Stress Alters the Discriminative Stimulus and Response Rate Effects of Cocaine Differentially in Lewis and Fischer Inbred Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Baseline Vehicle and Cocaine Discrimination Tests
2.1.1. Vehicle Test in Cocaine Test Groups
| Segment | % Cocaine-appropriate responding | Response rate | ||
|---|---|---|---|---|
| F344 rats | Lewis rats | F344 rats | Lewis rats | |
| 1 | 0.7 (0.7) | 11.3 (7.6) | 0.68 (0.06) | 1.08 (0.07) |
| 2 | 1.9 (1.0) | 2.5 (1.3) | 0.73 (0.06) | 1.02 (0.08) |
| 3 | 15.0 (11.0) | 0.5 (0.5) | 0.73 (0.06) | 0.03) |
2.1.2. Vehicle Test in Vehicle Test Groups
| Segment | % Cocaine-appropriate responding | Response rate | ||
|---|---|---|---|---|
| F344 rats | Lewis rats | F344 rats | Lewis rats | |
| 1 | 1.7 (6.1) | 0.6 (0.2) | 0.47 (0.06) | 1.14 (0.14) |
| 2 | 1.3 (5.9) | 1.6 (0.8) | 0.47 (0.06) | 0.67 (0.19) |
| 3 | 0 (3.6) | 0 | 0.31 (0.04) | 0.85 (0.03) |
2.1.3. Cocaine Test in Cocaine Test Groups
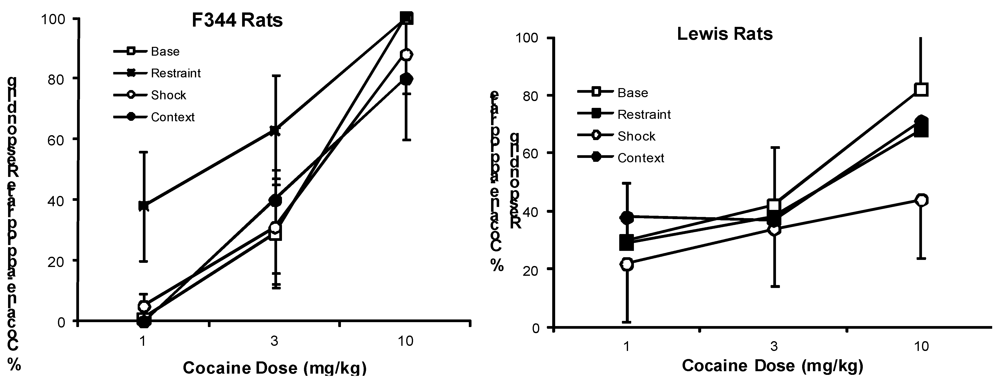
2.2. Effects of Stressors on Discrimination Performance
2.2.1. Cocaine Test Groups
2.2.2. Vehicle Test Groups
2.3. Effects of Stressors on Response Rates
2.3.1. Cocaine Test Groups

2.3.2. Vehicle Test Groups
2.4. Corticosterone Levels
| Condition | F344 | Lewis |
|---|---|---|
| Baseline | 249 (50) | 187 (20) |
| Cocaine | 519 (57) | 646 (46) |
| Restraint | 367 (33) | 220 (45) |
| Shock* | 455 (58) | 714 (45) |
| Exposure to shock context* | 516 (28) | 368 (40) |
3. Experimental Section
3.1. Subjects
3.2. Groups
3.3. Apparatus
3.4. Discrimination Training
3.5. Discrimination Testing
3.6. Stressors
3.7. Corticosterone Assay
3.8. Drugs
3.9. Data Analysis
4. Conclusions
Acknowledgments
References
- Antelman, S.M.; Eichler, A.J.; Black, C.A.; Kocan, D. Interchangeability of stress and amphetamine in sensitization. Science 1980, 207, 329–331. [Google Scholar]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Goeders, N.E. The impact of stress on addiction. Eur. J. Neuropsychopharmacol. 2003, 13, 435–441. [Google Scholar] [CrossRef]
- Capriles, N.; Cancella, L.M. Effect of acute and chronic stress restraint on amphetamine-associated place preference: Involvement of D1 and D2 receptors. Eur. J. Pharmacol. 1999, 386, 127–134. [Google Scholar] [CrossRef]
- Goeders, N.E.; Guerin, G.F. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology 1994, 114, 63–70. [Google Scholar] [CrossRef]
- Haney, M.; Maccari, S.; LeMoal, M.; Simon, H.; Piazza, P.V. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995, 698, 46–52. [Google Scholar] [CrossRef]
- Piazza, P.V.; Deminiere, J.-M.; LeMoal, M.; Simon, H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990, 514, 22–26. [Google Scholar] [CrossRef]
- Ramsey, N.F.; vanRee, J.M. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 1993, 608, 216–222. [Google Scholar] [CrossRef]
- Tidey, J.W.; Miczek, K.A. Acquisition of cocaine self-administration after social stress: Role of accumbens dopamine. Psychopharmacology 1997, 130, 203–212. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Koob, G.F. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology 1997, 132, 289–295. [Google Scholar] [CrossRef]
- Erb, S.; Shaham, Y.; Stewart, J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology 1996, 128, 408–412. [Google Scholar] [CrossRef]
- Najavits, L.M.; Gastfriend, D.R.; Barber, J.P.; Reif, S.; Muenz, L.R.; Blaine, J.; Frank, A.; Crits-Christoph, P.; Thase, M.; Weiss, R.D. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Am. J. Psychiatry 1998, 155, 214–219. [Google Scholar]
- Rhoads, D.L. A longitudinal study of life stress and social support among drug abusers. Int. J. Addict. 1983, 18, 195–222. [Google Scholar]
- Wilsnack, S.C.; Vogeltanz, N.D.; Klassen, A.D.; Harris, T.R. Childhood sexual abuse and women’s substance abuse: National survey findings. J. Stud. Alcohol 1997, 58, 264–271. [Google Scholar]
- Winhusen, T.; Somoza, E. The HPA axis cocaine use: Implications for pharmacotherapy. J. Addict. Dis. 2001, 20, 105–119. [Google Scholar] [CrossRef]
- Borowsky, B.; Kuhn, C.M. Monoamine mediation of cocaine-induced hypothalamic-pituitary-adrenal activation. J. Pharmacol. Exp. Ther. 1991, 256, 204–210. [Google Scholar]
- Kosten, T.A.; Ambrosio, E. HPA axis function and drug addictive behaviors: Insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 2002, 27, 35–69. [Google Scholar] [CrossRef]
- Moldow, R.L.; Fischman, A.J. Cocaine induced secretion of ACTH, beta-endorphin and corticosterone. Peptides 1987, 8, 819–822. [Google Scholar] [CrossRef]
- Rivier, C.L.; Vale, W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing (CRF)-mediated mechanism. Brain Res. 1987, 422, 403–406. [Google Scholar] [CrossRef]
- Saphier, D.; Welch, J.E.; Farrar, G.E.; Goeders, N.E. Effects of intracerebroventricular and intrahypothalamic cocaine administration on adrenocortical secretion. Neuroendocrinology 1993, 57, 54–62. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Biro, E.; Gardi, J.; Vecsernyes, M.; Julesz, J.; Telegdy, G. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res. 1992, 589, 154–156. [Google Scholar] [CrossRef]
- Koob, G.F.; Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 1988, 242, 715–723. [Google Scholar]
- Piazza, P.V.; LeMoal, M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 1998, 19, 67–74. [Google Scholar] [CrossRef]
- Wise, R.A.; Rompre, P.P. Brain Dopamine and Reward. In Annual Review of Psychology; Rosenzweig, M.R., Porter, L.W., Eds.; Annual Reviews Inc: Palo Alto, CA, USA, 1989; Volume 40, pp. 191–225. [Google Scholar]
- Deutch, A.Y.; Tam, S.Y.; Roth, R.H. Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985, 616, 89–98. [Google Scholar]
- Sorg, B.A.; Kalivas, P.W. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991, 559, 29–36. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; McEwen, B.S.; Spencer, R.L. Stress response, adrenal steroid receptor levels an corticosteroid-binding globulin levels—A comparison between Sprague-Dawley, Fischer 344, and Lewis rats. Brain Res. 1993, 616, 89–98. [Google Scholar] [CrossRef]
- Griffin, A.C.; Whitacre, C.C. Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rats: Implications for rodent models of autoimmune disease. J. Neuroimmunol. 1991, 35, 53–64. [Google Scholar]
- Ortiz, J.; DeCaprio, J.L.; Kosten, T.A.; Nestler, E.J. Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience 1995, 67, 383–397. [Google Scholar] [CrossRef]
- Glowa, J.R.; Geyer, M.A.; Gold, P.W.; Sternberg, E.M. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology 1992, 56, 719–723. [Google Scholar] [CrossRef]
- Sternberg, E.M.; Glowa, J.R.; Smith, M.A.; Calogero, A.E.; Listwak, S.J.; Aksentijevich, S.; Chrousos, C.P.; Wilder, R.I.; Gold, P.W. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992, 570, 54–60. [Google Scholar] [CrossRef]
- Stohr, T.; Szuran, T.; Welzl, H.; Pliska, V.; Feldon, J.; Pryce, C.R. Lewis/Fischer rat strain differences in endocrine and behavioural responses to environmental challenge. Pharmacol. Biochem. Behav. 2000, 67, 809–819. [Google Scholar] [CrossRef]
- Flores, G.; Wood, G.K.; Barbeau, D.; Quirion, R.; Srivastava, L.K. Lewis and Fischer rats: A comparison of dopamine transporter and receptors levels. Brain Res. 1998, 814, 34–40. [Google Scholar] [CrossRef]
- Haile, C.N.; Hiroi, N.; Nestler, E.J.; Kosten, T.A. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic proteins in Lewis and Fischer 344 rats. Synapse 2001, 41, 179–190. [Google Scholar] [CrossRef]
- Minabe, Y.; Emori, K.; Ashby, C.R. Significant differences in the activity of midbrain dopamine neurons between male Fischer 344 (F344) and Lewis rats: An in vivo electrophysiological study. Life Sci. 1995, 56, PL261–PL267. [Google Scholar] [CrossRef]
- Kosten, T.A.; Miserendino, M.J.D.; Chi, S.; Nestler, E.J. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J. Pharmacol. Exp. Ther. 1994, 269, 137–144. [Google Scholar]
- Kosten, T.A.; Miserendino, M.J.D.; Haile, C.N.; DeCaprio, J.L.; Jatlow, P.I.; Nestler, E.J. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997, 778, 418–429. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Goeders, N.E. Generalization of a restraint-induced discriminative stimulus to cocaine in rat. Psychopharmacology 1998, 135, 423–426. [Google Scholar] [CrossRef]
- Miczek, K.A.; Mutschler, N.H.; vanErp, A.M.M.; Blank, A.D.; McInerney, S.C. d-amphetamine “cue” generalizes to social defeat stress: Behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology 1999, 147, 190–199. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Watanabe, S. Footshock facilitates discrimination of stimulus properties of morphine. Life Sci. 1997, 61, 1045–1049. [Google Scholar] [CrossRef]
- Tomie, A.; Shultz, P.L.; Quartarolo, N.M.; Cunha, C. Presession noise increases sensitivity to chlordiazepoxide’s discriminative stimulus in pigeons. Progress Neuro Psychopharmacol. Biol. Psychiatry 1997, 21, 1155–1168. [Google Scholar] [CrossRef]
- Haile, C.N.; Kosten, T.A. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J. Pharmacol. Exp. Ther. 2001, 299, 509–518. [Google Scholar]
- Extance, K.; Goudie, A.J. Inter-animal olfactory cues in operant drug discrimination procedures in rats. Psychopharmacology 1981, 73, 363–371. [Google Scholar] [CrossRef]
- Haile, C.N.; Grandpre, T.N.; Kosten, T.A. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine. Psychopharmacology 2001, 154, 213–220. [Google Scholar] [CrossRef]
- Woolfolk, D.R.; Holtzman, S.G. μ–, δ– and κ–opioid receptor agonists do not alter the discriminative stimulus effects of cocaine or d-amphetamine in rats. Drug Alcohol Depend. 1997, 48, 209–220. [Google Scholar] [CrossRef]
- Lu, L.; Shepard, J.D.; Hall, F.S.; Shaham, Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neurosci. Biobehav. Rev. 2003, 27, 457–491. [Google Scholar] [CrossRef]
- Mantsch, M.R.; Goeders, N.E. Ketoconzaole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: Relationship to the discriminative stimulus effects of cocaine. Psychopharmacology 1999, 142, 399–407. [Google Scholar] [CrossRef]
- Glowa, J.R. Some effects of d-amphetamine, caffeine, nicotine and cocaine on schedule-controlled responding of the mouse. Neuropharmacology 1986, 25, 1127–1135. [Google Scholar] [CrossRef]
- Gonzalez, F.A.; Goldberg, S.R. Effects of cocaine and d-amphetamine on behavior maintained under various schedules of food presentation in squirrel monkeys. J. Pharmacol. Exp. Ther. 1977, 201, 33–43. [Google Scholar]
- Zuccarelli, R.; Barrett, J.E. A comparison of the effects of d-amphetamine, cocaine, imipramine, and pentobarbital on local and overall rates of responding maintained under a four-component multiple fixed-interval schedule. Pharmacol. Biochem. Behav. 1980, 12, 899–907. [Google Scholar] [CrossRef]
- Simar, M.R.; Saphier, D.; Goeders, N.E. Differential neuroendocrine and behavioral responses to cocaine in Lewis and Fischer rats. Neuroendocrinology 1996, 63, 93–100. [Google Scholar] [CrossRef]
- Baumann, M.H.; Elmer, G.I.; Goldberg, S.R.; Ambrosio, E. Differential neuroendocrine responsiveness to morphine in Lewis, Fischer 344, and ACI inbred rats. Brain Res. 2000, 858, 320–326. [Google Scholar] [CrossRef]
- Katzev, R.D.; Mills, S.K. Strain differences in avoidance conditioning as a function of the classical CS-US contingency. J. Comp. Physiol. Psychol. 1974, 87, 661–671. [Google Scholar] [CrossRef]
- Pryce, C.R.; Lehmann, J.; Feldon, J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis, and Fischer rat strains. Pharmacol. Biochem. Behav. 1999, 64, 753–759. [Google Scholar] [CrossRef]
- DeVries, A.C.; Taymans, S.E.; Sundstrom, J.M.; Pert, A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998, 786, 39–46. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kosten, T.A.; Miserendino, M.J.D. Stress Alters the Discriminative Stimulus and Response Rate Effects of Cocaine Differentially in Lewis and Fischer Inbred Rats. Behav. Sci. 2012, 2, 23-37. https://doi.org/10.3390/bs2010023
Kosten TA, Miserendino MJD. Stress Alters the Discriminative Stimulus and Response Rate Effects of Cocaine Differentially in Lewis and Fischer Inbred Rats. Behavioral Sciences. 2012; 2(1):23-37. https://doi.org/10.3390/bs2010023
Chicago/Turabian StyleKosten, Therese A., and Mindy J. D. Miserendino. 2012. "Stress Alters the Discriminative Stimulus and Response Rate Effects of Cocaine Differentially in Lewis and Fischer Inbred Rats" Behavioral Sciences 2, no. 1: 23-37. https://doi.org/10.3390/bs2010023
APA StyleKosten, T. A., & Miserendino, M. J. D. (2012). Stress Alters the Discriminative Stimulus and Response Rate Effects of Cocaine Differentially in Lewis and Fischer Inbred Rats. Behavioral Sciences, 2(1), 23-37. https://doi.org/10.3390/bs2010023




