Reflection Rumination Reduces Negative Emotional Processing During Goal-Directed Behavior: An ERP Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Rumination
2.2.2. Depression Severity
2.2.3. Emotional Delayed Response WM Task and Stimuli
2.3. EEG Recording and Processing
3. Results
3.1. Behavioral Data
3.2. ERP Data
3.2.1. Analysis Steps
3.2.2. Emotion and Region Effects
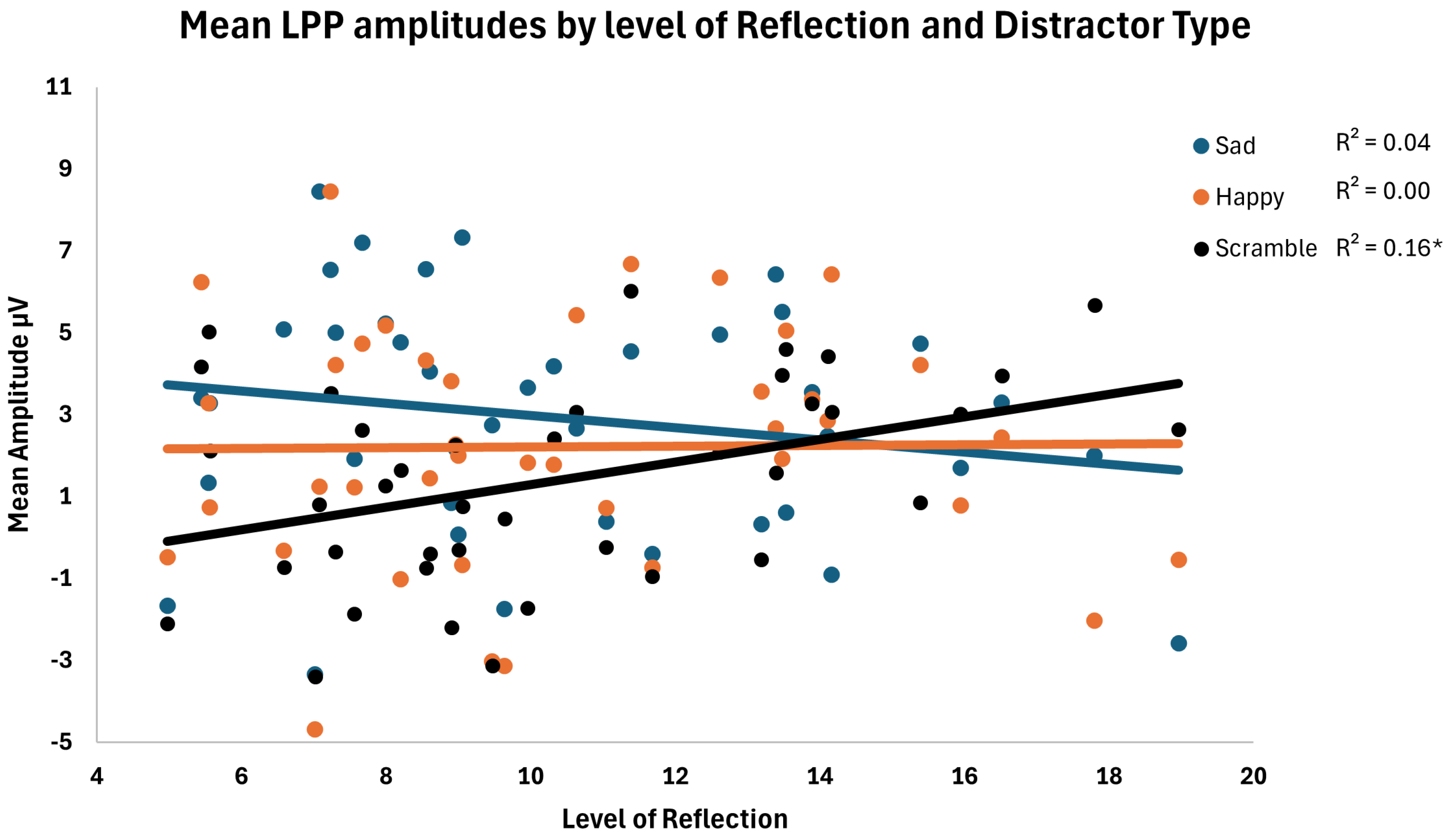
3.2.3. Brooding and Reflection Analyses
4. Discussion
Avenues for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alderman, B. L., Olson, R. L., Bates, M. E., Selby, E. A., Buckman, J. F., Brush, C. J., Panza, E. A., Kranzler, A., Eddie, D., & Shors, T. J. (2015). Rumination in major depressive disorder is associated with impaired neural activation during conflict monitoring. Frontiers in Human Neuroscience, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Beck, A. T., Steer, R. A., Ball, R., & Ranieri, W. F. (1996). Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). L. Erlbaum Associates Publisher. [Google Scholar]
- Cuthbert, B. N., & Kozak, M. J. (2013). Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology, 122(3), 928–937. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [Google Scholar] [CrossRef]
- Dolcos, F., & Denkova, E. (2016). Dissociating between enhancing and impairing effects of emotion on cognition. Sante Mentale au Quebec, 41(1), 15–34. [Google Scholar] [CrossRef]
- Duque, A., Sanchez, A., & Vazquez, C. (2014). Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition and Emotion, 28(8), 1347–1366. [Google Scholar] [CrossRef]
- Gan, S. Z., Chen, S., & Shen, X. R. (2019). The emotion regulation effect of cognitive control is related to depressive state through the mediation of rumination: An ERP study. PLoS ONE, 14(11), e0225285. [Google Scholar] [CrossRef]
- Grahek, I., Shenhav, A., Musslick, S., Krebs, R. M., & Koster, E. H. W. (2019). Motivation and cognitive control in depression. Neuroscience & Biobehavioral Reviews, 102, 371–381. [Google Scholar] [CrossRef]
- Hajcak, G., & Foti, D. (2020). Significance? & Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), e13570. [Google Scholar] [CrossRef]
- Hamilton, J. P., Furman, D. J., Chang, C., Thomason, M. E., Dennis, E., & Gotlib, I. H. (2011). Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–333. [Google Scholar] [CrossRef]
- Hsu, T. Y., Liu, T. L., Cheng, P. Z., Lee, H. C., Lane, T. J., & Duncan, N. W. (2021). Depressive rumination is correlated with brain responses during self-related processing. Journal of Psychiatry & Neuroscience, 46(5), E518–E527. [Google Scholar] [CrossRef]
- Jacob, Y., Morris, L. S., Huang, K. H., Schneider, M., Rutter, S., Verma, G., Murrough, J. W., & Balchandani, P. (2020). Neural correlates of rumination in major depressive disorder: A brain network analysis. NeuroImage Clinical, 25, 102142. [Google Scholar] [CrossRef] [PubMed]
- Joormann, J., & Gotlib, I. H. (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion, 24(2), 281–298. [Google Scholar] [CrossRef] [PubMed]
- Joormann, J., & Tanovic, E. (2015). Cognitive vulnerability to depression: Examining cognitive control and emotion regulation. Current Opinion in Psychology, 4, 86–92. [Google Scholar] [CrossRef]
- Jung, T.-P., Makeig, S., Humphries, C., Lee, T.-W., Mckeown, M. J., Iragui, V., & Sejnowski, T. J. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–178. [Google Scholar] [CrossRef]
- Koster, E. H., De Lissnyder, E., Derakshan, N., & De Raedt, R. (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31(1), 138–145. [Google Scholar] [CrossRef]
- Koster, E. H., De Raedt, R., Goeleven, E., Franck, E., & Crombez, G. (2005). Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emotion, 5(4), 446–455. [Google Scholar] [CrossRef]
- Krompinger, J. W., & Simons, R. F. (2011). Cognitive inefficiency in depressive undergraduates: Stroop processing and ERPs. Biological Psychology, 86(3), 239–246. [Google Scholar] [CrossRef]
- Leppänen, J. M., Milders, M., Bell, J. S., Terriere, E., & Hietanen, J. K. (2004). Depression biases the recognition of emotionally neutral faces. Psychiatry Research, 128(2), 123–133. [Google Scholar] [CrossRef]
- Lo, B. C. Y., Lau, S., Cheung, S. H., & Allen, N. B. (2012). The impact of rumination on internal attention switching. Cognition and Emotion, 26(2), 209–223. [Google Scholar] [CrossRef]
- Lopez-Calderon, J., & Luck, S. J. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Luck, S. J., & Gaspelin, N. (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology, 54(1), 146–157. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, D., Flykt, A., & Öhman, A. (1998). Karolinska directed emotional faces. Cognition and Emotion. [Google Scholar] [CrossRef]
- Ma, D. S., Correll, J., & Wittenbrink, B. (2015). The Chicago face database: A free stimulus set of faces and norming data. Behavior Research Methods, 47, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, R. C., Zhang, S., Preacher, K. J., & Rucker, D. D. (2002). On the practice of dichotomization of quantitative variables. Psychol Methods, 7(1), 19–40. [Google Scholar] [CrossRef]
- MacNamara, A., Joyner, K., & Klawohn, J. (2022). Event-related potential studies of emotion regulation: A review of recent progress and future directions. International Journal of Psychophysiology, 176, 73–88. [Google Scholar] [CrossRef]
- MacNamara, A., Schmidt, J., Zelinsky, G. J., & Hajcak, G. (2012). Electrocortical and ocular indices of attention to fearful and neutral faces presented under high and low working memory load. Biological Psychology, 91(3), 349–356. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S., Wisco, B. E., & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–424. [Google Scholar] [CrossRef]
- Owens, M., & Gibb, B. E. (2017). Brooding rumination and attentional biases in currently non-depressed individuals: An eye-tracking study. Cognition and Emotion, 31(5), 1062–1069. [Google Scholar] [CrossRef]
- Owens, M., Harrison, A. J., Burkhouse, K. L., McGeary, J. E., Knopik, V. S., Palmer, R. H., & Gibb, B. E. (2016). Eye tracking indices of attentional bias in children of depressed mothers: Polygenic influences help to clarify previous mixed findings. Development and Psychopathology, 28(2), 385–397. [Google Scholar] [CrossRef]
- Palmwood, E. N., Krompinger, J. W., & Simons, R. F. (2017). Electrophysiological indicators of inhibitory control deficits in depression. Biological Psychology, 130, 1–10. [Google Scholar] [CrossRef]
- Park, H., Kuplicki, R., Paulus, M. P., & Guinjoan, S. M. (2024). Rumination and overrecruitment of cognitive control circuits in depression. Biological Psychiatry Cognitive Neuroscience and Neuroimaging, 9(8), 800–808. [Google Scholar] [CrossRef]
- Proudman, D., Greenberg, P., & Nellesen, D. (2021). The growing burden of Major Depressive Disorders (MDD): Implications for researchers and policy makers. Pharmacoeconomics, 39(6), 619–625. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L., Russell, K., Yung, C. S. E., Dobson, K. S., & Sears, C. R. (2024). Associations between attentional biases for emotional images and rumination in depression. Cognition & Emotion, 1–18. [Google Scholar] [CrossRef]
- Stewart, T. M., Hunter, S. C., & Rhodes, S. M. (2019). Reflective pondering is associated with executive control for emotional information: An adolescent prospective study. Journal of Behavior Therapy and Experimental Psychiatry, 65, 101486. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., Marcus, D. J., Westerlund, A., Casey, B. J., & Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [Google Scholar] [CrossRef]
- Treynor, W., Gonzalez, R., & Nolen-Hoeksema, S. (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. [Google Scholar] [CrossRef]
- Villalobos, D., Pacios, J., & Vazquez, C. (2021). Cognitive control, cognitive biases and emotion regulation in depression: A new proposal for an integrative interplay model. Frontiers in Psychology, 12, 628416. [Google Scholar] [CrossRef]
- Watkins, E. R., & Roberts, H. (2020). Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behaviour Research and Therapy, 127, 103573. [Google Scholar] [CrossRef]
- Webb, C. A., Auerbach, R. P., Bondy, E., Stanton, C. H., Foti, D., & Pizzagalli, D. A. (2017). Abnormal neural responses to feedback in depressed adolescents. Journal of Abnormal Psychology, 126(1), 19–31. [Google Scholar] [CrossRef]
- Weinberg, A., Correa, K. A., Stevens, E. S., & Shankman, S. A. (2021). The emotion-elicited late positive potential is stable across five testing sessions. Psychophysiology, 58(11), e13904. [Google Scholar] [CrossRef]
- Weinberg, A., & Hajcak, G. (2010). Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion, 10(6), 767–782. [Google Scholar] [CrossRef]
- Whitmer, A. J., & Gotlib, I. H. (2013). An attentional scope model of rumination. Psychological Bulletin, 139(5), 1036–1061. [Google Scholar] [CrossRef]
- WHO. (2025). Fact sheet—Depressive disorder (depression). World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 July 2025).
- Wong, G., Dolcos, S., Denkova, E., Morey, R., Wang, L., McCarthy, G., & Dolcos, F. (2012). Brain imaging investigation of the impairing effect of emotion on cognition. Journal of Visualized Experiments, 60, e2434. [Google Scholar] [CrossRef]
- Yang, Y., Cao, S., Shields, G. S., Teng, Z., & Liu, Y. (2017). The relationships between rumination and core executive functions: A meta-analysis. Depression and Anxiety, 34(1), 37–50. [Google Scholar] [CrossRef]
- Zetsche, U., Burkner, P. C., & Schulze, L. (2018). Shedding light on the association between repetitive negative thinking and deficits in cognitive control—A meta-analysis. Clinical Psychology Review, 63, 56–65. [Google Scholar] [CrossRef]
- Zhou, H. X., Chen, X., Shen, Y. Q., Li, L., Chen, N. X., Zhu, Z. C., Castellanos, F. X., & Yan, C. G. (2020). Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage, 206, 116287. [Google Scholar] [CrossRef]

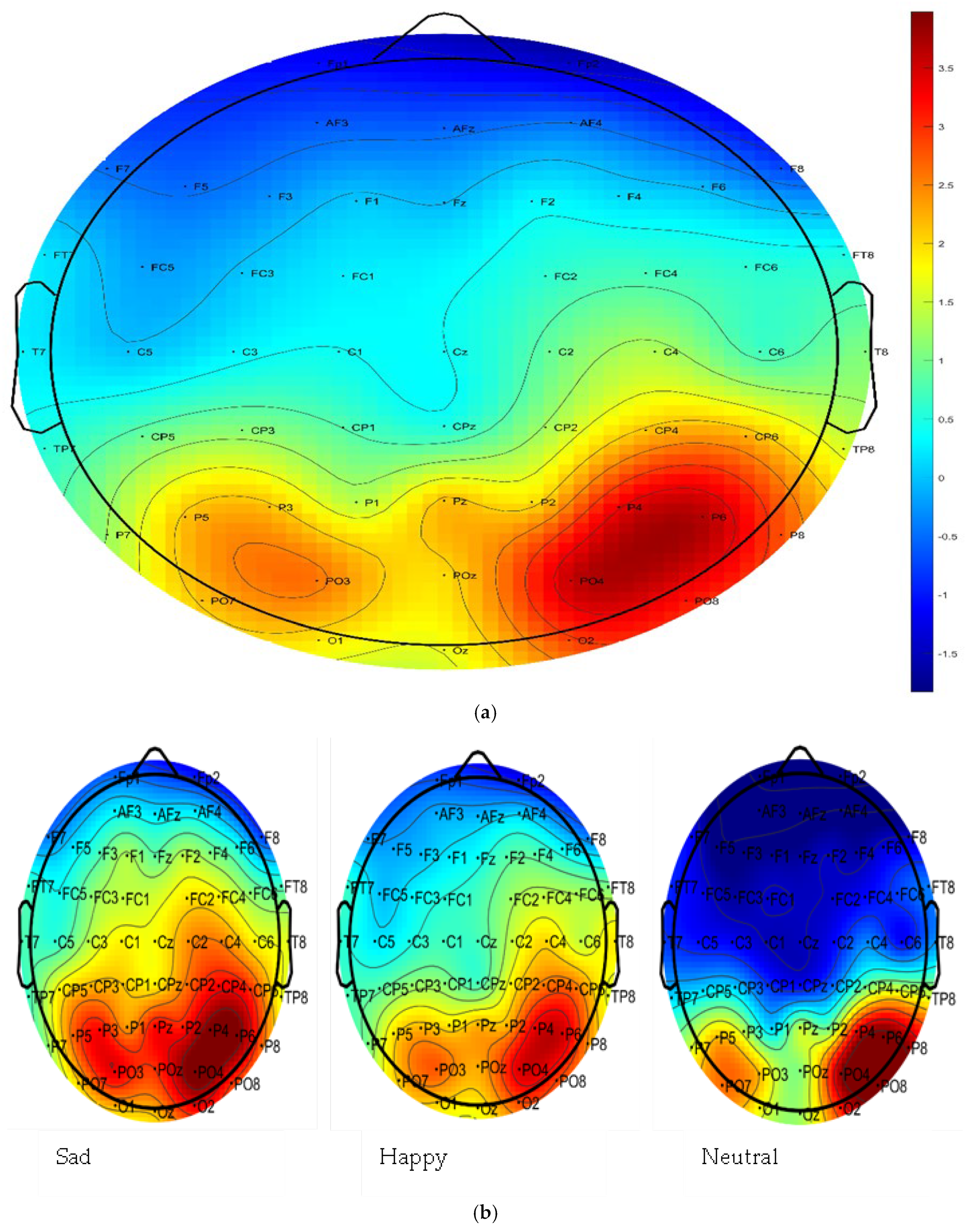

| Source | DF | F | p-Value | Partial η2 | |
|---|---|---|---|---|---|
| Within-subjects | Emotion | 2.78 | 4.46 | 0.01 | 0.10 |
| Region | 2.78 | 9.18 | 0.002 | 0.19 | |
| Emotion × Region | 4.156 | 20.07 | <0.001 | 0.34 | |
| Brooding | 1.38 | 0.000 | 0.986 | 0.00 | |
| ×Emotion | 2.76 | 2.55 | 0.086 | 0.06 | |
| ×Region | 2.76 | 0.18 | 0.74 | 0.005 | |
| ×Emotion × Region | 4.152 | 0.228 | 0.88 | 0.006 | |
| Reflection | 1.38 | 0.19 | 0.66 | 0.005 | |
| ×Emotion | 2.76 | 5.14 | 0.009 | 0.12 | |
| ×Region | 2.76 | 2.13 | 0.12 | 0.05 | |
| ×Emotion × Region | 4.152 | 1.60 | 0.19 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owens, M.; Renaud, J.; Healy, A.S. Reflection Rumination Reduces Negative Emotional Processing During Goal-Directed Behavior: An ERP Study. Behav. Sci. 2025, 15, 1081. https://doi.org/10.3390/bs15081081
Owens M, Renaud J, Healy AS. Reflection Rumination Reduces Negative Emotional Processing During Goal-Directed Behavior: An ERP Study. Behavioral Sciences. 2025; 15(8):1081. https://doi.org/10.3390/bs15081081
Chicago/Turabian StyleOwens, Max, Jessica Renaud, and Ashly S. Healy. 2025. "Reflection Rumination Reduces Negative Emotional Processing During Goal-Directed Behavior: An ERP Study" Behavioral Sciences 15, no. 8: 1081. https://doi.org/10.3390/bs15081081
APA StyleOwens, M., Renaud, J., & Healy, A. S. (2025). Reflection Rumination Reduces Negative Emotional Processing During Goal-Directed Behavior: An ERP Study. Behavioral Sciences, 15(8), 1081. https://doi.org/10.3390/bs15081081





