Cognitive and Non-Cognitive Predictors of Response to Cognitive Stimulation Interventions in Dementia: A Systematic Review Aiming for Personalization †
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy

2.2. Inclusion Criteria
- (a)
- studies that included participants older than 65 years of age and with a diagnosis of mild to moderate dementia. From a clinical and epidemiological standpoint, dementia occurring before the age of 65 is often classified as young-onset dementia (YOD) (Rossor et al., 2010), also known as early-onset dementia (EOD) (Johannessen & Möller, 2013), which may involve different etiologies, care needs, and psychosocial implications compared to late-onset dementia (Harvey et al., 2003; van Vliet et al., 2010). So, including younger individuals would therefore have introduced significant heterogeneity and potentially confounded our analysis. We also restricted inclusion to individuals with mild to moderate dementia, as cognitive stimulation interventions are specifically recommended for this subgroup. Evidence from both clinical trials and international guidelines indicates that cognitive stimulation interventions are most effective—and most appropriate—for people with mild to moderate levels of cognitive impairment (Spector et al., 2003; NICE, 2018). In people with severe dementia, cognitive stimulation interventions may be less feasible or have diminished efficacy due to greater functional limitations and lower cognitive reserve.
- (b)
- studies that included isolated cognitive stimulation treatment, according to Clare and Woods’ definition of cognitive stimulation as “engagement in a range of group activities and discussions aimed at general enhancement of cognitive and social functioning,” rather than interventions targeting a specific cognitive function. This type of multi-domain, non-specific stimulation is distinct from cognitive training (which targets specific domains such as memory or attention), cognitive rehabilitation (which is goal-oriented and individualized), and other psychosocial interventions (Clare & Woods, 2004; Spector et al., 2003; Woods et al., 2012). This category includes both standardized Cognitive Stimulation Therapy (CST) protocols as well as other cognitive stimulation interventions sharing similar principles but not strictly following manualized CST.
- (c)
- studies that included a passive control group that received standard care (treatment as usual) or no active treatment. This choice was made to ensure that any observed effects could be more confidently attributed to the cognitive stimulation intervention, minimizing potential confounding effects introduced by other simultaneous activities.
- (d)
- studies which evaluated multiple outcome domains—including functional, cognitive, psychological, and affective outcomes—assessed both before and after cognitive stimulation intervention and that underlined the influence of cognitive and non-cognitive aspects of people with dementia on the gains resulting from cognitive stimulation intervention. We specifically included studies that evaluated the effects of cognitive stimulation on multiple cognitive domains rather than on a single cognitive function, because dementia typically affects a range of cognitive abilities simultaneously. Measuring improvement across multiple domains allows for a more comprehensive and ecologically valid assessment of the intervention’s effectiveness. This approach aligns with existing literature emphasizing the importance of multi-domain cognitive assessments in dementia research to detect meaningful and generalizable changes (Clare & Woods, 2004; Yates et al., 2018). Therefore, our inclusion criteria aimed to capture studies that evaluate cognitive outcomes more comprehensively to inform the development of personalized and effective cognitive stimulation interventions. Furthermore, a key inclusion criterion was that studies had to explore the influence of individual cognitive and/or non-cognitive characteristics (e.g., baseline cognitive level, mood, education, age) on the outcomes of the intervention. This focus reflects the increasing recognition in dementia care and research of person-centered approaches, which emphasize the importance of identifying which individuals are more likely to benefit from specific interventions (Clare et al., 2019; Yates et al., 2018).
- (e)
- studies whose design was that of a randomized controlled trial (RCT) to select studies with demonstrated evidence of efficacy with higher standards. RCTs are widely considered the gold standard for evaluating the efficacy of interventions and for minimizing selection bias and confounding (Moher et al., 2009). This choice was made to ensure that the evidence reviewed was based on robust and methodologically sound designs capable of supporting causal inferences.
2.3. Exclusion Criteria
- (a)
- studies focusing on an adult population younger than 65 years, or patients without a diagnosis of dementia, or those presenting with other medical or psychiatric conditions such as major psychiatric disorders, stroke, or traumatic brain injury. This last decision was grounded in standard diagnostic criteria for dementia. DSM-5 and ICD-10 require that cognitive decline not be better explained by other neurological, psychiatric, or systemic medical disorders (APA, 2013; WHO, 1992).
- (b)
- studies that included isolated cognitive stimulation interventions focused on a single cognitive function (e.g., memory-only tasks), multifactorial intervention (e.g., combining physical activity, diet, and cognitive tasks without isolating the cognitive stimulation component), other psychosocial interventions (e.g., reminiscence therapy or Reality Orientation Therapy (ROT)) that do not involve cognitive stimulation, or combined cognitive stimulation interventions with pharmacological treatments, as this would have made it difficult to isolate the effect of the cognitive component. The decision to exclude studies focusing on cognitive stimulation targeting a single cognitive domain is supported by existing literature emphasizing the superior efficacy of multi-domain cognitive stimulation interventions. Clare and Woods (2004) clarify that cognitive stimulation is characterized by engaging multiple cognitive domains, distinguishing it from domain-specific cognitive training or rehabilitation. Systematic reviews, including those by Woods et al. (2012) and Bahar-Fuchs et al. (2013), provide evidence that multi-domain cognitive stimulation leads to broader improvements in cognitive functioning and daily living activities compared to interventions focusing on a single cognitive domain. Furthermore, clinical guidelines such as those from NICE (2018) recommend multi-domain approaches as standard practice for cognitive interventions in dementia care, reinforcing the rationale behind our exclusion criteria.
- (c)
- studies with an active control group, such as those engaging participants in alternative cognitive, social, or behavioral activities (e.g., recreational groups, psychoeducation, or other non-specific engagement strategies). Active controls, while useful in some contexts, may reduce the ability to isolate the unique contribution of cognitive stimulation, particularly in a systematic review aiming to explore moderators of response.
- (d)
- studies that did not include cognitive and non-cognitive outcomes or that focused solely on improvement of a single cognitive function or did not explore the relationship between individual characteristics and intervention outcomes (e.g., studies reporting only overall group-level effects, without analysis of influencing factors).
- (e)
- studies without a control group or with only pre- and post-treatment comparison within a single group.
- (f)
- furthermore, protocol studies, abstracts, or posters from congresses and studies with no full text available were excluded.
2.4. Study Selection and Data Extraction
2.5. Study Risk of Bias Assessment
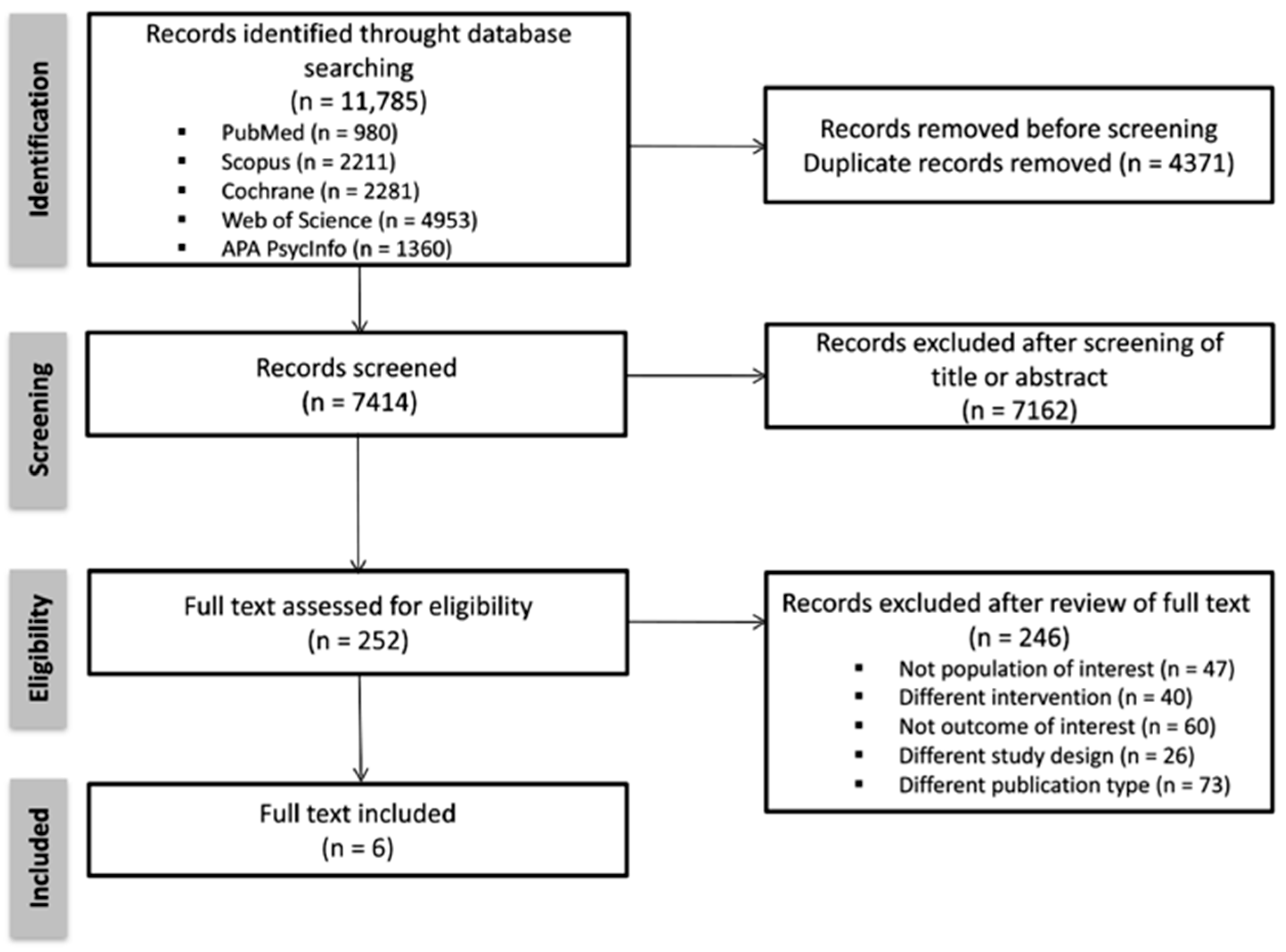
3. Results
3.1. Study Selection
3.2. Risk of Bias Analysis
3.3. Study Descriptions
3.4. Cognitive and Non-Cognitive Factors Associated with Greater Benefit from Cognitive Stimulation
4. Discussion
5. Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAS-Cog | Alzheimer’s Disease Assessment Scale–Cognitive Subscale |
| ADCS-ADL | Alzheimer’s Disease Cooperative Study–Activities of Daily Living |
| BDI | Beck’s Depression Inventory |
| CDRS | Clinical Dementia Rating Scale |
| SF-12 | Short Form 12 items Health Survey Questionnaire |
| DRS | Dementia Rating Scale |
| HADS | Hospital Anxiety and Depression Scale |
| MMSE | Mini Mental State Examination |
| NPI-NH | Neuropsychiatric Inventory–Nursing Home version |
| QCPR | Quality of the Caregiving Relationship Questionnaire |
| QoL | Quality of life |
| QoL-AD | Quality of Life–Alzheimer Disease |
| WAIS-R | Wechsler Adult Intelligence Scale–Revised |
| WHODAS 2.0 | World Health Organization Disability Assessment Schedule 2.0 |
| WHOQOL-Brief | World Health Organization–Quality of Life |
| WMS-R | Wechsler Memory Scale–Revised |
| ZBI | Zarit Burden Inventory |
References
- Aguirre, E., Evans, S. C., & Orrel, M. (2014). An evaluation of the FMAP (Formative Method for Adapting Psychotherapy) to improve the cultural relevance of cognitive stimulation therapy. Dementia, 13(5), 630–645. [Google Scholar]
- Aguirre, E., Woods, R. T., Spector, A., & Orrell, M. (2013). Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Research Reviews, 12(1), 253–262. [Google Scholar] [CrossRef]
- Alvares-Pereira, G., Silva-Nunes, M. V., & Spector, A. (2021). Validation of the cognitive stimulation therapy (CST) program for people with dementia in Portugal. Aging Ment Health, 25(6), 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Alvares Pereira, S., Duarte Santos, J., Sousa, L., & Orrell, M. (2022). Adaptation and implementation of Cognitive Stimulation Therapy for older adults with dementia in Portugal: A feasibility study. Dementia, 21(8), 2703–2719. [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- Bahar-Fuchs, A., Clare, L., & Woods, B. (2013). Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. The Cochrane Database of Systematic Reviews, 2013(6), CD003260. [Google Scholar] [CrossRef]
- Booth, A., Curran, J., Jureidini, R., & Saini, P. (2023). Personalization in non-pharmacological interventions for dementia: A systematic review. Journal of Dementia Care, 31(2), 45–56. [Google Scholar]
- Buckley, J. S., & Salpeter, S. R. (2015). A risk-benefit assessment of dementia medications: Systematic review of the evidence. Drugs & Aging, 32(6), 453–467. [Google Scholar] [CrossRef]
- Buschert, V. C., Friese, U., Teipel, S. J., Schneider, P., Merensky, W., Rujescu, D., Möller, H. J., Hampel, H., & Buerger, K. (2011). Effects of a newly developed cognitive intervention in Amnestic Mild Cognitive Impairment and Mild Alzheimer’s disease: A pilot study. Journal of Alzheimer’s Disease, 25(4), 679–694. [Google Scholar] [CrossRef]
- Cafferata, R. M. T., Hicks, B., & von Bastian, C. C. (2021). Effectiveness of cognitive stimulation for dementia: A systematic review and meta-analysis. Psychological Bulletin, 147(5), 455–476. [Google Scholar] [CrossRef]
- Carbone, E., Piras, F., Pastore, M., & Borella, E. (2022). The role of individual characteristics in predicting short-and long-term cognitive stimulation therapy for mild-to-moderate dementia. Frontiers in Aging Neuroscience, 13, 811127. [Google Scholar] [CrossRef]
- Chen, X., Zhao, Y., He, C., & Cao, Y. (2022). Cognitive stimulation therapy for dementia: A systematic review and meta-analysis of randomized controlled trials. Aging & Mental Health, 26(9), 1812–1824. [Google Scholar]
- Clare, L., Kudlicka, A., Oyebode, J. R., Jones, R. W., Bayer, A., Leroi, I., Kopelman, M., James, I. A., Culverwell, A., Pool, J., Brand, A., Henderson, C., Hoare, Z., Knapp, M., & Woods, B. (2019). Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: A multicentre randomised controlled trial (the GREAT trial). International Journal of Geriatric Psychiatry, 34(5), 709–721. [Google Scholar] [CrossRef]
- Clare, L., & Woods, R. (2004). Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: A review. Neuropsychological Rehabilitation, 14(4), 385–401. [Google Scholar] [CrossRef]
- Cove, J., Jacobi, N., Donovan, H., Orrell, M., Stott, J., & Spector, A. (2014). Effectiveness of weekly cognitive stimulation therapy for people with dementia and the additional impact of enhancing cognitive stimulation therapy with a carer training program. Clinical Interventions in Aging, 11(9), 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M., Faleafa, M., Orrell, M., & Fa’alau, F. (2025). Cognitive stimulation therapy for Māori with dementia: A culturally adapted pilot study in Aotearoa New Zealand. Australasian Psychiatry, 33(1), 102–110. [Google Scholar]
- Geda, Y. E., Schneider, L. S., Gitlin, L. N., Miller, D. S., Smith, G. S., Bell, J., Evans, J., Lee, M., Porsteinsson, A., Lanctôt, K. L., Rosenberg, P. B., Sultzer, D. L., Francis, P. T., Brodaty, H., Padala, P. P., Onyike, C. U., Ortiz, L. A., Ancoli-Israel, S., Bliwise, D. L., … Lyketsos, C. G. (2013). Neuropsychiatric symptoms in alzheimer’s disease: Past progress and anticipation of the future. Alzheimer’s & Dementia, 9(5), 602–608. [Google Scholar]
- Gitlin, L. N., Kales, H. C., & Lyketsos, C. G. (2020). Nonpharmacologic management of behavioral symptoms in dementia. JAMA, 322(9), 857–865. [Google Scholar] [CrossRef]
- Gomez-Soria, I., Iguacel, I., Aguilar-Latorre, A., Peralta-Marrupe, P., Latorre, E., Cuenca Zaldivar, J. N., & Calatayud, E. (2023). Cognitive stimulation and cognitive results in older adults: A systematic review and meta-analysis. Archives of Gerontology and Geriatrics, 104, 104807. [Google Scholar] [CrossRef]
- Hall, L., Orrell, M., Stott, J., & Spector, A. (2013). Cognitive stimulation therapy (CST): Neuropsychological mechanisms of change. International Psychogeriatrics, 25(3), 479–489. [Google Scholar] [CrossRef]
- Harvey, R. J., Skelton-Robinson, M., & Rossor, M. N. (2003). The prevalence and causes of dementia in people under the age of 65 years. Journal of Neurology, Neurosurgery & Psychiatry, 74(9), 1206–1209. [Google Scholar] [CrossRef]
- Hess, T. M., Hinson, J. T., & Statham, J. A. (2004). Explicit and implicit stereotype activation effects on memory: Do age and awareness moderate the impact of priming? Psychology and Aging, 19(3), 495–505. [Google Scholar] [CrossRef]
- Holopainen, A., Siltanen, H., & Okkonen, A. (2017). Factors associated with the quality of life of people with dementia and quality of life-improving interventions: Scoping review. Dementia, 18(4), 1507–1537. [Google Scholar] [CrossRef]
- Hong, Q. N., Pluye, P., Fabregues, S., Bartlett, G., Boardman, F., Cargo, M., Dagenais, P., Gagnon, M. P., Griffiths, F., Nicolau, B., O’Cathain, A., Rousseau, M. C., & Vedel, I. (2018). Mixed Methods Appraisal Tool (MMAT), version 2018 (Registration of Copiright (#1148552)). Canadian Intellectual Property Office, Industry Canada. [Google Scholar]
- Hsieh, S., Mohan, A., Sato, K., Tay, J., & Yeo, T. (2024). Effects of a 12-month multicomponent cognitive intervention on cognition and activities of daily living in mild dementia: Results from the SADEM trial. Alzheimer’s Research & Therapy, 16(1), 12. [Google Scholar]
- ISTAT. (2018). La salute mentale nelle varie fasi della vita (Report). Istituto Nazionale di Statistica. Available online: https://www.istat.it/it/files/2018/07/Report_Salute_mentale.pdf (accessed on 3 August 2025).
- Istituto Superiore di Sanità (ISS). (2024). Linea Guida Nazionale per la Diagnosi e il Trattamento della Demenza. Istituto Superiore di Sanità. [Google Scholar]
- Johannessen, A., & Möller, A. (2013). Experiences of persons with early-onset dementia in everyday life: A qualitative study. Dementia, 12(4), 410–424. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, P. A., Aquino, J. P., Bérard, A., Gzil, F., Andrieu, S., Banerjee, S., Brémond, F., Buée, L., Cohens-Mansfield, J., Mangialasche, F., Platel, H., Salmon, E., & Robert, P. (2016). Dementia beyond 2025: Knowledge and uncertainties. Dementia: The International Journal of Social Research and Practice, 15(1), 6–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. J., Yang, Y. S., Oh, J. G., Oh, S., Choi, H., Kim, K. H., & Kim, S. H. (2016). Effectiveness of a community-based multidomain cognitive intervention program in patients with Alzheimer’s disease. Geriatrics Gerontology, 16(2), 191–199. [Google Scholar] [CrossRef]
- Knapp, M., Thorgrimsen, L., Patel, A., Spector, A., Hallam, A., Woods, B., & Orrel, M. (2006). Cognitive stimulation therapy for people with dementia: Cost-effectiveness analysis. British Journal of Psychiatry, 118, 574–580. [Google Scholar] [CrossRef]
- Kwok, T., Wong, A., Chan, G., Shiu, Y. Y., Lam, K. C., Toung, D., Ho, D. W. H., & Ho, F. (2013). Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clinical Interventions in Aging, 8, 213–219. [Google Scholar] [CrossRef]
- Leroi, I., Vatter, S., & Carter, L. A. (2019). Parkinson’s-adapted cognitive stimulation therapy: A pilot randomized controlled clinical trial. Therapeutic Advances in Neurological Disorders, 12, 1756286419852217. [Google Scholar] [CrossRef]
- Leung, P., Yates, L., Orgeta, V., Hamidi, F., & Orrell, M. (2017). The experiences of people with dementia and their carers participating in individual cognitive stimulation therapy. International Journal of Geriatric Psychiatry, 32(12), e34–e42. [Google Scholar] [CrossRef]
- Livingston, G., Huntley, J., Liu, K. Y., Costafreda, S. G., Selbæk, G., Alladi, S., Ames, D., Banerjee, S., Burns, A., Brayne, C., Fox, N. C., Ferri, C. P., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Nakasujja, N., Rockwood, K., … Mukadam, N. (2024). Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. The Lancet, 404(10452), 572–628. [Google Scholar] [CrossRef]
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., Ballard, C., Banerjee, S., Burns, A., Cohen-Mansfield, J., Cooper, C., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Larson, E. B., Ritchie, K., Rockwood, K., Sampson, E. L., … Mukadam, N. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734. [Google Scholar] [CrossRef]
- Lopez, C., Sànchez, J. L., & Martìn, J. (2020). The effect of cognitive stimulation on the progression of cognitive impairment in subjects with Alzheimer’s disease. Applied Neuropsychology: Adult, 29(1), 90–99. [Google Scholar] [CrossRef]
- Lu, S., Zhang, A. Y., Liu, T., Choy, J. C. P., Ma, M. S. L., Wong, G., & Lum, T. (2021). Degree of personalisation in tailored activities and its effect on behavioural and psychological symptoms and quality of life among people with dementia: A systematic review and meta-analysis. BMJ Open, 11(11), e048917. [Google Scholar] [CrossRef]
- Lu, Y., Zhu, M., Liu, W., & Li, J. (2021). Personalization of non-pharmacological interventions for dementia: Conceptual framework and practice. Aging & Mental Health, 25(10), 1885–1893. [Google Scholar]
- McDermott, O., Charlesworth, G., Hogervorst, E., Stoner, C., Moniz-Cook, E., Spector, A., Csipke, E., & Orrell, M. (2019). Psychosocial interventions for people with dementia: A synthesis of systematic reviews. Aging & Mental Health, 23(4), 393–403. [Google Scholar]
- Middelstadt, J., Folkerts, A. K., Blawath, S., & Kalbe, E. (2016). Cognitive stimulation for people with dementia in long-term care facilities: Baseline cognitive level predicts cognitive gains, moderated by depression. Journal of Alzheimer’s Disease, 54(1), 253–268. [Google Scholar] [CrossRef] [PubMed]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S., Madella, I., Zangrossi, A., Bigolin, A., Tomasi, C., Michieletto, M., Villani, D., Di Giovanni, G., & Mapelli, D. (2016). Cognitive reserve in dementia: Implications for cognitive training. Frontiers in Aging Neurosciences, 8, 84. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. (2018). Dementia: Assessment, management and support for people living with dementia and their carers (NICE guideline NG97). National Institute for Health and Care Excellence. [Google Scholar]
- Neely, A., Vikstrom, S., & Josephsson, S. (2009). Collaborative memory intervention in dementia: Caregiver participation matters. Neuropsychological Rehabilitation, 19(5), 696–715. [Google Scholar] [CrossRef]
- Norton, M. C., Piercy, K. W., Rabins, R. C., Green, R. C., Breitner, J. C. S., Ostbye, T., Corcoran, C., Welsh-Bohmer, K. A., Lyketsos, C. G., & Tschanz, J. T. (2009). Caregiver-recipient closeness and symptom progression in alzheimer disease. The cache country dementia progression study. Journal of Gerontology: Psychological Sciences, 64(5), 560–568. [Google Scholar] [CrossRef]
- Olazarán, J., Muñiz, R., Reisberg, B., Peña-Casanova, J., del Ser, T., Cruz-Jentoft, A. J., Serrano, P., Navarro, E., García de la Rocha, M. L., Frank, A., Galiano, M., Fernández-Bullido, Y., Serra, J. A., González-Salvador, M. T., & Sevilla, C. (2004). Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology, 63(12), 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Onder, G., Zanetti, O., Giacobini, E., Frisoni, G. B., Bartorelli, L., Carbone, G., Lambertucci, P., Silveri, M. C., & Bernabei, R. (2005). Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: Randomised control trial. The British Journal of Psychiatry, 187, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, S., Gibbor, L., Carr, C., & Spector, A. (2021). Group-based cognitive stimulation therapy for dementia: A qualitative study on experiences of group interactions. Aging Ment Health, 25(6), 991–998. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V., Mukadam, N., Sommerlad, A., & Livingston, G. (2015). Psychosocial interventions for people with dementia: A case report on personalized cognitive stimulation. Dementia, 14(3), 395–404. [Google Scholar]
- Orrell, M., Yates, L., Leung, P., Kang, S., Hoare, Z., Whitaker, C., Burns, A., Knapp, M., Leroi, I., Moniz-Cook, E., Pearson, S., Simpson, S., Spector, A., Roberts, S., Russel, I., de Waal, H., Woods, R. T., & Orgeta, V. (2012). The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial. PLoS Medicine, 14(3), e1002269. [Google Scholar] [CrossRef]
- Paddick, S. M., Mkenda, S., Mbowe, G., Kisoli, A., Gray, W. K., Dotchin, C. L., Ternet, L., Ogunniyi, A., Kissima, J., Olakehinde, O., Mushi, D., & Walker, R. W. (2017). Cognitive stimulation therapy as a sustainable intervention for dementia in Sub-Saharan Africa: Feasibility and clinical efficacy using a stepped-wedge design. International Psychogeriatrics, 29(6), 979–989. [Google Scholar] [CrossRef]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. [Google Scholar] [CrossRef]
- Parke, B., & Hunter, S. (2023). The impact of tailored activities on quality of life and behavior in dementia: A Cochrane review update. Cochrane Database of Systematic Reviews, 3, CD012345. [Google Scholar]
- Quayhagen, M. P., & Quayhagen, M. (2001). Testing of a cognitive stimulation intervention for dementia caregiving dyads. Neuropsychological Rehabilitation, 11(3–4), 319–332. [Google Scholar] [CrossRef]
- Quayhagen, M. P., Quayhagen, M., Corbeil, R. R., Roth, P. A., & Rodgers, J. A. (1995). A dyadic remediation program for care recipients with dementia. Nursing Research, 44(3), 153–159. [Google Scholar] [CrossRef]
- Rossor, M. N., Fox, N. C., Mummery, C. J., Schott, J. M., & Warren, J. D. (2010). The diagnosis of young-onset dementia. The Lancet Neurology, 9(8), 793–806. [Google Scholar] [CrossRef]
- Saito, T., Murata, C., Saito, M., Takeda, T., & Kondo, K. (2018). Influence of social relationship domains and their combinations on incident dementia: A prospective cohort study. J Epidemiol Community Health, 72(1), 7–12. [Google Scholar] [CrossRef]
- Sikkes, S. A. M., Tang, Y., Jutten, R. J., Wesselman, L. M. P., Turkstra, L. S., Brodaty, H., Clare, L., Cassidy-Eagle, E., Cox, K. L., Chételat, G., Dautricourt, S., Dhana, K., Dodge, H., Dröes, R. M., Hampstead, B. M., Holland, T., Lampit, A., Laver, K., Lutz, A., … Bahar-Fuchs, A. (2021). Toward a theory-based specification of non-pharmacological treatments in aging and dementia: Focused reviews and methodological recommendations. Alzheimer’s & Dementia, 17(2), 255–270. [Google Scholar]
- Sopina, E., & Sørensen, J. (2018). Decision modelling of non-pharmacological interventions for individuals with dementia: A systematic review of methodologies. Health Economics Review, 8(1), 8. [Google Scholar] [CrossRef] [PubMed]
- Spector, A., Gardner, C., & Orrell, M. (2011). The impact of Cognitive Stimulation Therapy groups on people with dementia: Views from participants, their carers and group facilitators. Aging Ment Health, 15(8), 945–949. [Google Scholar] [CrossRef] [PubMed]
- Spector, A., Thorgrimsen, L., Woods, B., Royan, L., Davies, S., Butterworth, M., & Orrell, M. (2003). Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: Randomised controlled trial. The British Journal of Psychiatry, 183(3), 248–254. [Google Scholar] [CrossRef]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. [Google Scholar] [CrossRef]
- Stern, Y. (2006). Cognitive reserve and Alzheimer disease. Alzheimer Disease & Associated Disorders, 20(2), 112–117. [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. [Google Scholar] [CrossRef]
- Sun, Y., Zhang, X., & Wang, Z. (2022). Comparative Effectiveness of 3 Settings of Cognitive Stimulation Therapy on Cognition and Quality of Life for People With Dementia: A Systematic Review and Network Meta-analysis. Journal of the American Medical Directors Association, 23(3), 461–467.e1. [Google Scholar] [CrossRef]
- van Vliet, D., de Vugt, M. E., Bakker, C., Koopmans, R. T., & Verhey, F. R. (2010). Impact of early onset dementia on caregivers: A review. International Journal of Geriatric Psychiatry, 25(11), 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Warmoth, K., Morgan-Trimmer, S., Kudlicka, A., Toms, G., James, I. A., Woods, B., & GREAT Trial Team. (2022). Reflections on personalized cognitive rehabilitation intervention: Experiences of people living with dementia and their carers participating in the GREAT trial. Neuropsychological Rehabilitation, 32(2), 268–286. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, L. A., McLaughlin, A. C., & Allaire, J. C. (2012). Individual differences in response to cognitive training: Using a multi-modal, attentionally demanding game-based intervention for older adults. Computers in Human Behavior, 28(4), 1091–1096. [Google Scholar] [CrossRef]
- WHO. (1992). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. World Health Organization. [Google Scholar]
- Wong, G. H. Y., Chan, C. L. W., Yip, B. H. K., & Lam, L. C. W. (2018). Cultural adaptation of Cognitive Stimulation Therapy (CST) in Hong Kong: A qualitative study. Aging & Mental Health, 22(6), 768–774. [Google Scholar]
- Wong, Y. L., Cheng, C. P. W., Wong, C. S. M., Wong, S. N., Wong, H. L., Tse, S., Wong, G. H. Y., & Chan, W. C. (2021). Cognitive stimulation for persons with dementia: A systematic review and meta-analysis. East Asian Archives of Psychiatry, 31(3), 55–66. [Google Scholar] [CrossRef]
- Woods, B., Aguirre, E., Spector, A., & Orrell, M. (2012). Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Review, 2, CD005562. [Google Scholar] [CrossRef]
- Woods, B., Thorgrimsen, L., Spector, A., Royan, L., & Orrell, M. (2006). Improved quality of life and cognitive stimulation in dementia. Aging and Mental Health, 10, 219–226. [Google Scholar] [CrossRef]
- World Health Organization. (2017). Global action plan on the public health response to dementia 2017–2025. World Health Organization. [Google Scholar]
- Xiang, C., & Zhang, Y. (2024). Comparison of cognitive intervention strategies for individuals with alzheimer’s disease: A systematic review and network meta-analysis. Neuropsychology Review, 34(2), 402–416. [Google Scholar] [CrossRef]
- Yates, L. A., Yates, J., Orrell, M., Spector, A., & Woods, B. (2018). Cognitive stimulation therapy for dementia. Clinics in Geriatric Medicine, 34(4), 653–665. [Google Scholar] [CrossRef]
- Young, D. K., Kwok, T. C., & Ng, P. Y. (2014). A single blind randomized control trial on support groups for Chinese persons with mild dementia. Clinical Interventions in Aging, 9, 2105–2112. [Google Scholar] [CrossRef]
| Parameters | |
|---|---|
| Participants | Adults aged 65 years old or more and with a diagnosis of mild to moderate dementia. |
| Interventions | Cognitive Stimulation (CS) interventions (including manualized CST protocols and other cognitive stimulation programs). |
| Control | Treatment as usual (standard care) or no active treatment. |
| Outcomes | Functional, cognitive, psychological, and affective outcomes evaluated before and after the cognitive stimulation intervention. Influence of cognitive and non-cognitive aspects of people with dementia on the gains resulting from cognitive stimulation intervention. |
| Study design | Randomized controlled trials (RCTs). |
| Category of Study Design | Methodological Quality Criteria | Cove et al. (2014) Quantitative RCT | Kwok et al. (2013) Quantitative RCT | Middelstadt et al. (2016) Quantitative RCT | Neely et al. (2009) Quantitative RCT | Paddick et al. (2017) Quantitative RCT | Quayhagen et al. (1995) Quantitative RCT |
|---|---|---|---|---|---|---|---|
| 1. Screening questions | S1. Are there clear research questions? | Yes | Yes | Yes | Yes | Yes | Yes |
| S2. Do the collected data allow the study to address the research questions? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 2. Quantitative randomized controlled trials | 2.1. Is randomization appropriately performed? | Yes | Cannot tell | Yes | Cannot tell | Yes | Cannot tell |
| 2.2. Are the groups comparable at baseline? | Yes | No | Yes | Yes | Yes | Yes | |
| 2.3. Are there complete outcome data? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 2.4. Are the outcome assessors blinded to the intervention provided? | Yes | Yes | Yes | Cannot tell | Yes | Cannot | |
| 2.5. Did the participants adhere to the assigned intervention? | Yes | Yes | Yes | Yes | Yes | Yes |
| Authors, Region, and Study Design | Total Sample Size | Intervention Group | Control Group | Frequency and Duration of Intervention | Content of Intervention | Assessment | Main Results | |
|---|---|---|---|---|---|---|---|---|
| Experimental Group | Control Group | |||||||
| Cove et al. (2014) UK RCT | 68 | 21 (CST plus carer training) 10 F/11 M Age 75.4 ± 5.56 24 (CST) 9 F/15 M Age 76.8 ± 6.62 | 23 13 F/10 M Age 77.8 ± 7.47 | 45 min One time a week 14 weeks | Standardized CST manual with an RO board | Waitlist | MMSE ADAS-Cog QoL-AD QCPR | No changes in cognition (MMSE), quality of life (QoL-AD), or quality of carer–patient relationship (QCPR) over time and no significant differences between groups at follow-up. Significant decline in cognition between baseline and follow-up as assessed by ADAS-Cog, but no differences between groups. |
| Kwok et al. (2013) Hong Kong RCT | 176 150 F/26 M Age 75.41 ± 7.31 Years of Education 3.54 ± 3.77 | 86 75 F 7 11 M Age 77.41 ± 6.75 Years of education 2.92 ± 3.36 | 90 75 F/15 M Age 73.50 ± 7.35 Years of Education 4.17 ± 4.05 | 60 min One time a week 8 weeks | Active Mind, CST version targeted to Chinese culture | Treatment as usual | CDRS MMSE Cantonese version SF12 | Improvements in cognition (CDRS) and QoL (SF12) in the IG. Both the IG and the CG showed improvement after intervention, but this was more prominent in the IG. |
| Middelstadt et al. (2016) Germany RCT | 71 60 F/11 M Age 86.37 ± 4.45 | 36 30 F/6 M Age 86.25 ± 4.76 | 35 30 F/5 M Age 86.49 ± 4.17 | 60 min Two times a week 8 weeks | NEUROvitalis Sinnreich | Usual care | ADAS-Cog QoL NPI-NH ADCS-ADL | No significant interaction effects regarding Time x Group. Significant within-subject effect regarding Time (pre-test to follow-up) for QoL and ADL scale, indicating that both worsened. |
| Neely et al. (2009) Sweden RCT | 30 couples | Collaborative Intervention: 10 7 F/3 M Age 74.4 ± 6.0 Individual Intervention: 10 4 F/6 M Age 74.8 ± 6.7 | 10 4 F/6 M Age 77.0 ± 6.6 | 60 min One time a week 8 weeks | Cognitive training | Did not receive any intervention | Objective recall random/clustered recall of non-categorizable words ZBI BDI Collaborative object recall random Health Questionnaire MMSE Digit Span forward and backward WAIS-R Verbal fluency task Digit Symbol WAIS-R Verbal Ability Swedish Synonym Test | People with dementia in the collaborative group improved their memory performance from pre-test to post-test compared to the two other groups. No improvements in collaborative memory performance as a function of training. Separately, caregivers showed a reliable decrease in recall performance from pre-test to post-test, whereas their spouses with dementia showed an improvement in object recall. No changes in measures of reported depressive symptoms or in perceived caregiving burden for the caregivers as a function of the intervention; however, there was an increase in depression scores for all groups, which may reflect a response to disease progression. |
| Paddick et al. (2017) Sub-Saharan Africa Stepped-wedge design | 34 29 F/5 M Age 80.0 Years of Education 10 | Immediate Start Group 1: 8 8 F/0 M Age 84.0 Years of Education 1 Group 2: 8 6 F/2 M Age 80.0 Years of Education 7 | Delayed Start Group 3: 8 5 F/3 M Age 83.5 Years of Education 1 Group 4: 10 10 F/0 M Age 80.0 Years of Education 1 | Two times a week 7 weeks | CST-SSA (CST adapted version for sub-Saharan Africa) | Delayed start groups acted as control | WHOQOL-Brief WHODAS 2.0 ADAS-Cog HADS NPI ZBI | Significant improvements in cognition (ADAS-Cog) and in the physical health domain of the WHOQOL-Bref. According to the caregivers, there were significant improvements in symptoms of anxiety and the number and severity of BPS and distress caused by BPS of dementia in the person they cared for, as assessed by the NPI. |
| Quayhagen et al. (1995) USA RCT | 78 27 F/51 M Age 73.6 ± 8.0 Years of Education 12.6 ± 4.1 | 25 | 28 placebo group 25 control group | 60 min One time a day 6 days | Active cognitive stimulation program | Placebo: activities similar to those in the experimental group Control: wait-list | DRS WMS-R F-A-S test Geriatric Coping Schedule Visual Memory Span Digit Span Memory and Behavior Problems Checklist | Care recipients in the IG had improvements post-treatment in overall cognitive functioning (word fluency, recall of non-verbal material). There was a tendency in all outcomes for the IG to regress toward baseline by the ninth month. Instead, the CG declined post-treatment and at the 9-month follow-up; the placebo group remained at baseline maintenance level over time. |
| Demographic Factors | Cognitive Factors | Emotional Factors | Social Factors | Quality of Life | |
|---|---|---|---|---|---|
| Cove et al. (2014) | Baseline level of cognitive functioning | ||||
| Kwok et al. (2013) | Education level | Baseline level of depressive symptoms | Baseline level of quality of life | ||
| Middelstadt et al. (2016) | Baseline level of cognitive functioning | ||||
| Neely et al. (2009) | Active participation of caregiver during CS session | ||||
| Paddick et al. (2017) | Education level | ||||
| Quayhagen et al. (1995) | Gender |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, L.; Despini, G.; Quartarone, M.; Calabrese, L.; Brigiano, M.; Trolese, S.; Annini, A.; Chirico, I.; Ottoboni, G.; Casagrande, M.; et al. Cognitive and Non-Cognitive Predictors of Response to Cognitive Stimulation Interventions in Dementia: A Systematic Review Aiming for Personalization. Behav. Sci. 2025, 15, 1069. https://doi.org/10.3390/bs15081069
Forte L, Despini G, Quartarone M, Calabrese L, Brigiano M, Trolese S, Annini A, Chirico I, Ottoboni G, Casagrande M, et al. Cognitive and Non-Cognitive Predictors of Response to Cognitive Stimulation Interventions in Dementia: A Systematic Review Aiming for Personalization. Behavioral Sciences. 2025; 15(8):1069. https://doi.org/10.3390/bs15081069
Chicago/Turabian StyleForte, Ludovica, Giulia Despini, Martina Quartarone, Lara Calabrese, Marco Brigiano, Sara Trolese, Alice Annini, Ilaria Chirico, Giovanni Ottoboni, Maria Casagrande, and et al. 2025. "Cognitive and Non-Cognitive Predictors of Response to Cognitive Stimulation Interventions in Dementia: A Systematic Review Aiming for Personalization" Behavioral Sciences 15, no. 8: 1069. https://doi.org/10.3390/bs15081069
APA StyleForte, L., Despini, G., Quartarone, M., Calabrese, L., Brigiano, M., Trolese, S., Annini, A., Chirico, I., Ottoboni, G., Casagrande, M., & Chattat, R. (2025). Cognitive and Non-Cognitive Predictors of Response to Cognitive Stimulation Interventions in Dementia: A Systematic Review Aiming for Personalization. Behavioral Sciences, 15(8), 1069. https://doi.org/10.3390/bs15081069








