Examining the Role of Neuroticism Polygenic Risk in Late Life Cognitive Change: A UK Biobank Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measures
- (1)
- Reasoning: Reasoning or fluid intelligence refers to logic and reasoning abilities that are distinct from acquired knowledge and are crucial for problem-solving. In the assessment, participants were presented with 13 multiple-choice questions and were instructed to complete as many questions as possible within a two-minute timeframe. The questions covered numeric addition (e.g., adding the numbers 1, 2, 3, 4, 5), word interpolation (e.g., Bud is to flower as child is to?), arithmetic sequence recognition (e.g., 150… 137… 125… 114… 104… What comes next?), and subset inclusion logic (e.g., If some flinks are plinks and some plinks are stinks, then some flinks are definitely stinks?). Subsequently, fluid intelligence was measured by totaling the number of questions answered correctly.
- (2)
- Processing Speed: The symbol digit substitution test was employed as a web-based questionnaire to assess processing speed [27]. During the test, participants were allotted approximately three minutes to match symbols in a series of grids to corresponding numbers based on a provided key. The processing speed score was determined by the number of matches made correctly.
- (3)
- Visual Attention: The trail making test (part A), implemented as a web-based questionnaire in the UK Biobank, was employed to assess visual attention. In this test, participants were asked to draw lines to connect circled numbers in a numerical sequence (e.g., 1–2–3). The score was derived from the time in seconds taken to accurately connect all the circles, with a higher time indicating poorer performance. For some participants, a value of 0 was recorded as the time taken to complete the test. These participants were excluded from the analysis as a time 0 indicated that they did not complete the test.
- (4)
- Memory: Episodic memory was evaluated using the pairs matching test. During the assessment, participants were tasked with memorizing the positions of as many matching pairs of cards as possible. Subsequently, the cards were turned face down, and participants were required to identify as many pairs as they could with the fewest attempts. The test consisted of two or three rounds: the first round with 3 pairs of cards, the second with 6 pairs, and the third with 8 pairs. Participants progressed to the third round only if they made 0 errors or 1 error in the second round. The score for this test was determined by the number of incorrect matches in a round, where a higher score indicated poorer performance.
2.3. DNA Collection, Genotyping and Genetic Quality Control
2.4. Summary Statistics Quality Control
2.5. Polygenic Score Calculation
2.6. Statistical Analysis
2.7. Sensitivity Analysis
3. Results
3.1. Demographic Characteristics
3.2. Cross-Sectional Analysis
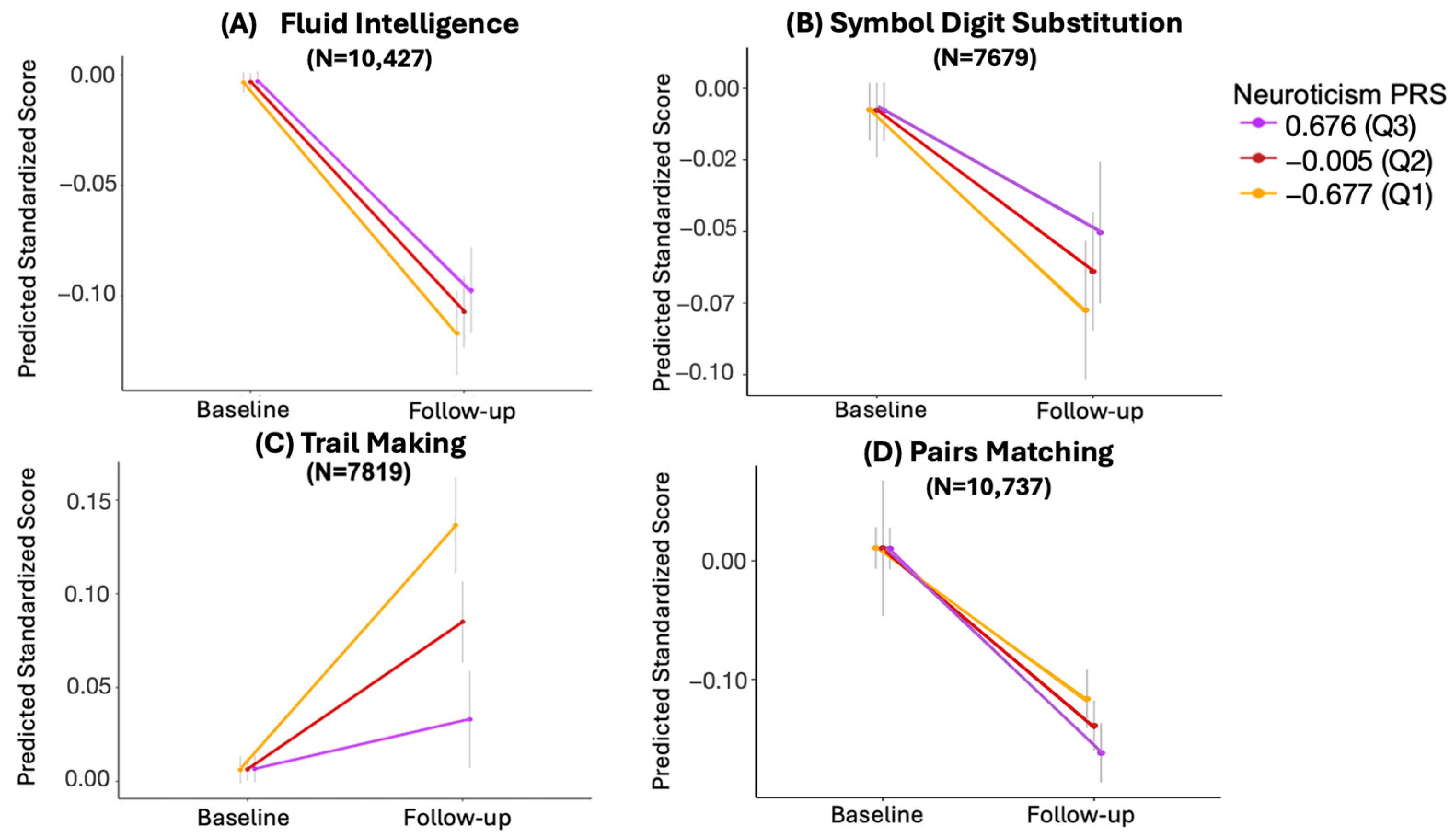
3.3. Longitudinal Analysis
3.3.1. Trail Making
3.3.2. Pairs Matching
3.3.3. Symbol Digit Substitution
3.3.4. Fluid Intelligence
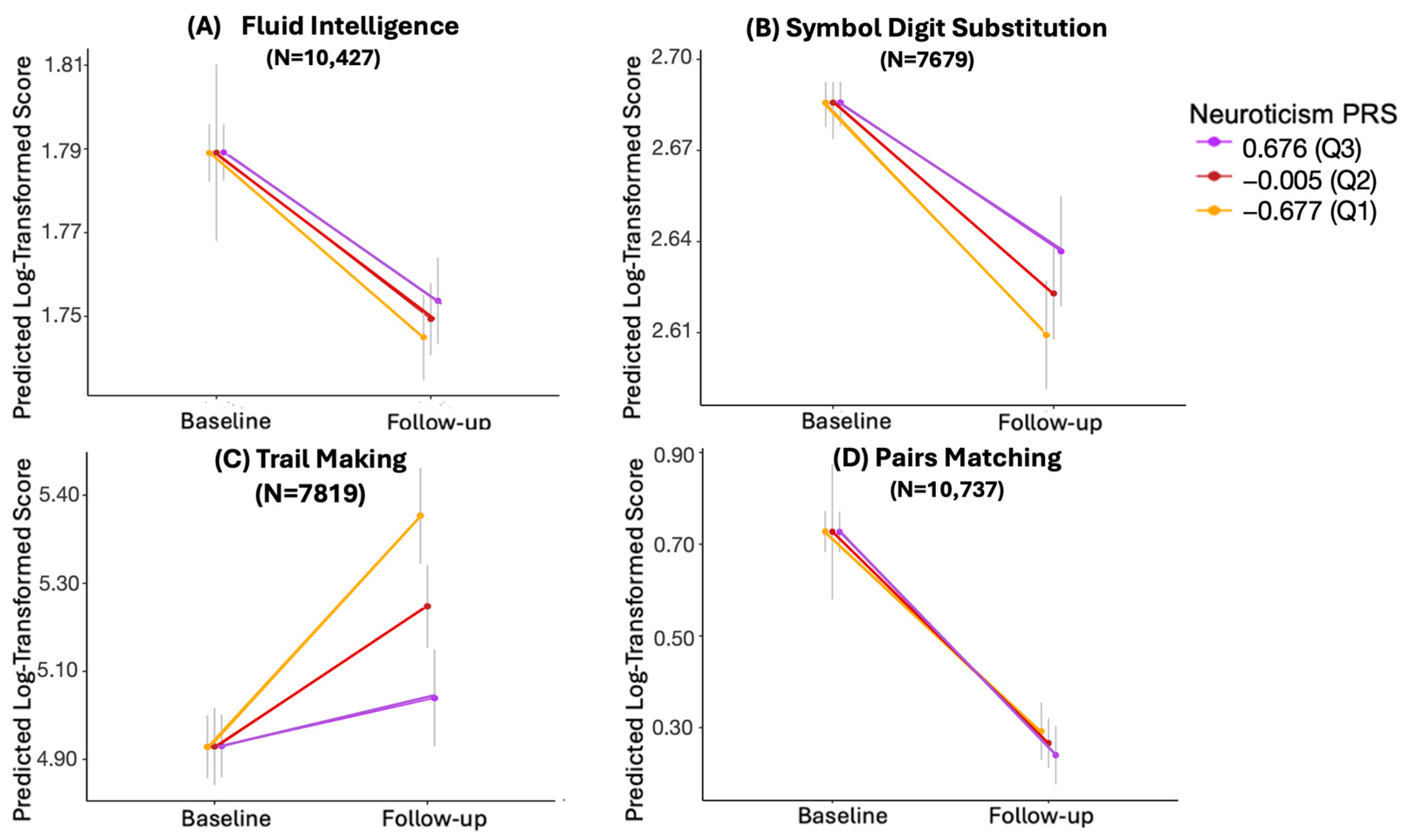
3.4. Longitudinal Analysis with Log-Transformed Values
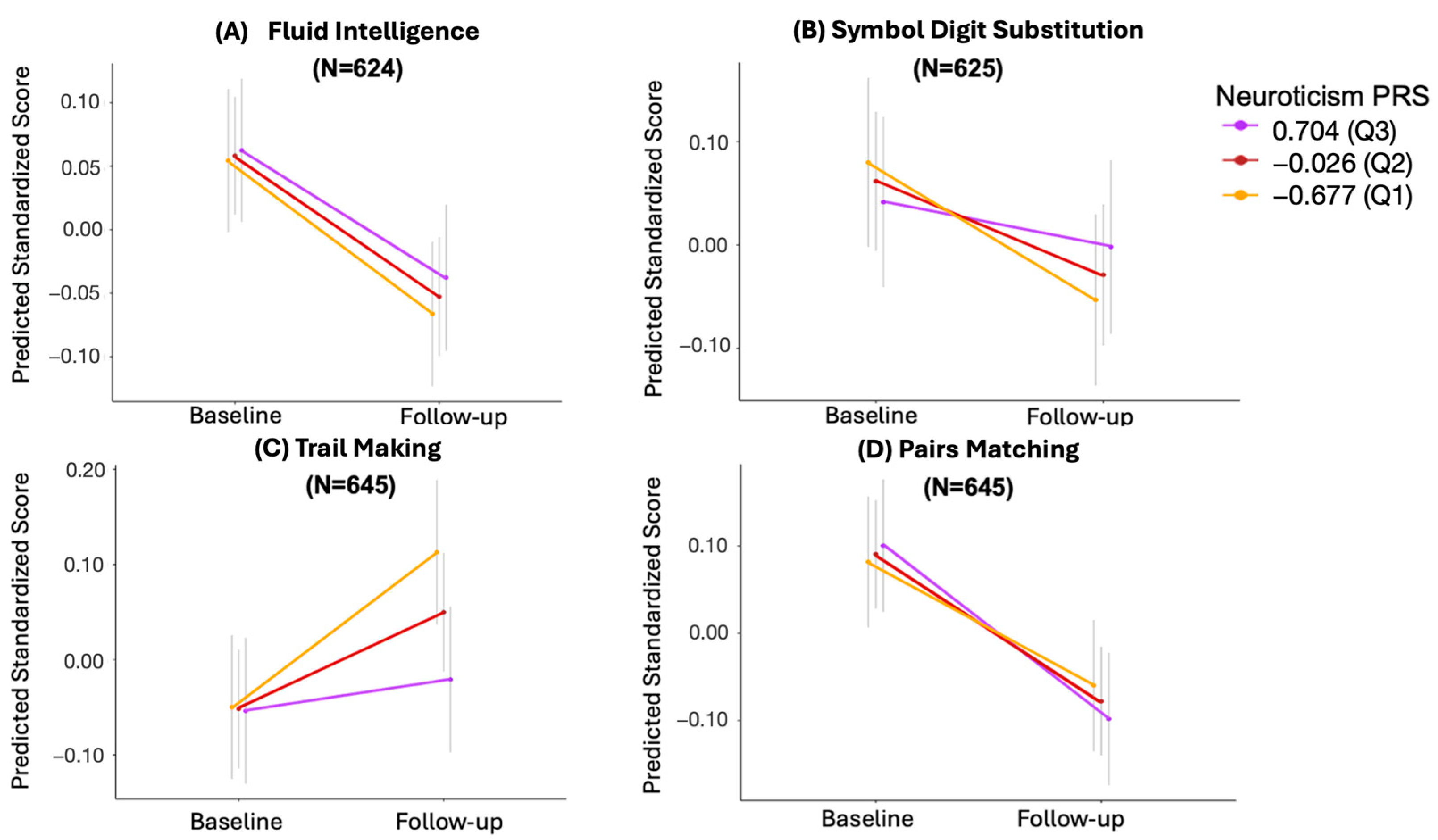
3.5. The Effect of Missing Longitudinal Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Danti, S.; Picchi, L.; Nuti, A.; Fiorino, M.D. Daily functioning and dementia. Dement. Neuropsychol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Leibson, C.L.; Long, K.H.; Ransom, J.E.; Roberts, R.O.; Hass, S.L.; Duhig, A.M.; Smith, C.Y.; Emerson, J.A.; Pankratz, V.S.; Petersen, R.C. Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimer’s Dement. 2015, 11, 917–932. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines on Risk Reduction of Cognitive Decline and Dementia; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241550543 (accessed on 1 August 2024).
- Brito, D.V.C.; Esteves, F.; Rajado, A.T.; Silva, N.; Araújo, I.; Bragança, J.; Castelo-Branco, P.; Nóbrega, C.; Consortium, A.s. Assessing cognitive decline in the aging brain: Lessons from rodent and human studies. NPJ Aging 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Hsu, H.C.; Bai, C.H. Individual and environmental factors associated with cognitive function in older people: A longitudinal multilevel analysis. BMC Geriatr. 2022, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.H.; Ellard, K.K.; Sauer-Zavala, S.; Bullis, J.R.; Carl, J.R. The Origins of Neuroticism. Perspect. Psychol. Sci. 2014, 9, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Duberstein, P.; Tindle, H.A.; Sink, K.M.; Robbins, J.; Tancredi, D.J.; Franks, P.; Investigators, G.E.o.M.S. Personality predicts cognitive function over 7 years in older persons. Am. J. Geriatr. Psychiatry 2012, 20, 612–621. [Google Scholar] [CrossRef]
- Aschwanden, D.; Sutin, A.R.; Luchetti, M.; Allemand, M.; Stephan, Y.; Terracciano, A. A Systematic Review and Meta-Analysis of the Association between Personality and Cognitive Failures/Complaints. Soc. Personal. Psychol. Compass 2020, 14, e12565. [Google Scholar] [CrossRef]
- Aschwanden, D.; Strickhouser, J.E.; Luchetti, M.; Stephan, Y.; Sutin, A.R.; Terracciano, A. Is personality associated with dementia risk? A meta-analytic investigation. Ageing Res. Rev. 2021, 67, 101269. [Google Scholar] [CrossRef]
- Terracciano, A.; Aschwanden, D.; Passamonti, L.; Toschi, N.; Stephan, Y.; Luchetti, M.; Lee, J.H.; Sesker, A.; O’Súilleabháin, P.S.; Sutin, A.R. Is neuroticism differentially associated with risk of Alzheimer’s disease, vascular dementia, and frontotemporal dementia? J. Psychiatr. Res. 2021, 138, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.D.; Yoneda, T.; James, B.D.; Bennett, D.A.; Hassenstab, J.; Katz, M.J.; Lipton, R.B.; Morris, J.; Mroczek, D.K.; Graham, E.K. Personality predictors of dementia diagnosis and neuropathological burden: An individual participant data meta-analysis. Alzheimer’s Dement. 2024, 20, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.C. Personality and Incident Alzheimer’s Disease: Theory, Evidence, and Future Directions. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 513–521. [Google Scholar] [CrossRef] [PubMed]
- de Moor, M.H.; van den Berg, S.M.; Verweij, K.J.; Krueger, R.F.; Luciano, M.; Arias Vasquez, A.; Matteson, L.K.; Derringer, J.; Esko, T.; Amin, N.; et al. Meta-analysis of Genome-wide Association Studies for Neuroticism, and the Polygenic Association With Major Depressive Disorder. JAMA Psychiatry 2015, 72, 642–650. [Google Scholar] [CrossRef]
- Nagel, M.; Jansen, P.R.; Stringer, S.; Watanabe, K.; de Leeuw, C.A.; Bryois, J.; Savage, J.E.; Hammerschlag, A.R.; Skene, N.G.; Muñoz-Manchado, A.B.; et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018, 50, 920–927. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Luchetti, M.; Caille, P.; Terracciano, A. Polygenic Score for Alzheimer Disease and cognition: The mediating role of personality. J. Psychiatr. Res. 2018, 107, 110–113. [Google Scholar] [CrossRef]
- Hill, W.D.; Weiss, A.; Liewald, D.C.; Davies, G.; Porteous, D.J.; Hayward, C.; McIntosh, A.M.; Gale, C.R.; Deary, I.J. Genetic contributions to two special factors of neuroticism are associated with affluence, higher intelligence, better health, and longer life. Mol. Psychiatry 2020, 25, 3034–3052. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.R.; Beck, A.; Gillespie, N.A.; Reynolds, C.A.; Elman, J.A.; Williams, M.E.; Gustavson, D.E.; Lyons, M.J.; Neale, M.C.; Kremen, W.S.; et al. A Traitlike Dimension of Subjective Memory Concern Over 30 Years Among Adult Male Twins. JAMA Psychiatry 2023, 80, 718–727. [Google Scholar] [CrossRef]
- Hindley, G.; Shadrin, A.A.; van der Meer, D.; Parker, N.; Cheng, W.; O’Connell, K.S.; Bahrami, S.; Lin, A.; Karadag, N.; Holen, B.; et al. Multivariate genetic analysis of personality and cognitive traits reveals abundant pleiotropy. Nat. Hum. Behav. 2023, 7, 1584–1600. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Hill, W.D.; Marioni, R.E.; Davies, G.; Hagenaars, S.P.; Harris, S.E.; Cox, S.R.; Taylor, A.M.; Corley, J.; Pattie, A.; et al. Polygenic predictors of age-related decline in cognitive ability. Mol. Psychiatry 2020, 25, 2584–2598. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Gorkom, K.N.; Zaki, N.; Almansoori, T.M.; Al Zahmi, F.; Ljubisavljevic, M.R.; Belghali, M. Proportional Changes in Cognitive Subdomains During Normal Brain Aging. Front. Aging Neurosci. 2021, 13, 673469. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, S.B.G.; Eysenck, H.J.; Barrett, P. A revised version of the psychoticism scale. Personal. Individ. Differ. 1985, 6, 21–29. [Google Scholar] [CrossRef]
- Gow, A.J.; Whiteman, M.C.; Pattie, A.; Deary, I.J. Goldberg’s ‘IPIP’ Big-Five factor markers: Internal consistency and concurrent validation in Scotland. Personal. Individ. Differ. 2005, 39, 317–329. [Google Scholar] [CrossRef]
- Fawns-Ritchie, C.; Deary, I.J. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 2020, 15, e0231627. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- RStudio. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2024. [Google Scholar]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience 2019, 8, giz082. [Google Scholar] [CrossRef]
- Choi, S.W.; Mak, T.S.; O’Reilly, P.F. Tutorial: A guide to performing polygenic risk score analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ormel, J.; Jeronimus, B.F.; Kotov, R.; Riese, H.; Bos, E.H.; Hankin, B.; Rosmalen, J.G.M.; Oldehinkel, A.J. Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clin. Psychol. Rev. 2013, 33, 686–697. [Google Scholar] [CrossRef]
- Almas, A.; Moller, J.; Iqbal, R.; Forsell, Y. Effect of neuroticism on risk of cardiovascular disease in depressed persons—A Swedish population-based cohort study. BMC Cardiovasc. Disord. 2017, 17, 185. [Google Scholar] [CrossRef]
- Friedman, H.S. Long-term relations of personality and health: Dynamisms, mechanisms, tropisms. J. Pers. 2000, 68, 1089–1107. [Google Scholar] [CrossRef]
- Friedman, H.S. Neuroticism and health as individuals age. Personal. Disord. 2019, 10, 25–32. [Google Scholar] [CrossRef]
- Gale, C.R.; Čukić, I.; Batty, G.D.; McIntosh, A.M.; Weiss, A.; Deary, I.J. When Is Higher Neuroticism Protective Against Death? Findings From UK Biobank. Psychol. Sci. 2017, 28, 1345–1357. [Google Scholar] [CrossRef]
- Weston, S.J.; Jackson, J.J. The role of vigilance in the relationship between neuroticism and health: A registered report. J. Res. Pers. 2018, 73, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Smit, F.; Penninx, B.W.; de Graaf, R.; ten Have, M.; Beekman, A.T. Economic costs of neuroticism: A population-based study. Arch. Gen. Psychiatry 2010, 67, 1086–1093. [Google Scholar] [CrossRef]
- Dhinakaran, J.; De Vos, M.; Thorne, J.D.; Kranczioch, C. Neuroticism focuses attention: Evidence from SSVEPs. Exp. Brain Res. 2014, 232, 1895–1903. [Google Scholar] [CrossRef]
- Flehmig, H.C.; Steinborn, M.B.; Westhoff, K.; Langner, R. Neuroticism and Speed-Accuracy Tradeoff in Self-Paced Speeded Mental Addition and Comparison. J. Individ. Differ. 2010, 31, 130–137. [Google Scholar] [CrossRef]
- Pearman, A. Neuroticism Predicts Reasoning Performance in Young but Not Older Adults. Exp. Aging Res. 2020, 46, 236–243. [Google Scholar] [CrossRef]
- Mandell, A.R.; Becker, A.; VanAndel, A.; Nelson, A.; Shaw, T.H. Neuroticism and vigilance revisited: A transcranial doppler investigation. Conscious. Cogn. 2015, 36, 19–26. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Holliday, J.; Gibson, L.M.; Garratt, S.; Oesingmann, N.; Alfaro-Almagro, F.; Bell, J.D.; Boultwood, C.; Collins, R.; Conroy, M.C.; et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat. Commun. 2020, 11, 2624. [Google Scholar] [CrossRef]
- Privé, F.; Arbel, J.; Vilhjálmsson, B.J. LDpred2: Better, faster, stronger. Bioinformatics 2021, 36, 5424–5431. [Google Scholar] [CrossRef]
- Privé, F.; Aschard, H.; Blum, M.G.B. Efficient Implementation of Penalized Regression for Genetic Risk Prediction. Genetics 2019, 212, 65–74. [Google Scholar] [CrossRef]
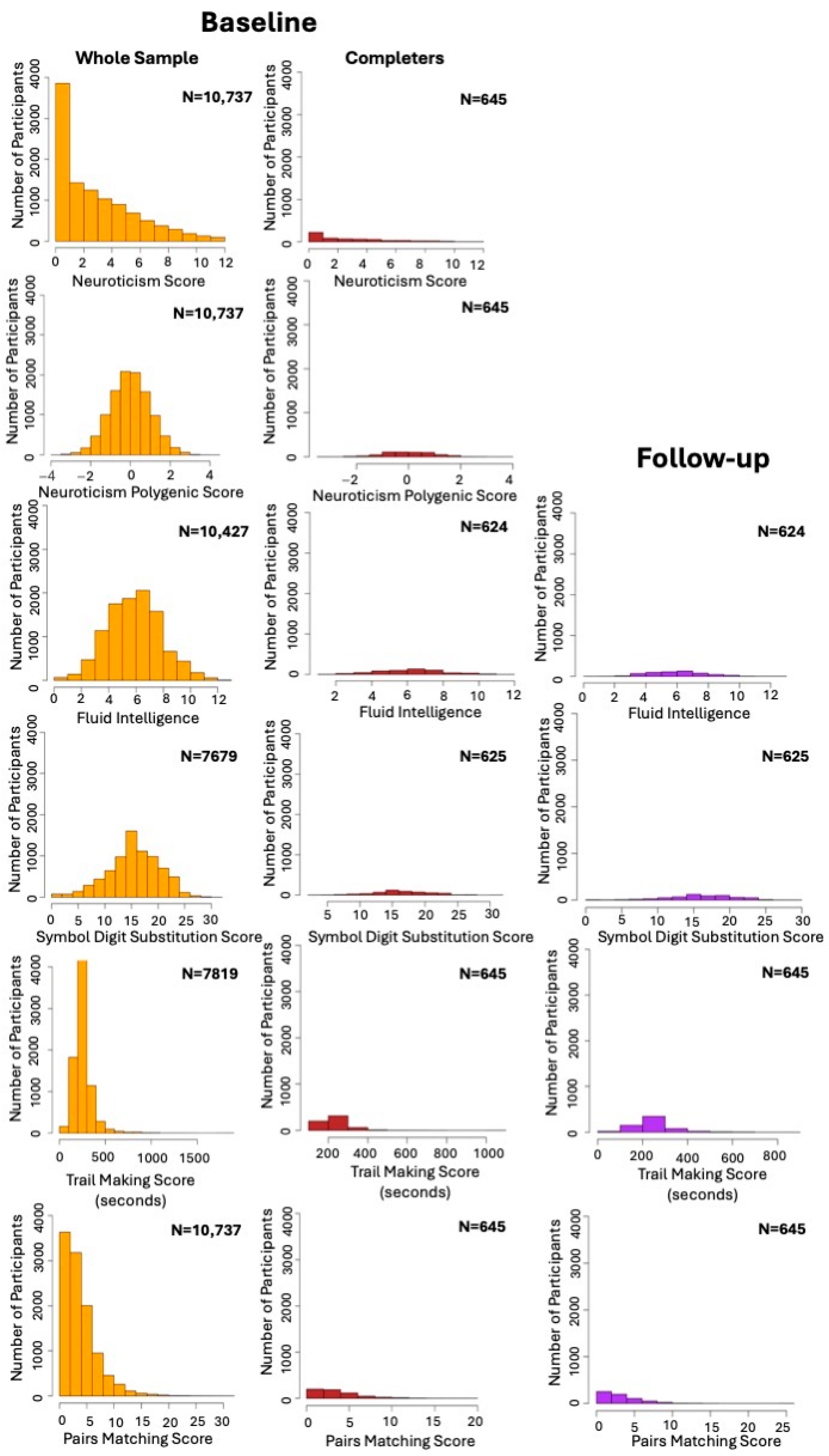
| Demographic Characteristics at Baseline | |||||||
|---|---|---|---|---|---|---|---|
| Completers (N = 645) | Lost to Follow Up (N = 10,092) | Overall (N = 10,737) | |||||
| Sex | |||||||
| Female | 262 (40.6%) | 4424 (43.8%) | 4686 (43.6%) | ||||
| Male | 383 (59.4%) | 5668 (56.2%) | 6051 (56.4%) | ||||
| Age (Years) | |||||||
| Mean (SD) | 62.9 (2.44) | 63.5 (2.72) | 63.4 (2.71) | ||||
| Neuroticism | |||||||
| Mean (SD) | 3.26 (2.88) | 3.20 (2.92) | 3.21 (2.91) | ||||
| Neuroticism PRS | |||||||
| Median [Min, Max] | −0.0261 [−3.10, 3.71] | −0.0047 [−3.51, 4.02] | −0.00486 [−3.50, 4.01] | ||||
| Cognitive Scores | |||||||
| Baseline | Follow-up | ||||||
| Completers (N = 645) | Lost to Follow Up (N = 10,092) | Overall (N = 10,737) | Completers (N = 645) | ||||
| Fluid Intelligence | Fluid Intelligence | ||||||
| Mean (SD) | 6.71 (1.97) | 6.35 (1.99) | 6.37 (2.00) | Mean (SD) | 6.47 (1.89) | ||
| Missing | 8 (1.2%) | 302 (3.0%) | 310 (2.9%) | Missing | 21 (3.1%) | ||
| Symbol Digit Substitution | Symbol Digit Substitution | ||||||
| Mean (SD) | 17.7 (4.08) | 16.8 (4.44) | 16.0 (4.85) | Mean (SD) | 16.4 (4.95) | ||
| Missing | 44 (6.8%) | 3014 (29.9%) | 3058 (28.5%) | Missing | 20 (3.1%) | ||
| Trail Making | Trail Making | ||||||
| Mean (SD) | 238 (88.4) | 259 (114) | 258 (113) | Mean (SD) | 250 (101) | ||
| Missing | 35 (5.4%) | 2883 (28.6%) | 2918 (27.2%) | ||||
| Pairs Matching | Pairs Matching | ||||||
| Mean (SD) | 4.10 (2.90) | 4.18 (3.27) | 4.18 (3.25) | Mean (SD) | 3.61 (2.79) | ||
| Estimate | 95% CI | SE | t | p-Value | Adjusted p-Value | N | |
|---|---|---|---|---|---|---|---|
| Fluid Intelligence | 0.014 | −0.0019, 0.0301 | 8.174 × 10−3 | 1.715 | 0.086 | 0.086 | 10427 |
| Symbol Digit Substitution | 0.020 | 0.0006, 0.0405 | 1.033 × 10−2 | 1.964 | 0.050 | 0.066 | 7679 |
| Trail Making | −0.077 | −0.0985, −0.0553 | 1.104 × 10−2 | −6.966 | 3.53 × 10−12 | 1.41 × 10−11 | 7819 |
| Pairs Matching | −0.033 | −0.0535, −0.0131 | 1.032 × 10−2 | −3.229 | 0.001 | 0.003 | 10737 |
| Estimate | 95% CI | SE | t | p-Value | Adjusted p-Value | N | |
|---|---|---|---|---|---|---|---|
| Fluid Intelligence | 0.006 | −0.0020, 0.0147 | 4.277 × 10−3 | 1.483 | 0.138 | 0.152 | 10,427 |
| Symbol Digit Substitution | 0.020 | 0.0055, 0.0352 | 7.585 × 10−3 | 2.679 | 0.007 | 0.0148 | 7679 |
| Trail Making | −0.159 | −0.2130, −0.1040 | 2.782 × 10−2 | −5.698 | 1.26 × 10−8 | 5.04 × 10−8 | 7819 |
| Pairs Matching | −0.038 | −0.0892, 0.0138 | 2.631 × 10−2 | −1.433 | 0.152 | 0.152 | 10,737 |
| Estimate | 95% CI | SE | t | p | Adjusted p-Value | N | |
|---|---|---|---|---|---|---|---|
| Fluid Intelligence | 0.015 | −0.0509 0.0803 | 3.365 × 10−2 | 0.434 | 0.665 | 0.665 | 624 |
| Symbol Digit Substitution | 0.021 | −0.0530, 0.0954 | 3.812 × 10−2 | 0.557 | 0.578 | 0.665 | 625 |
| Trail Making | −0.094 | −0.1818, −0.0058 | 4.514 × 10−2 | −2.079 | 0.038 | 0.152 | 645 |
| Pairs Matching | −0.041 | −0.1277, 0.0455 | 4.444 × 10−2 | −0.926 | 0.354 | 0.665 | 645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarian, N.; Ebrahimi, M.; Dos Santos, F.C.; Afjeh, S.S.; Abdelhack, M.; Sanches, M.; Diaconescu, A.O.; Rajji, T.K.; Felsky, D.; Zai, C.C.; et al. Examining the Role of Neuroticism Polygenic Risk in Late Life Cognitive Change: A UK Biobank Study. Behav. Sci. 2024, 14, 876. https://doi.org/10.3390/bs14100876
Akbarian N, Ebrahimi M, Dos Santos FC, Afjeh SS, Abdelhack M, Sanches M, Diaconescu AO, Rajji TK, Felsky D, Zai CC, et al. Examining the Role of Neuroticism Polygenic Risk in Late Life Cognitive Change: A UK Biobank Study. Behavioral Sciences. 2024; 14(10):876. https://doi.org/10.3390/bs14100876
Chicago/Turabian StyleAkbarian, Niki, Mahbod Ebrahimi, Fernanda C. Dos Santos, Sara Sadat Afjeh, Mohamed Abdelhack, Marcos Sanches, Andreea O. Diaconescu, Tarek K. Rajji, Daniel Felsky, Clement C. Zai, and et al. 2024. "Examining the Role of Neuroticism Polygenic Risk in Late Life Cognitive Change: A UK Biobank Study" Behavioral Sciences 14, no. 10: 876. https://doi.org/10.3390/bs14100876
APA StyleAkbarian, N., Ebrahimi, M., Dos Santos, F. C., Afjeh, S. S., Abdelhack, M., Sanches, M., Diaconescu, A. O., Rajji, T. K., Felsky, D., Zai, C. C., & Kennedy, J. L. (2024). Examining the Role of Neuroticism Polygenic Risk in Late Life Cognitive Change: A UK Biobank Study. Behavioral Sciences, 14(10), 876. https://doi.org/10.3390/bs14100876







