behav. Process. 2017, 134, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Naguib, M.; Trillmich, F.; Schraderb, L. The effects of short term enrichment on learning in chickens from a laying strain (Gallus gallus domesticus). Appl. Anim. Behav. Sci. 2006, 101, 318–327. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnham, L.; Løvlie, H. Sophisticated Fowl: The Complex Behaviour and Cognitive Skills of Chickens and Red Junglefowl. Behav. Sci. 2018, 8, 13. https://doi.org/10.3390/bs8010013
Garnham L, Løvlie H. Sophisticated Fowl: The Complex Behaviour and Cognitive Skills of Chickens and Red Junglefowl. Behavioral Sciences. 2018; 8(1):13. https://doi.org/10.3390/bs8010013
Chicago/Turabian StyleGarnham, Laura, and Hanne Løvlie. 2018. "Sophisticated Fowl: The Complex Behaviour and Cognitive Skills of Chickens and Red Junglefowl" Behavioral Sciences 8, no. 1: 13. https://doi.org/10.3390/bs8010013
APA StyleGarnham, L., & Løvlie, H. (2018). Sophisticated Fowl: The Complex Behaviour and Cognitive Skills of Chickens and Red Junglefowl. Behavioral Sciences, 8(1), 13. https://doi.org/10.3390/bs8010013



