Physiological Consequences of Repeated Exposures to Conditioned Fear
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experiment 1: Physiological Indices of Chronic Stress
2.2. Experiment 2: Prazosin and Propranolol Administration during CFT
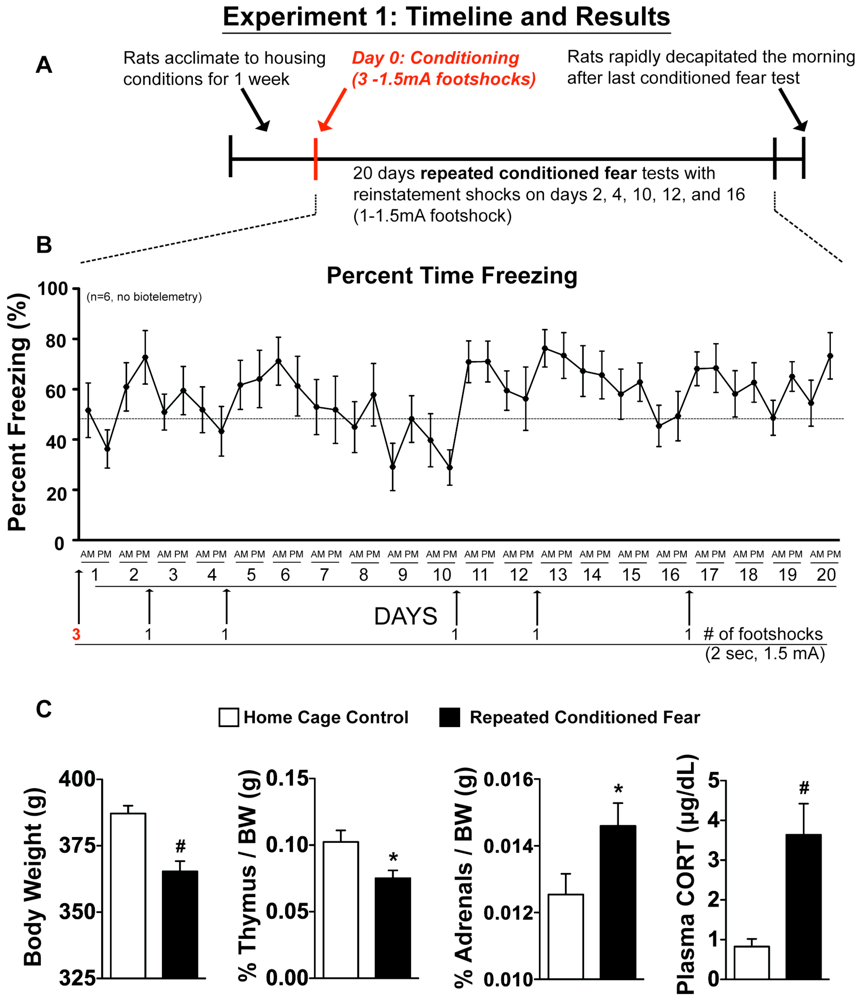
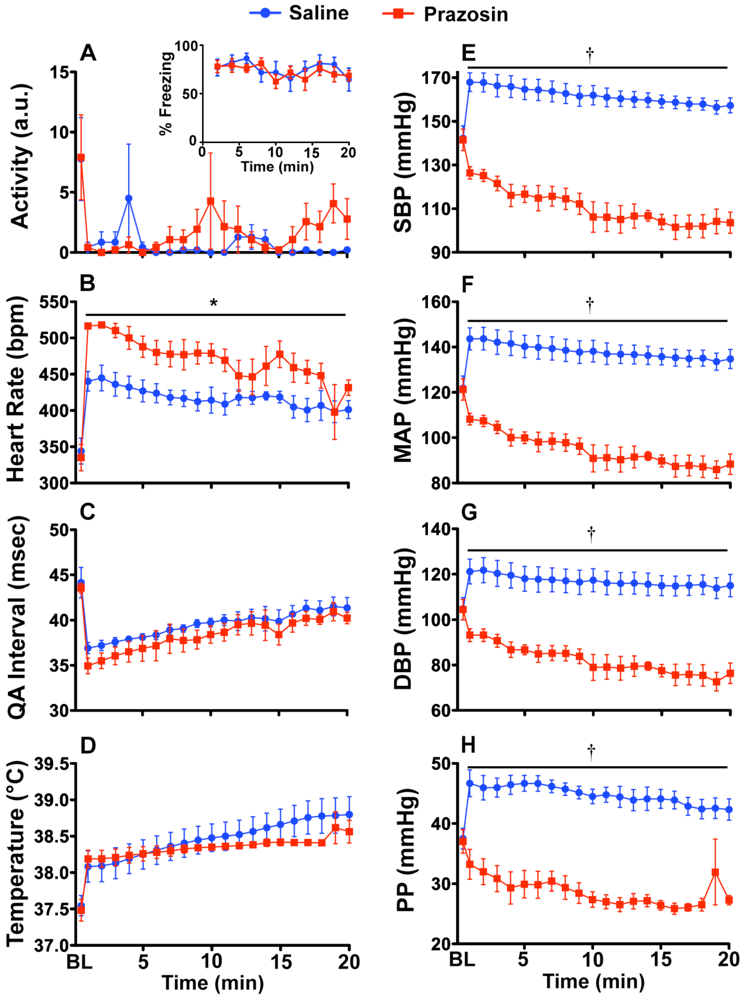
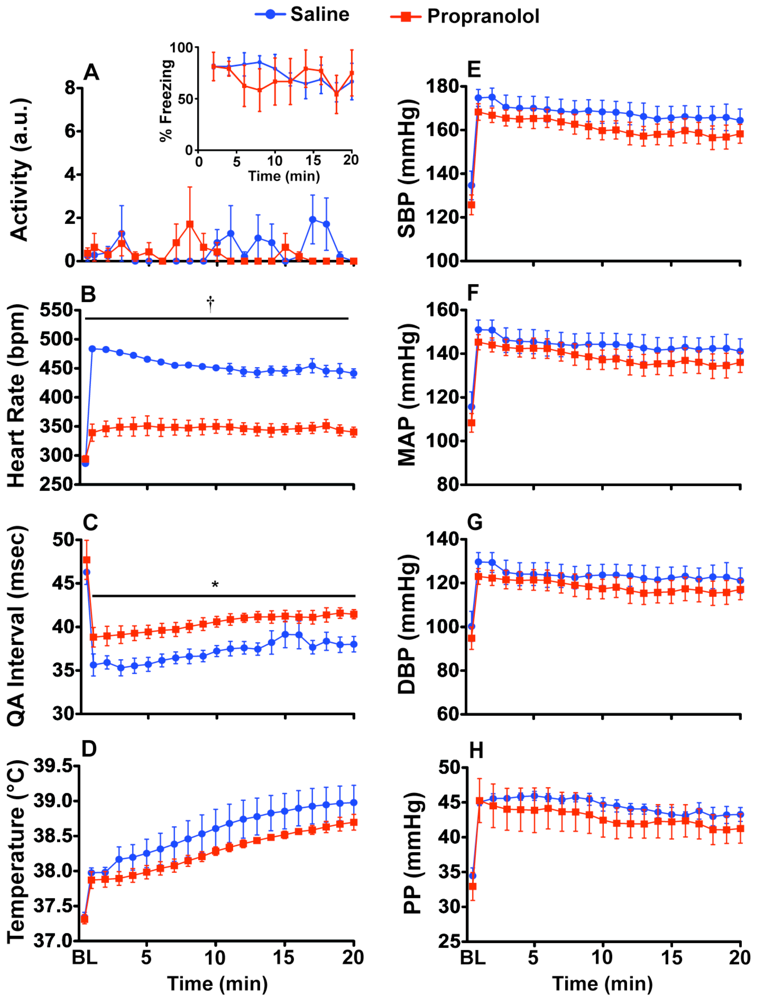
2.3. Experiment 3: In vivo Biotelemetry Monitoring during Repeated CFT and 5 Days after Stressor Termination
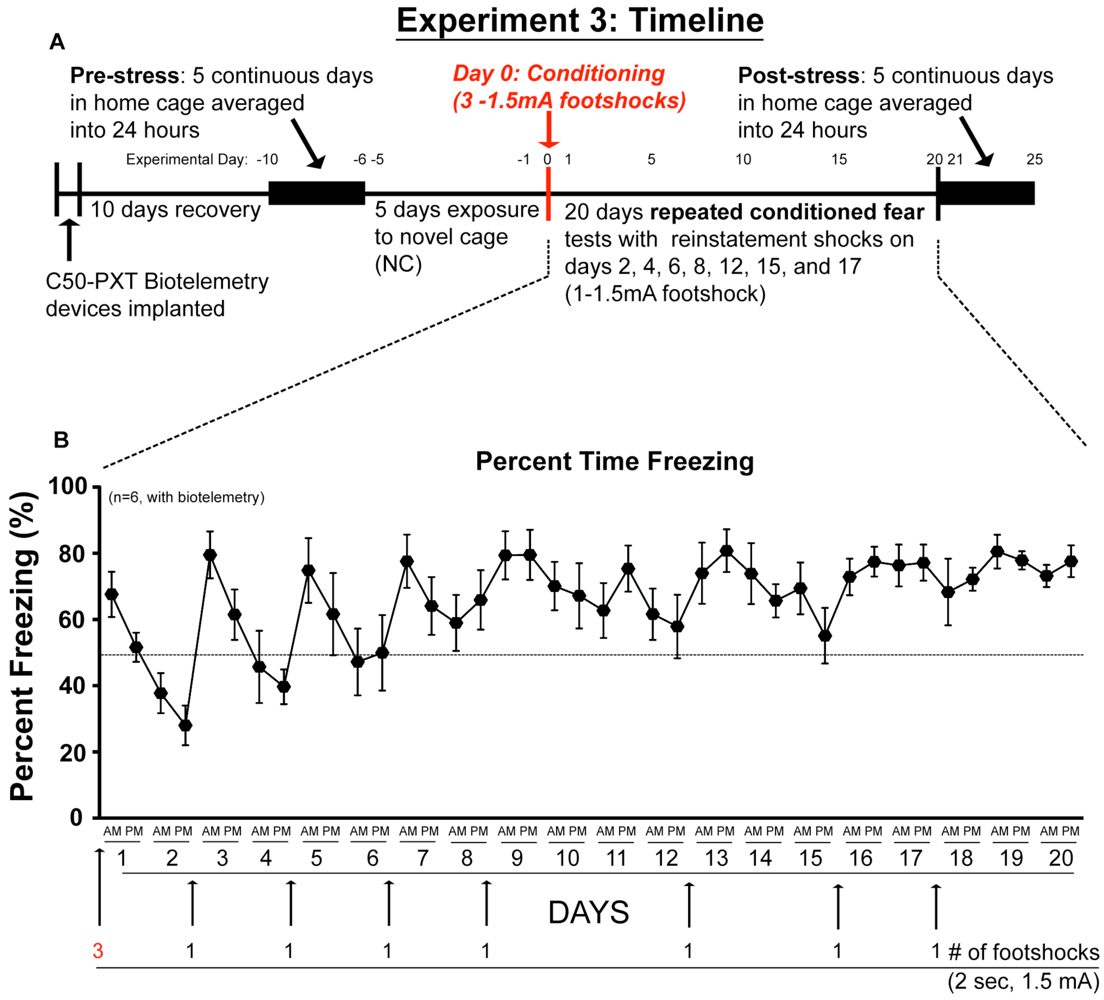
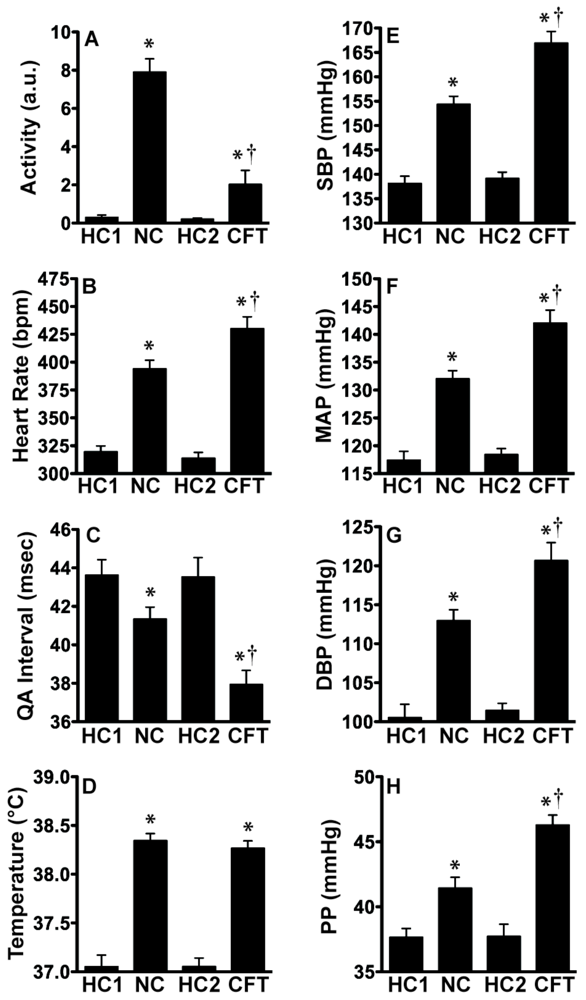
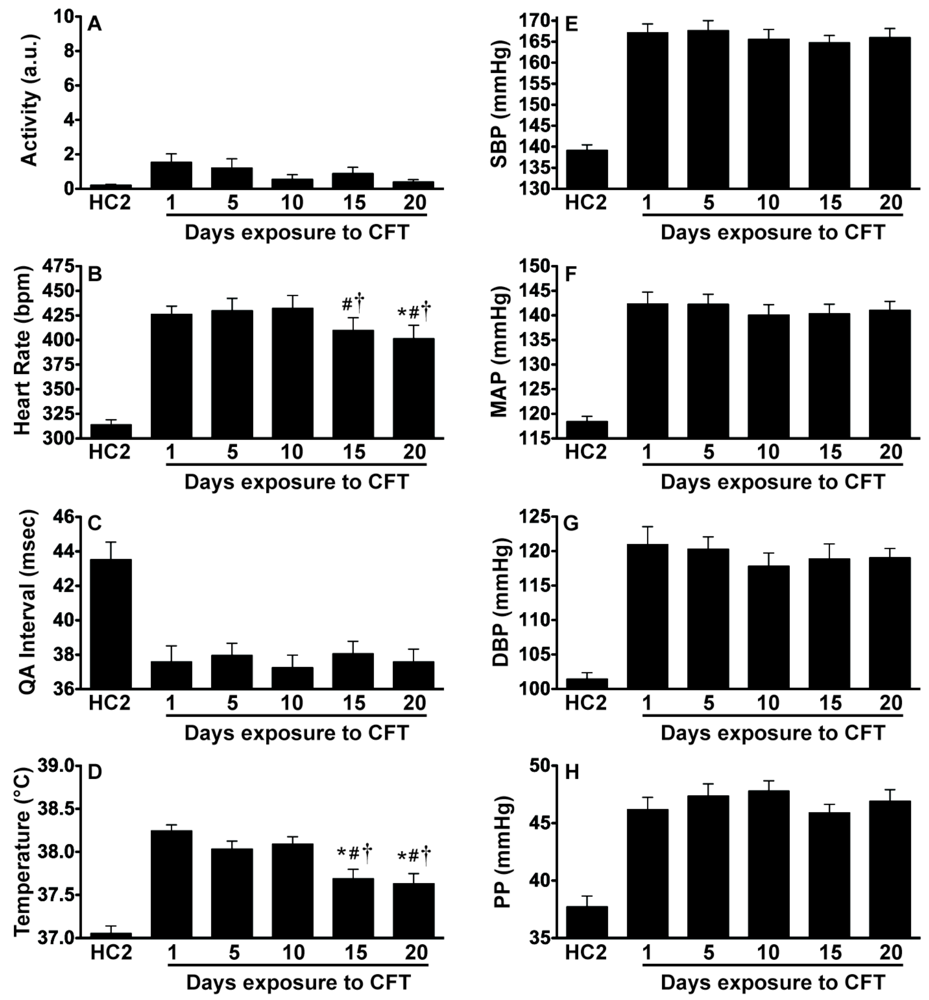
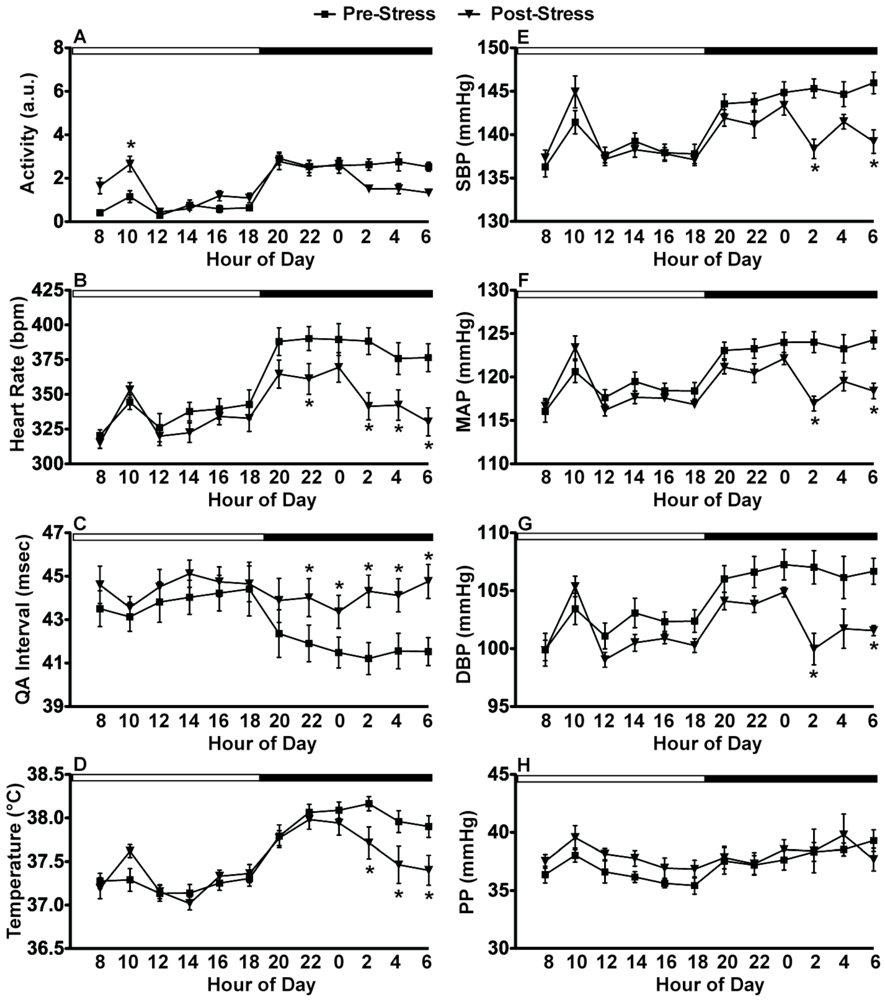
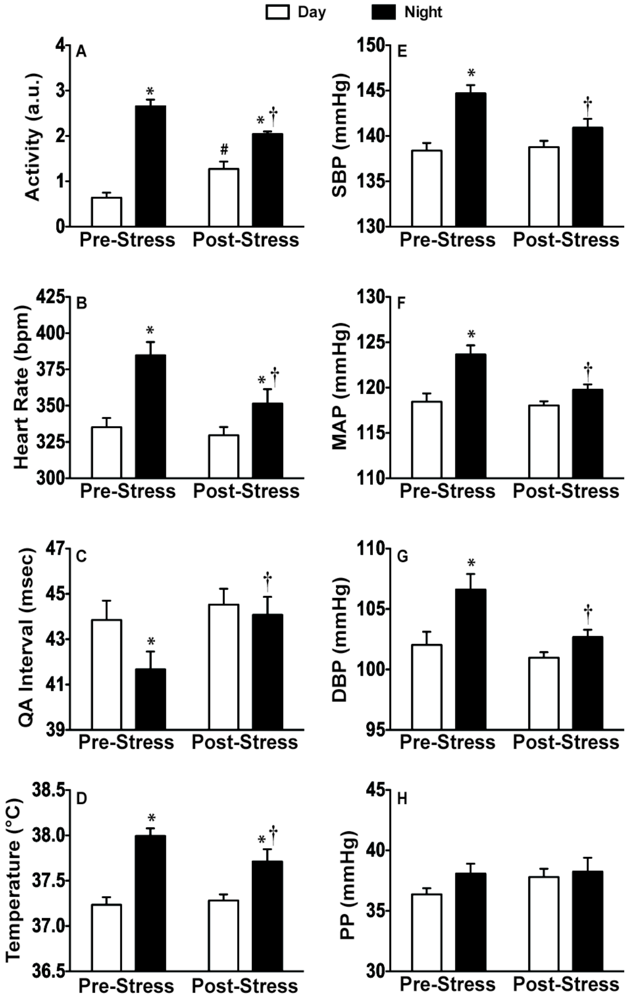
2.4. Discussion of Results
3. Experimental Section
3.1. Animals
3.2. Biotelemetry Surgeries
3.3. Data Acquisition and Analysis
3.4. Contextual Conditioned Fear Test (CFT)
3.5. Experiment 1: Repeated Exposure to CFT and Tissue Collection
3.6. Experiment 2: Verification of SNS Involvement during CFT
3.7. Experiment 3: Biotelemetry Monitoring during Repeated CFT
3.8. Statistical Analysis
4. Conclusions
References
- Silverthorn, D.U. Human Physiology: An Integrated Approach; Pearson Benjamin Cummings: San Fransisco, CA, USA, 2007; pp. 800–802. [Google Scholar]
- Forsman, L.; Lindblad, L.E. Effect of mental stress on baroreceptor-mediated changes in blood pressure and heart rate and on plasma catecholamines and subjective responses in healthy men and women. Psychosom. Med. 1983, 45, 435–445. [Google Scholar]
- Hamer, M.; Gibson, E.L.; Vuononvirta, R.; Williams, E.; Steptoe, A. Inflammatory and hemostatic responses to repeated mental stress: Individual stability and habituation over time. Brain, Behav. Immunity 2006, 20, 456–459. [Google Scholar] [CrossRef]
- Hjemdahl, P.; Fagius, J.; Freyschuss, U.; Wallin, B.G.; Daleskog, M.; Bohlin, G.; Perski, A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am. J. Physiol. 1989, 257, E654–E664. [Google Scholar]
- Kubzansky, L.D.; Adler, G.K. Aldosterone: A forgotten mediator of the relationship between psychological stress and heart disease. Neurosci. Biobehav. Rev. 2010, 34, 80–86. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA: J. Am. Med. Assoc. 2007, 298, 1685–1687. [Google Scholar]
- Dimsdale, J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar] [CrossRef]
- Rozanski, A.; Blumenthal, J.A.; Davidson, K.W.; Saab, P.G.; Kubzansky, L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. J. Am. Coll.Cardiol. 2005, 45, 637–651. [Google Scholar]
- Grippo, A.J.; Moffitt, J.A.; Johnson, A.K. Evaluation of baroreceptor reflex function in the chronic mild stress rodent model of depression. Psychosom. Med. 2008, 70, 435–443. [Google Scholar] [CrossRef]
- Iso, H.; Date, C.; Yamamoto, A.; Toyoshima, H.; Tanabe, N.; Kikuchi, S.; Kondo, T.; Watanabe, Y.; Wada, Y.; Ishibashi, T.; et al. Perceived mental stress and mortality from cardiovascular disease among japanese men and women: The japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (jacc study). Circulation 2002, 106, 1229–1236. [Google Scholar]
- Rosengren, A.; Tibblin, G.; Wilhelmsen, L. Self-perceived psychological stress and incidence of coronary artery disease in middle-aged men. Am. J. Cardiol. 1991, 68, 1171–1175. [Google Scholar] [CrossRef]
- Smith, T.W.; Ruiz, J.M. Psychosocial influences on the development and course of coronary heart disease: Current status and implications for research and practice. J. Consult. Clin. Psychol. 2002, 70, 548–568. [Google Scholar] [CrossRef]
- Grippo, A.J.; Johnson, A.K. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009, 12, 1–21. [Google Scholar] [CrossRef]
- Baumert, M.; Schlaich, M.P.; Nalivaiko, E.; Lambert, E.; Sari, C.I.; Kaye, D.M.; Elser, M.D.; Sanders, P.; Lambert, G. Relation between qt interval variability and cardiac sympathetic activity in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1412–H1417. [Google Scholar] [CrossRef]
- Nalivaiko, E. Animal models of psychogenic cardiovascular disorders: What we can learn from them and what we cannot. Clin. Exp. Pharmacol. Physiol. 2011, 38, 115–125. [Google Scholar] [CrossRef]
- Branch, C.A.; Knuepfer, M.M. Causes of differential cardiovascular sensitivity to cocaine. Ii: Sympathetic, metabolic and cardiac effects. J. Pharmacol. Exp. Ther. 1994, 271, 1103–1113. [Google Scholar]
- Knuepfer, M.M.; Purcell, R.M.; Gan, Q.; Le, K.M. Hemodynamic response patterns to acute behavioral stressors resemble those to cocaine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1778–R1786. [Google Scholar]
- Light, K.C.; Dolan, C.A.; Davis, M.R.; Sherwood, A. Cardiovascular responses to an active coping challenge as predictors of blood pressure patterns 10 to 15 years later. Psychosom. Med. 1992, 54, 217–230. [Google Scholar]
- Muller, J.R.; Le, K.M.; Haines, W.R.; Gan, Q.; Knuepfer, M.M. Hemodynamic response pattern predicts susceptibility to stress-induced elevation in arterial pressure in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R31–R37. [Google Scholar]
- Chrousos, G.P. Stress, chronic inflammation, and emotional and physical well-being: Concurrent effects and chronic sequelae. J. Allergy Clin. Immunol. 2000, 106, S275–S291. [Google Scholar] [CrossRef]
- Pertsov, S.S.; Koplik, E.V.; Stepanyuk, V.L.; Simbirtsev, A.S. Blood cytokines in rats with various behavioral characteristics during emotional stress and treatment with interleukin-1beta. Bull. Exp. Biol. Med. 2009, 148, 196–199. [Google Scholar] [CrossRef]
- Picardi, A.; Mazzotti, E.; Gaetano, P.; Cattaruzza, M.S.; Baliva, G.; Melchi, C.F.; Biondi, M.; Pasquini, P. Stress, social support, emotional regulation, and exacerbation of diffuse plaque psoriasis. Psychosomatics 2005, 46, 556–564. [Google Scholar] [CrossRef]
- Shao, F.; Lin, W.; Wang, W.; Washington, W.C., Jr.; Zheng, L. The effect of emotional stress on the primary humoral immunity of rats. J. Psychopharmacol. 2003, 17, 179–183. [Google Scholar] [CrossRef]
- Amiragova, M.G. Neurophysiological analysis of the development of endocrine and hypertensive reactions in prolonged emotional stress. Brain Res. 1985, 344, 303–315. [Google Scholar] [CrossRef]
- Shoji, H.; Mizoguchi, K. Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav. Brain Res. 2010, 211, 169–177. [Google Scholar] [CrossRef]
- Batalha, V.L.; Pego, J.M.; Fontinha, B.M.; Costenla, A.R.; Valadas, J.S.; Baqi, Y.; Radjainia, H.; Muller, C.E.; Sebastiao, A.M.; Lopes, L.V. Adenosine a(2a) receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol. Psychiatry 2012, in press. [Google Scholar]
- Christiansen, S.; Bouzinova, E.V.; Palme, R.; Wiborg, O. Circadian activity of the hypothalamic-pituitary-adrenal axis is differentially affected in the rat chronic mild stress model of depression. Stress 2012, in press. [Google Scholar]
- Koresh, O.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Matar, M.A.; Cohen, H. The long-term abnormalities in circadian expression of period 1 and period 2 genes in response to stress is normalized by agomelatine administered immediately after exposure. Eur. Neuropsychopharmacol. 2012, 22, 205–221. [Google Scholar] [CrossRef]
- Richards, R.S.; Nwose, E.U.; Bwititi, P. Biochemical basis of circadian rhythms and diseases: With emphasis on post-traumatic stress disorder. Med. Hypotheses 2011, 77, 605–609. [Google Scholar] [CrossRef]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian disruption and metabolic disease: Findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef]
- Albrecht, U. Circadian rhythms and sleep—The metabolic connection. Pflugers Arch. 2012, 463, 23–30. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. Circadian clock and cardiovascular disease. J. Cardiol. 2011, 57, 249–256. [Google Scholar] [CrossRef]
- Portaluppi, F.; Tiseo, R.; Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Fabbian, F. Circadian rhythms and cardiovascular health. Sleep Med. Rev. 2012, 16, 151–166. [Google Scholar]
- McClung, C.A. Clock genes and bipolar disorder: Implications for therapy. Pharmacogenomics 2007, 8, 1097–1100. [Google Scholar] [CrossRef]
- McClung, C.A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther. 2007, 114, 222–232. [Google Scholar] [CrossRef]
- Bunney, J.N.; Potkin, S.G. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br. Med. Bull. 2008, 86, 23–32. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Blanchard, R.J. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972, 81, 281–290. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Gale, G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003, 985, 125–134. [Google Scholar] [CrossRef]
- Godsil, B.P.; Quinn, J.J.; Fanselow, M.S. Body temperature as a conditional response measure for pavlovian fear conditioning. Learn. Mem. 2000, 7, 353–356. [Google Scholar] [CrossRef]
- Carrive, P. Conditioned fear to environmental context: Cardiovascular and behavioral components in the rat. Brain Res. 2000, 858, 440–445. [Google Scholar] [CrossRef]
- Carrive, P. Cardiovascular and behavioural components of conditioned fear to context after ganglionic and alpha-adrenergic blockade. Auton. Neurosci. 2002, 98, 90–93. [Google Scholar] [CrossRef]
- Choi, E.A.; Leman, S.; Vianna, D.M.; Waite, P.M.; Carrive, P. Expression of cardiovascular and behavioural components of conditioned fear to context in t4 spinally transected rats. Auton. Neurosci. 2005, 120, 26–34. [Google Scholar] [CrossRef]
- Vianna, D.M.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef]
- Carrive, P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1251–1254. [Google Scholar] [CrossRef]
- Dias-Ferreira, E.; Sousa, J.C.; Melo, I.; Morgado, P.; Mesquita, A.R.; Cerqueira, J.J.; Costa, R.M.; Sousa, N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009, 325, 621–625. [Google Scholar]
- Vandekerckhove, M.; Cluydts, R. The emotional brain and sleep: An intimate relationship. Sleep Med. Rev. 2010, 14, 219–226. [Google Scholar] [CrossRef]
- Nishino, S. Hypothalamus, hypocretins/orexin, and vigilance control. Handb. Clin. Neurol. 2011, 99, 765–782. [Google Scholar] [CrossRef]
- Morimoto, K.; Tan, N.; Nishiyasu, T.; Sone, R.; Murakami, N. Spontaneous wheel running attenuates cardiovascular responses to stress in rats. Pflugers Arch. 2000, 440, 216–222. [Google Scholar]
- Murphy, H.M.; Wideman, C.H.; Aquila, L.A.; Nadzam, G.R. Telemetry provides new insights into entrainment of activity wheel circadian rhythms and the role of body temperature in the development of ulcers in the activity-stress paradigm. Integr. Physiol. Behav. Sci. 2002, 37, 228–241. [Google Scholar] [CrossRef]
- Soszynski, D.; Kozak, W.; Conn, C.A.; Rudolph, K.; Kluger, M.J. Beta-adrenoceptor antagonists suppress elevation in body temperature and increase in plasma il-6 in rats exposed to open field. Neuroendocrinology 1996, 63, 459–467. [Google Scholar] [CrossRef]
- Leman, S.; Dielenberg, R.A.; Carrive, P. Effect of dorsal periaqueductal gray lesion on cardiovascular and behavioural responses to contextual conditioned fear in rats. Behav. Brain Res. 2003, 143, 169–176. [Google Scholar] [CrossRef]
- Glavin, G.B.; Pare, W.P.; Sandbak, T.; Bakke, H.K.; Murison, R. Restraint stress in biomedical research: An update. Neurosci. Biobehav. Rev. 1994, 18, 223–249. [Google Scholar] [CrossRef]
- Simpkiss, J.L.; Devine, D.P. Responses of the hpa axis after chronic variable stress: Effects of novel and familiar stressors. Neuroendocrinol. Lett. 2003, 24, 97–103. [Google Scholar]
- Babygirija, R.; Bulbul, M.; Cerjak, D.; Ludwig, K.; Takahashi, T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci. Lett. 2011, 495, 77–81. [Google Scholar] [CrossRef]
- Girotti, M.; Pace, T.W.; Gaylord, R.I.; Rubin, B.A.; Herman, J.P.; Spencer, R.L. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 2006, 138, 1067–1081. [Google Scholar] [CrossRef]
- Brooks, B.A.; Fahey, T.D.; Baldwin, K.M. Exercise Physiology: Human Bioenergetics and Its Applications, 4th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Cambridge, D.; Whiting, M.V. Evaluation of the qa interval as an index of cardiac contractility in anaesthetised dogs: Responses to changes in cardiac loading and heart rate. Cardiovasc. Res. 1986, 20, 444–450. [Google Scholar] [CrossRef]
- Chang, C.C.; Hwang, J.S.; Chan, C.C.; Wang, P.Y.; Hu, T.H.; Cheng, T.J. Effects of concentrated ambient particles on heart rate, blood pressure, and cardiac contractility in spontaneously hypertensive rats. Inhal. Toxicol. 2004, 16, 421–429. [Google Scholar] [CrossRef]
- Light, K.C.; Dolan, C.A.; Davis, M.R.; Sherwood, A. Cardiovascular responses to an active coping challenge as predictors of blood pressure patterns 10 to 15 years later. Psychosom. Med. 1992, 54, 217–230. [Google Scholar]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef]
- Irigoyen, M.C.; Krieger, E.M. Baroreflex control of sympathetic activity in experimental hypertension. Brazilian J. Med. Biol. Res. 1998, 31, 1213–1220. [Google Scholar] [CrossRef]
- Conti, L.H.; Shannon, M.H.; Murry, J.D.; Printz, M.P. Repeated restraint stress-induced increase in baroreceptor reflex sensitivity: Role of corticotropin-releasing factor. Neuropeptides 2001, 35, 71–81. [Google Scholar] [CrossRef]
- Foster, D.O.; Frydman, M.L. Nonshivering thermogenesis in the rat. Ii. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 1978, 56, 110–122. [Google Scholar] [CrossRef]
- Marks, A.; Vianna, D.M.; Carrive, P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am. J. Physiol. Regul., Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Revi. Genet. 2008, 9, 764–775. [Google Scholar]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar]
- Arntz, H.R.; Willich, S.N.; Oeff, M.; Bruggemann, T.; Stern, R.; Heinzmann, A.; Matenaer, B.; Schroder, R. Circadian variation of sudden cardiac death reflects age-related variability in ventricular fibrillation. Circulation 1993, 88, 2284–2289. [Google Scholar]
- Sheward, W.J.; Naylor, E.; Knowles-Barley, S.; Armstrong, J.D.; Brooker, G.A.; Seckl, J.R.; Turek, F.W.; Holmes, M.C.; Zee, P.C.; Harmar, A.J. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: Behavioral effects are more significant than direct outputs. PLoS One 2010, 5, e9783. [Google Scholar]
- Vianna, D.M.; Allen, C.; Carrive, P. Cardiovascular and behavioral responses to conditioned fear after medullary raphe neuronal blockade. Neuroscience 2008, 153, 1344–1353. [Google Scholar] [CrossRef]
- Rudy, J.W.; Huff, N.C.; Matus-Amat, P. Understanding contextual fear conditioning: Insights from a two-process model. Neurosci. Biobehav. Rev. 2004, 28, 675–685. [Google Scholar] [CrossRef]
- Morris, R.W.; Westbrook, R.F.; Killcross, A.S. Reinstatement of extinguished fear by beta-adrenergic arousal elicited by a conditioned context. Behav. Neurosci. 2005, 119, 1662–1671. [Google Scholar] [CrossRef]
- Dirikx, T.; Beckers, T.; Muyls, C.; Eelen, P.; Vansteenwegen, D.; Hermans, D.; D'Hooge, R. Differential acquisition, extinction, and reinstatement of conditioned suppression in mice. Q. J. Exp. Psychol. 2007, 60, 1313–1320. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Strong, P.V.; Foley, T.E.; Fleshner, M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus 2009, 19, 988–1001. [Google Scholar] [CrossRef]
- Cordero, M.I.; Merino, J.J.; Sandi, C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav. Neurosci. 1998, 112, 885–891. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1978. [Google Scholar]
- Campeau, S.; Nyhuis, T.J.; Sasse, S.K.; Kryskow, E.M.; Herlihy, L.; Masini, C.V.; Babb, J.A.; Greenwood, B.N.; Fleshner, M.; Day, H.E. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J. Neuroendocrinol. 2010, 22, 872–888. [Google Scholar]
- Swenson, R.M.; Vogel, W.H. Plasma catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol. biochem. Behav. 1983, 18, 689–693. [Google Scholar] [CrossRef]
- Bechtold, A.G.; Patel, G.; Hochhaus, G.; Scheuer, D.A. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am. J. Physiol. Regul., Integr. Comp. Physiol. 2009, 296, R1445–R1454. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thompson, R.S.; Strong, P.V.; Fleshner, M. Physiological Consequences of Repeated Exposures to Conditioned Fear. Behav. Sci. 2012, 2, 57-78. https://doi.org/10.3390/bs2020057
Thompson RS, Strong PV, Fleshner M. Physiological Consequences of Repeated Exposures to Conditioned Fear. Behavioral Sciences. 2012; 2(2):57-78. https://doi.org/10.3390/bs2020057
Chicago/Turabian StyleThompson, Robert S., Paul V. Strong, and Monika Fleshner. 2012. "Physiological Consequences of Repeated Exposures to Conditioned Fear" Behavioral Sciences 2, no. 2: 57-78. https://doi.org/10.3390/bs2020057
APA StyleThompson, R. S., Strong, P. V., & Fleshner, M. (2012). Physiological Consequences of Repeated Exposures to Conditioned Fear. Behavioral Sciences, 2(2), 57-78. https://doi.org/10.3390/bs2020057



