MoCA Domain-Specific Pattern of Cognitive Impairment in Stroke Patients Attending Intensive Inpatient Rehabilitation: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
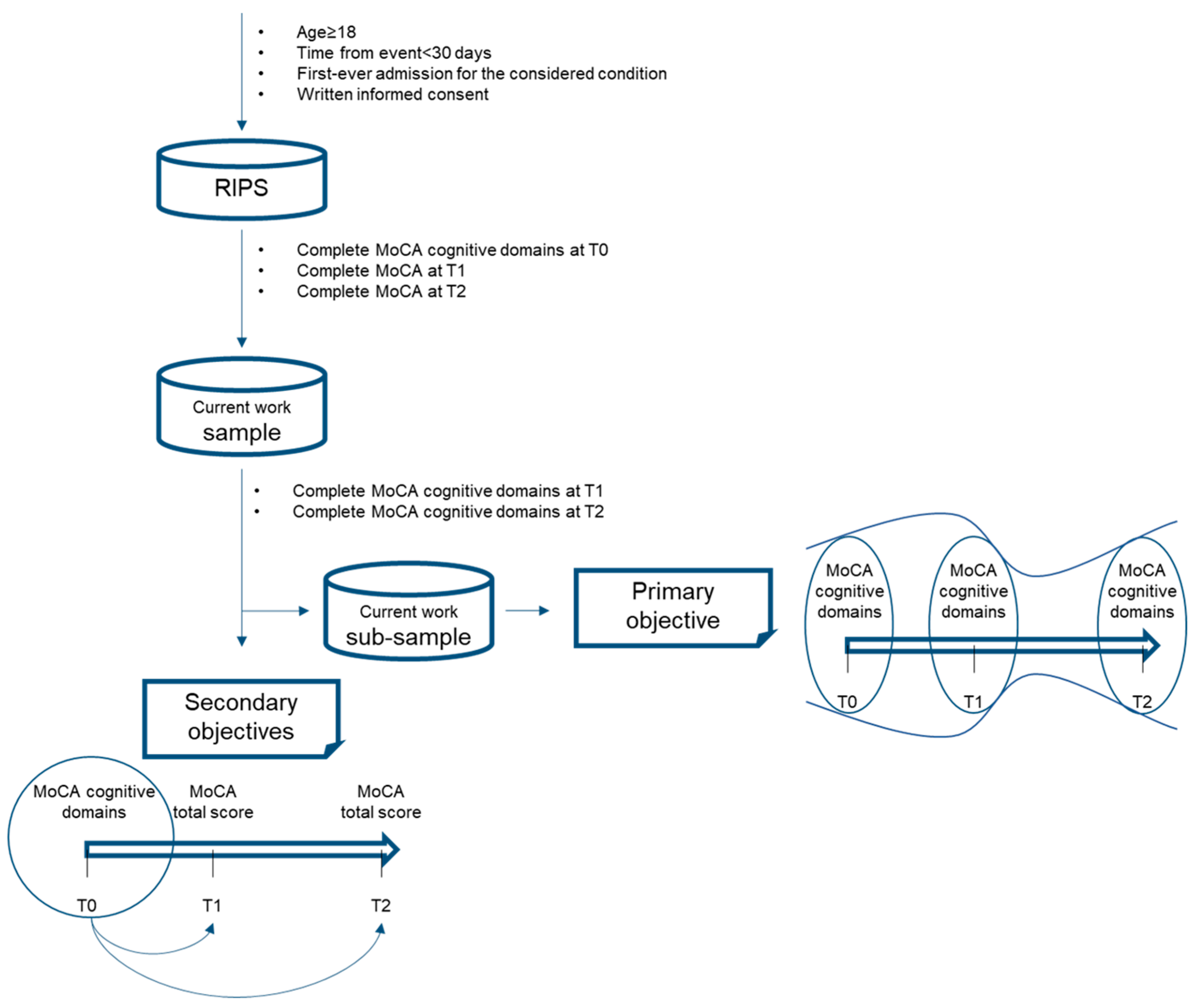
2.1. Participants
2.2. Intervention
2.3. Assessment
- Executive Functioning: this cognitive domain is investigated by three tests: (a) an alternation task adapted from the trail-making B task, (b) phonemic fluency, (c) a verbal abstraction task;
- Attention: investigated with three tests: (a) serial backward subtraction, (b) letter detection by tapping, (c) forward/backward digit span task;
- Language: assessed through two tests: (a) naming of three images of low-familiarity animals, (b) repetition of two syntactically complex sentences;
- Visuospatial: composed of two tests: (a) three-dimension cube copy, (b) clock drawing task;
- Orientation: composed of a single task in which the patient is asked to answer specific questions over time and place;
- Memory: consisting of a single memory test composed of delayed recall of five nouns after approximately five minutes from a learning trial.
2.4. Procedure of Data Collection
2.5. Statistical Analyses
3. Results
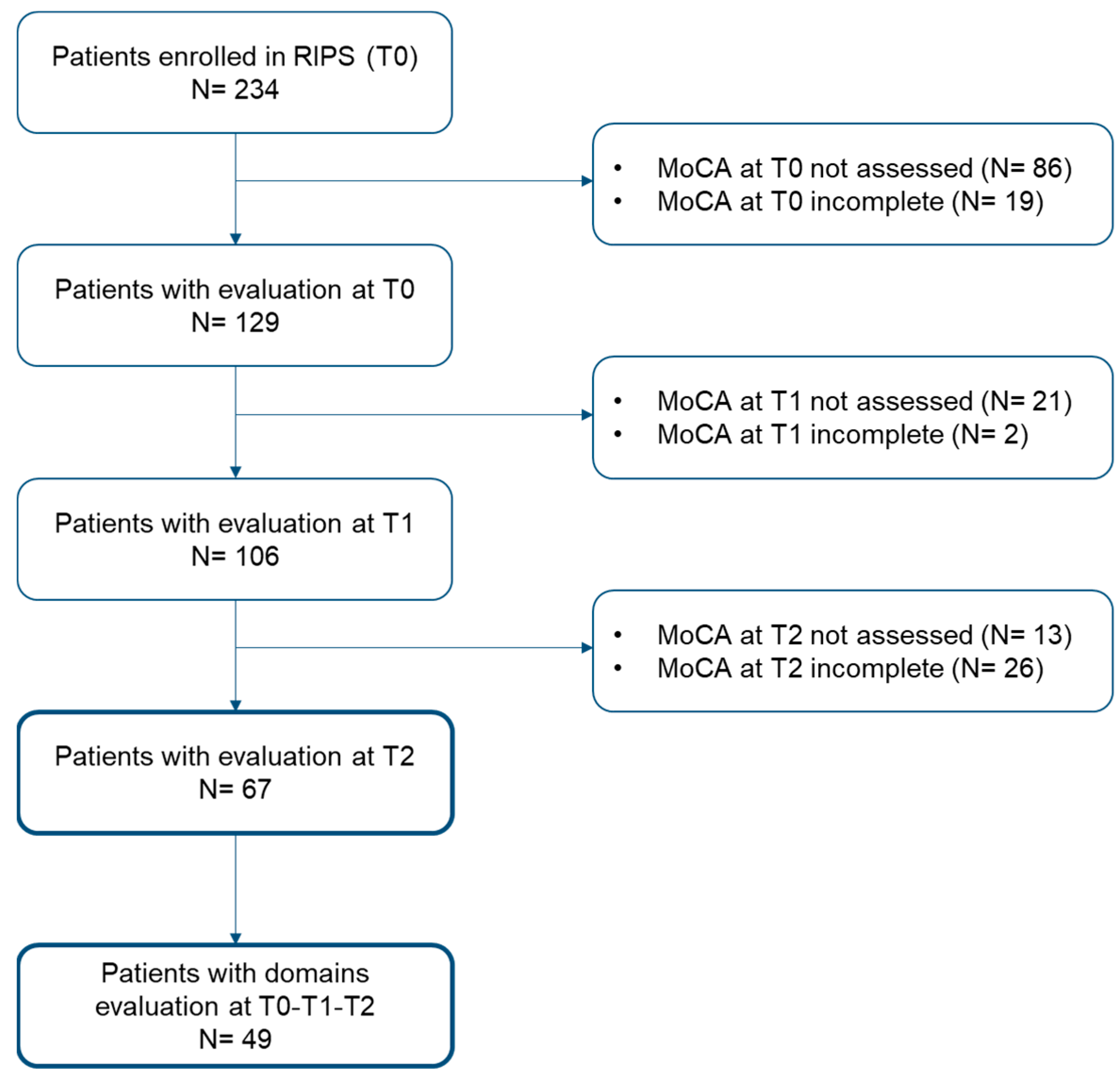
3.1. Participants
3.2. Patterns of Cognitive Impairment
3.3. Domain-Specific Cognitive Predictors of Patients’ Global Cognitive Functioning at Baseline (T0), Discharge (T1), and Follow-Up (T2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Barbay, M.; Diouf, M.; Roussel, M.; Godefroy, O.; GRECOGVASC Study Group. Systematic Review and Meta-Analysis of Prevalence in Post-Stroke Neurocognitive Disorders in Hospital-Based Studies. Dement. Geriatr. Cogn. Disord. 2018, 46, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Sexton, E.; McLoughlin, A.; Williams, D.J.; Merriman, N.A.; Donnelly, N.; Rohde, D.; Hickey, A.; Wren, M.-A.; Bennett, K. Systematic Review and Meta-Analysis of the Prevalence of Cognitive Impairment No Dementia in the First Year Post-Stroke. Eur. Stroke J. 2019, 4, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.; Feigin, V. The Impact of Neuropsychological Deficits on Functional Stroke Outcomes. Neuropsychol. Rev. 2006, 16, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Achten, D.; Visser-Meily, J.M.A.; Post, M.W.M.; Schepers, V.P.M. Life Satisfaction of Couples 3 Years after Stroke. Disabil. Rehabil. 2012, 34, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Delavaran, H.; Jönsson, A.-C.; Lövkvist, H.; Iwarsson, S.; Elmståhl, S.; Norrving, B.; Lindgren, A. Cognitive Function in Stroke Survivors: A 10-Year Follow-up Study. Acta Neurol. Scand. 2017, 136, 187–194. [Google Scholar] [CrossRef]
- Shi, D.; Chen, X.; Li, Z. Diagnostic Test Accuracy of the Montreal Cognitive Assessment in the Detection of Post-Stroke Cognitive Impairment under Different Stages and Cutoffs: A Systematic Review and Meta-Analysis. Neurol. Sci. 2018, 39, 705–716. [Google Scholar] [CrossRef]
- Pasi, M.; Poggesi, A.; Salvadori, E.; Pantoni, L. Post-Stroke Dementia and Cognitive Impairment. Front. Neurol. Neurosci. 2012, 30, 65–69. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Pantoni, L.; Salvadori, E. Location of Infarcts and Post-Stroke Cognitive Impairment. Lancet Neurol. 2021, 20, 413–414. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hung, S.-J.; Lin, K.-C.; Chen, K.-H.; Chen, P.; Tsay, P.-K. Responsiveness, Minimal Clinically Important Difference, and Validity of the MoCA in Stroke Rehabilitation. Occup. Ther. Int. 2019, 2019, 2517658. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, J.; Ovcar Stante, K.; Rakusa, M. The Validity of the Montreal Cognitive Assessment (MoCA) for the Screening of Vascular Cognitive Impairment after Ischemic Stroke. Acta Neurol. Belg. 2020, 120, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Xiong, Y.Y.; Kwan, P.W.L.; Chan, A.Y.Y.; Lam, W.W.M.; Wang, K.; Chu, W.C.W.; Nyenhuis, D.L.; Nasreddine, Z.; Wong, L.K.S.; et al. The Validity, Reliability and Clinical Utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in Patients with Cerebral Small Vessel Disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Abzhandadze, T.; Rafsten, L.; Lundgren Nilsson, Å.; Palstam, A.; Sunnerhagen, K.S. Very Early MoCA Can Predict Functional Dependence at 3 Months After Stroke: A Longitudinal, Cohort Study. Front. Neurol. 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Verdelho, A.; Biessels, G.J.; Chabriat, H.; Charidimou, A.; Duering, M.; Godefroy, O.; Pantoni, L.; Pavlovic, A.; Wardlaw, J. Cerebrovascular Disease in Patients with Cognitive Impairment: A White Paper from the ESO Dementia Committee—A Practical Point of View with Suggestions for the Management of Cerebrovascular Diseases in Memory Clinics. Eur. Stroke J. 2021, 6, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Siciliano, M.; Chiorri, C.; Passaniti, C.; Sant’Elia, V.; Trojano, L.; Santangelo, G. Comparison of Alternate and Original Forms of the Montreal Cognitive Assessment (MoCA): An Italian Normative Study. Neurol. Sci. 2019, 40, 691–702. [Google Scholar] [CrossRef]
- Block, C.K.; Johnson-Greene, D.; Pliskin, N.; Boake, C. Discriminating Cognitive Screening and Cognitive Testing from Neuropsychological Assessment: Implications for Professional Practice. Clin. Neuropsychol. 2017, 31, 487–500. [Google Scholar] [CrossRef]
- Mole, J.A.; Demeyere, N. The Relationship between Early Post-Stroke Cognition and Longer Term Activities and Participation: A Systematic Review. Neuropsychol. Rehabil. 2020, 30, 346–370. [Google Scholar] [CrossRef]
- Turunen, K.E.A.; Laari, S.P.K.; Kauranen, T.V.; Uimonen, J.; Mustanoja, S.; Tatlisumak, T.; Poutiainen, E. Domain-Specific Cognitive Recovery after First-Ever Stroke: A 2-Year Follow-Up. J. Int. Neuropsychol. Soc. 2018, 24, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hurford, R.; Charidimou, A.; Fox, Z.; Cipolotti, L.; Werring, D.J. Domain-Specific Trends in Cognitive Impairment after Acute Ischaemic Stroke. J. Neurol. 2013, 260, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Nys, G.M.S.; van Zandvoort, M.J.E.; de Kort, P.L.M.; van der Worp, H.B.; Jansen, B.P.W.; Algra, A.; de Haan, E.H.F.; Kappelle, L.J. The Prognostic Value of Domain-Specific Cognitive Abilities in Acute First-Ever Stroke. Neurology 2005, 64, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Milosevich, E.T.; Moore, M.J.; Pendlebury, S.T.; Demeyere, N. Domain-Specific Cognitive Impairment 6 Months after Stroke: The Value of Early Cognitive Screening. Int. J. Stroke 2023, 14, 17474930231205788. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Masoli, J.; Hase, Y.; Akinyemi, R.; Ballard, C.; Kalaria, R.N.; Allan, L.M. Trajectories of Cognitive Change Following Stroke: Stepwise Decline towards Dementia in the Elderly. Brain Commun. 2022, 4, fcac129. [Google Scholar] [CrossRef]
- Tang, E.Y.; Amiesimaka, O.; Harrison, S.L.; Green, E.; Price, C.; Robinson, L.; Siervo, M.; Stephan, B.C. Longitudinal Effect of Stroke on Cognition: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e006443. [Google Scholar] [CrossRef]
- Aam, S.; Einstad, M.S.; Munthe-Kaas, R.; Lydersen, S.; Ihle-Hansen, H.; Knapskog, A.-B.; Ellekjær, H.; Seljeseth, Y.; Saltvedt, I. Post-Stroke Cognitive Impairment-Impact of Follow-Up Time and Stroke Subtype on Severity and Cognitive Profile: The Nor-COAST Study. Front. Neurol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Dharmasaroja, P.A. Temporal Changes in Cognitive Function in Early Recovery Phase of the Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106027. [Google Scholar] [CrossRef]
- Hakiki, B.; Paperini, A.; Castagnoli, C.; Hochleitner, I.; Verdesca, S.; Grippo, A.; Scarpino, M.; Maiorelli, A.; Mosca, I.E.; Gemignani, P.; et al. Predictors of Function, Activity, and Participation of Stroke Patients Undergoing Intensive Rehabilitation: A Multicenter Prospective Observational Study Protocol. Front. Neurol. 2021, 12, 632672. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Diverio, M.; Arienti, C.; Corbella, E.; Marrazzo, F.; Speranza, G.; Del Zotto, E.; Poggianti, G.; Gigliotti, F.; Polcaro, P.; et al. Development and Implementation of a Stroke Rehabilitation Integrated Care Pathway in an Italian No Profit Institution: An Observational Study. Eur. J. Phys. Rehabil. Med. 2020, 56, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Aiello, E.N.; Gramegna, C.; Esposito, A.; Gazzaniga, V.; Zago, S.; Difonzo, T.; Maddaluno, O.; Appollonio, I.; Bolognini, N. The Montreal Cognitive Assessment (MoCA): Updated Norms and Psychometric Insights into Adaptive Testing from Healthy Individuals in Northern Italy. Aging Clin. Exp. Res. 2022, 34, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Welch, S.J.V.; Cuthbertson, F.C.; Mariz, J.; Mehta, Z.; Rothwell, P.M. Telephone Assessment of Cognition after Transient Ischemic Attack and Stroke: Modified Telephone Interview of Cognitive Status and Telephone Montreal Cognitive Assessment versus Face-to-Face Montreal Cognitive Assessment and Neuropsychological Battery. Stroke 2013, 44, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative Data for the Montreal Cognitive Assessment in an Italian Population Sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Capitani, E.; Laiacona, M. Composite Neuropsychological Batteries and Demographic Correction: Standardization Based on Equivalent Scores, with a Review of Published Data. The Italian Group for the Neuropsychological Study of Ageing. J. Clin. Exp. Neuropsychol. 1997, 19, 795–809. [Google Scholar] [CrossRef]
- Appelros, P.; Karlsson, G.M.; Seiger, A.; Nydevik, I. Neglect and Anosognosia after First-Ever Stroke: Incidence and Relationship to Disability. J. Rehabil. Med. 2002, 34, 215–220. [Google Scholar] [CrossRef]
- Nijboer, T.C.W.; Kollen, B.J.; Kwakkel, G. Time Course of Visuospatial Neglect Early after Stroke: A Longitudinal Cohort Study. Cortex 2013, 49, 2021–2027. [Google Scholar] [CrossRef]
- Demeyere, N.; Riddoch, M.J.; Slavkova, E.D.; Bickerton, W.-L.; Humphreys, G.W. The Oxford Cognitive Screen (OCS): Validation of a Stroke-Specific Short Cognitive Screening Tool. Psychol. Assess. 2015, 27, 883–894. [Google Scholar] [CrossRef]
- Pedersen, P.M.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Orientation in the Acute and Chronic Stroke Patient: Impact on ADL and Social Activities. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1996, 77, 336–339. [Google Scholar] [CrossRef]
- Rehabilitation and Recovery of People with Aphasia after Stroke (RELEASE) Collaborators. Predictors of Poststroke Aphasia Recovery: A Systematic Review-Informed Individual Participant Data Meta-Analysis. Stroke 2021, 52, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.; Jørgensen, H.; Nakayama, H.; Raaschou, H.; Olsen, T. Impaired Orientation in Acute Stroke: Frequency, Determinants, and Time-Course of Recovery. Cerebrovasc. Dis. 1998, 8, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Park, J.; Seo, W. A 2-Year Prospective Follow-up Study of Temporal Changes Associated with Post-Stroke Cognitive Impairment. Int. J. Nurs. Pract. 2018, 24, e12618. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Park, W.; Kwon, S.R.; Lim, M.J.; Suh, Y.O.; Seo, W.S.; Park, J.S. Effects of gout web based self-management program on knowledge related to disease, medication adherence, and self-management. J. Korean Acad. Nurs. 2013, 43, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Berthier, M.L. Poststroke Aphasia: Epidemiology, Pathophysiology and Treatment. Drugs Aging 2005, 22, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L. Predictors of Functional Outcome Following Stroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, L.J.; Ferraro, M.K.; Veramonti, T.; Farne, A.; Whyte, J.; Ladavas, E.; Frassinetti, F.; Coslett, H.B. Hemispatial Neglect: Subtypes, Neuroanatomy, and Disability. Neurology 2004, 62, 749–756. [Google Scholar] [CrossRef]
- Katz, N.; Hartman-Maeir, A.; Ring, H.; Soroker, N. Functional Disability and Rehabilitation Outcome in Right Hemisphere Damaged Patients with and without Unilateral Spatial Neglect. Arch. Phys. Med. Rehabil. 1999, 80, 379–384. [Google Scholar] [CrossRef]
- Esposito, E.; Shekhtman, G.; Chen, P. Prevalence of Spatial Neglect Post-Stroke: A Systematic Review. Ann. Phys. Rehabil. Med. 2021, 64, 101459. [Google Scholar] [CrossRef]
- Rasquin, S.M.C.; Lodder, J.; Ponds, R.W.H.M.; Winkens, I.; Jolles, J.; Verhey, F.R.J. Cognitive Functioning after Stroke: A One-Year Follow-up Study. Dement. Geriatr. Cogn. Disord. 2004, 18, 138–144. [Google Scholar] [CrossRef]
- Leśniak, M.; Bak, T.; Czepiel, W.; Seniów, J.; Członkowska, A. Frequency and Prognostic Value of Cognitive Disorders in Stroke Patients. Dement. Geriatr. Cogn. Disord. 2008, 26, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Sinanović, O.; Mrkonjić, Z.; Zukić, S.; Vidović, M.; Imamović, K. Post-Stroke Language Disorders. Acta Clin. Croat. 2011, 50, 79–94. [Google Scholar] [PubMed]
- Chung, C.S.Y.; Pollock, A.; Campbell, T.; Durward, B.R.; Hagen, S. Cognitive Rehabilitation for Executive Dysfunction in Adults with Stroke or Other Adult Non-Progressive Acquired Brain Damage. Cochrane Database Syst. Rev. 2013, 2013, CD008391. [Google Scholar] [CrossRef] [PubMed]
- McDowd, J.M.; Filion, D.L.; Pohl, P.S.; Richards, L.G.; Stiers, W. Attentional Abilities and Functional Outcomes Following Stroke. J. Gerontol. B Psychol. Sci. Soc. Sci. 2003, 58, P45–P53. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, L.; Coetzer, R.; Turnbull, O.H. Building the Bond: Predictors of the Alliance in Neurorehabilitation. NeuroRehabilitation 2020, 46, 271–285. [Google Scholar] [CrossRef]
- VanGilder, J.L.; Hooyman, A.; Peterson, D.S.; Schaefer, S.Y. Post-Stroke Cognitive Impairments and Responsiveness to Motor Rehabilitation: A Review. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 461–468. [Google Scholar] [CrossRef]
- Stolwyk, R.J.; O’Neill, M.H.; McKay, A.J.D.; Wong, D.K. Are Cognitive Screening Tools Sensitive and Specific Enough for Use After Stroke? Stroke 2014, 45, 3129–3134. [Google Scholar] [CrossRef]
- Plummer, P.; Eskes, G.; Wallace, S.; Giuffrida, C.; Fraas, M.; Campbell, G.; Clifton, K.-L.; Skidmore, E.R.; American Congress of Rehabilitation Medicine Stroke Networking Group Cognition Task Force. Cognitive-Motor Interference during Functional Mobility after Stroke: State of the Science and Implications for Future Research. Arch. Phys. Med. Rehabil. 2013, 94, 2565–2574.e6. [Google Scholar] [CrossRef]
- Dong, Y.; Venketasubramanian, N.; Chan, B.P.-L.; Sharma, V.K.; Slavin, M.J.; Collinson, S.L.; Sachdev, P.; Chan, Y.H.; Chen, C.L.-H. Brief Screening Tests during Acute Admission in Patients with Mild Stroke Are Predictive of Vascular Cognitive Impairment 3–6 Months after Stroke. J. Neurol. Neurosurg. Psychiatry 2012, 83, 580–585. [Google Scholar] [CrossRef]
- Zietemann, V.; Georgakis, M.K.; Dondaine, T.; Müller, C.; Mendyk, A.-M.; Kopczak, A.; Hénon, H.; Bombois, S.; Wollenweber, F.A.; Bordet, R.; et al. Early MoCA Predicts Long-Term Cognitive and Functional Outcome and Mortality after Stroke. Neurology 2018, 91, e1838–e1850. [Google Scholar] [CrossRef]
- Bisogno, A.L.; Franco Novelletto, L.; Zangrossi, A.; De Pellegrin, S.; Facchini, S.; Basile, A.M.; Baracchini, C.; Corbetta, M. The Oxford Cognitive Screen (OCS) as an Acute Predictor of Long-Term Functional Outcome in a Prospective Sample of Stroke Patients. Cortex 2023, 166, 33–42. [Google Scholar] [CrossRef] [PubMed]

| Variables | Median [IQR] or Frequencies At Admission (T0) | Median [IQR] or Frequencies At Discharge (T1) | Median [IQR] or Frequencies At Follow-Up (T2) | p-Value |
|---|---|---|---|---|
| Age (years) | 76.0 [16.0] | - | - | - |
| Gender | Male: 35 (52.2%) Female: 32 (47.8%) | - | - | - |
| Schooling | 8.00 [8.00] | - | - | - |
| Time from the event (days) | 11.0 [9.00] | - | - | - |
| Type of stroke | Ischemic: 49 (73.1%) Haemorrhagic: 18 (26.9%) | - | - | - |
| Side of stroke | Right: 35 (52.2%) Left: 24 (35.8%) Bilateral: 6 (9.0%) | - | - | - |
| Area of the lesion | None: 4 (6.0%) Supratentorial: 52 (77.6%) Subtentorial: 8 (11.9%) Both: 3 (4.5%) | - | - | - |
| NIHSS score | 5.00 [6.00] | 2.00 [5.00] | - | <0.001 |
| NIHSS item 9 (language) | No aphasia: 53 (79.1%) Mild to moderate aphasia: 9 (13.4%) Severe aphasia: 4 (6.0%) Mute or global aphasia: 0 (0%) | No aphasia: 56 (83.6%) Mild to moderate aphasia: 9 (13.4%) Severe aphasia: 1 (1.5%) Mute or global aphasia: 0 (0%) | No aphasia: 42 (62.7%) Mild to moderate aphasia: 6 (9.0%) Severe aphasia: 1 (1.5%) Mute or global aphasia: 0 (0%) | 0.028 |
| mBI score | 37.0 [45.0] | 79.0 [46.0] | 93.0 [25.0] | <0.001 |
| MoCA_dichotomised | Altered: 34 (50.7%) Normal: 33 (49.3%) | Altered: 12 (17.9%) Normal: 55 (82.1%) | Altered: 25 (37.3%) Normal: 42 (62.7%) | <0.001 |
| Length of stay (days) | - | 32.0 [20.0] | - | - |
| Speech therapy treatment | - | No: 25 (37.3%) Yes: 42 (62.7%) | - | - |
| MoCA Subtests | Number of Altered Cases | p-Values of Pairwise Comparisons | p-Value | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0-T1 | T0-T2 | T1-T2 | ||
| Visuospatial | 18 (36.7%) | 5 (10.2%) | 8 (16.3%) | 0.001 | 0.012 | 1.000 | <0.001 |
| Attention | 21 (42.9%) | 12 (24.5%) | 9 (18.4%) | 0.034 | 0.002 | 1.000 | 0.002 |
| Language | 11 (22.4%) | 6 (12.2%) | 18 (36.7%) | 0.513 | 0.166 | 0.003 | 0.004 |
| Repetition | 0: 10 (20.4%) 1: 15 (30.6%) 2: 24 (49.0%) | 0: 8 (16.3%) 1: 17 (34.7%) 2: 24 (49.0%) | 0: 7 (14.3%) 1: 12 (24.5%) 2: 30 (61.2%) | 0.090 | 0.090 | 0.107 | 0.172 |
| Naming | 0: 0 (0%) 1: 2 (4.1%) 2: 6 (12.2%) 3: 41 (83.7%) | 0: 0 (0%) 1: 0 (0%) 2: 5 (10.2%) 3: 44 (89.8%) | 0: 0 (0%) 1: 4 (8.2%) 2: 7 (14.3%) 3: 38 (77.6%) | 0.003 | 0.302 | 0.003 | 0.031 |
| Executive | 13 (26.5%) | 7 (14.3%) | 5 (10.2%) | 0.125 | 0.020 | 1.000 | 0.018 |
| Memory (raw score) | 10 (20.4%) 1.0 [2.0] | - 2.0 [3.0] | - 1.0 [3.0] | - 0.043 | - 0.480 | - 0.189 | - 0.045 |
| Orientation | 21 (42.9%) | 8 (16.3%) | 20 (40.8%) | 0.007 | 1.000 | 0.014 | 0.003 |
| MoCA Subtests at T0 | Outcome: Dichotomised MoCA at T1 | ||
|---|---|---|---|
| 0: Altered Cognitive Status (N = 12) | 1: Normal Cognitive Status (N = 55) | p-Value | |
| Visuospatial | Altered: 9 (75.0%) Normal: 3 (25.0%) | Altered: 19 (34.5%) Normal: 36 (65.5%) | 0.021 |
| Attention | Altered: 10 (83.3%) Normal: 2 (16.7%) | Altered: 23 (41.8%) Normal: 32 (58.2%) | 0.011 |
| Language | Altered: 7 (58.3%) Normal: 5 (41.7%) | Altered: 8 (14.5%) Normal: 47 (85.5%) | 0.003 |
| Executive | Altered: 10 (83.3%) Normal: 2 (16.7%) | Altered: 10 (18.2%) Normal: 45 (81.8%) | <0.001 |
| Memory | Altered: 2 (16.7%) Normal: 10 (83.3%) | Altered: 10 (18.2%) Normal: 45 (81.8%) | 1.000 |
| Orientation | Altered: 9 (75.0%) Normal: 3 (25.0%) | Altered: 26 (47.3%) Normal: 29 (52.7%) | 0.114 |
| MoCA Subtests at T0 | Outcome: Dichotomised MoCA at T2 | ||
|---|---|---|---|
| 0: Altered Cognitive Status (N = 25) | 1: Normal Cognitive Status (N = 42) | p-Value | |
| Visuospatial | Altered: 14 (56.0%) Normal: 11 (44.0%) | Altered: 14 (33.3%) Normal: 28 (66.7%) | 0.080 |
| Attention | Altered: 19 (76.0%) Normal: 6 (24.0%) | Altered: 14 (33.3%) Normal: 28 (66.7%) | 0.001 |
| Language | Altered: 9 (36.0%) Normal: 16 (64.0%) | Altered: 6 (14.3%) Normal: 36 (85.7%) | 0.067 |
| Executive | Altered: 14 (56.0%) Normal: 11 (44.0%) | Altered: 6 (14.3%) Normal: 36 (85.7%) | 0.001 |
| Memory | Altered: 5 (20.0%) Normal: 20 (80.0%) | Altered: 7 (16.7%) Normal: 35 (83.3%) | 0.751 |
| Orientation | Altered: 19 (76.0%) Normal: 6 (24.0%) | Altered: 16 (38.1%) Normal: 26 (61.9%) | 0.005 |
| Steps | Independent Variables | B | Standard Error | Wald | p-Value | Exp(B) | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| 1st step (Nagelkerke’s R2 = 0.537) | Visuospatial | −1.370 | 0.925 | 2.192 | 0.139 | 0.254 | 0.041 | 1.558 |
| Attention | −0.921 | 1.004 | 0.842 | 0.359 | 0.398 | 0.056 | 2.847 | |
| Language | −1.678 | 0.940 | 3.188 | 0.074 | 0.187 | 0.030 | 1.178 | |
| Executive | −2.264 | 0.963 | 5.527 | 0.019 | 0.104 | 0.016 | 0.686 | |
| Constant | 4.514 | 1.086 | 17.264 | 0.000 | 91.288 | - | - | |
| 2nd step (Nagelkerke’s R2 = 0.523) | Visuospatial | −1.274 | 0.900 | 2.002 | 0.157 | 0.280 | 0.048 | 1.633 |
| Language | −1.897 | 0.893 | 4.510 | 0.034 | .150 | 0.026 | 0.864 | |
| Executive | −2.576 | 0.917 | 7.892 | 0.005 | 0.076 | 0.013 | 0.459 | |
| Constant | 4.146 | 0.962 | 18.566 | 0.000 | 63.209 | - | - | |
| 3rd step (Nagelkerke’s R2 = 0.487) | Language | −1.818 | 0.842 | 4.658 | 0.031 | 0.162 | 0.031 | 0.846 |
| Executive | −2.936 | 0.890 | 10.883 | 0.001 | 0.053 | 0.009 | 0.304 | |
| Constant | 3.639 | 0.832 | 19.129 | 0.000 | 38.035 | - | - | |
| Steps | Independent Variables | B | Standard Error | Wald | p-Value | Exp(B) | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| 1st step (Nagelkerke’s R2 = 0.384) | Attention | −1.282 | 0.632 | 4.113 | 0.043 | 0.277 | 0.080 | 0.958 |
| Executive | −1.528 | 0.649 | 5.543 | 0.019 | 0.217 | 0.061 | 0.774 | |
| Orientation | −1.064 | 0.637 | 2.785 | 0.095 | 0.345 | 0.099 | 1.204 | |
| Constant | 2.346 | 0.607 | 14.916 | 0.000 | 10.439 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basagni, B.; Malloggi, S.; Polito, C.; Pellicciari, L.; Campagnini, S.; Pancani, S.; Mannini, A.; Gemignani, P.; Salvadori, E.; Marignani, S.; et al. MoCA Domain-Specific Pattern of Cognitive Impairment in Stroke Patients Attending Intensive Inpatient Rehabilitation: A Prospective Study. Behav. Sci. 2024, 14, 42. https://doi.org/10.3390/bs14010042
Basagni B, Malloggi S, Polito C, Pellicciari L, Campagnini S, Pancani S, Mannini A, Gemignani P, Salvadori E, Marignani S, et al. MoCA Domain-Specific Pattern of Cognitive Impairment in Stroke Patients Attending Intensive Inpatient Rehabilitation: A Prospective Study. Behavioral Sciences. 2024; 14(1):42. https://doi.org/10.3390/bs14010042
Chicago/Turabian StyleBasagni, Benedetta, Serena Malloggi, Cristina Polito, Leonardo Pellicciari, Silvia Campagnini, Silvia Pancani, Andrea Mannini, Paola Gemignani, Emilia Salvadori, Sara Marignani, and et al. 2024. "MoCA Domain-Specific Pattern of Cognitive Impairment in Stroke Patients Attending Intensive Inpatient Rehabilitation: A Prospective Study" Behavioral Sciences 14, no. 1: 42. https://doi.org/10.3390/bs14010042
APA StyleBasagni, B., Malloggi, S., Polito, C., Pellicciari, L., Campagnini, S., Pancani, S., Mannini, A., Gemignani, P., Salvadori, E., Marignani, S., Giovannelli, F., Viggiano, M. P., Hakiki, B., Grippo, A., Macchi, C., & Cecchi, F. (2024). MoCA Domain-Specific Pattern of Cognitive Impairment in Stroke Patients Attending Intensive Inpatient Rehabilitation: A Prospective Study. Behavioral Sciences, 14(1), 42. https://doi.org/10.3390/bs14010042









