The Effects of Different Exercise Approaches on Attention Deficit Hyperactivity Disorder in Adults: A Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participant Recruitment
2.3. Ethics
2.4. Eligibility Criteria for Participants
2.4.1. Inclusion Criteria
2.4.2. Exclusion Criteria
2.5. Procedure
2.5.1. Online Screening
2.5.2. Randomisation
2.5.3. Intervention
2.5.4. Measures
2.5.5. Changes to Protocol
2.5.6. Statistical Analysis
3. Results
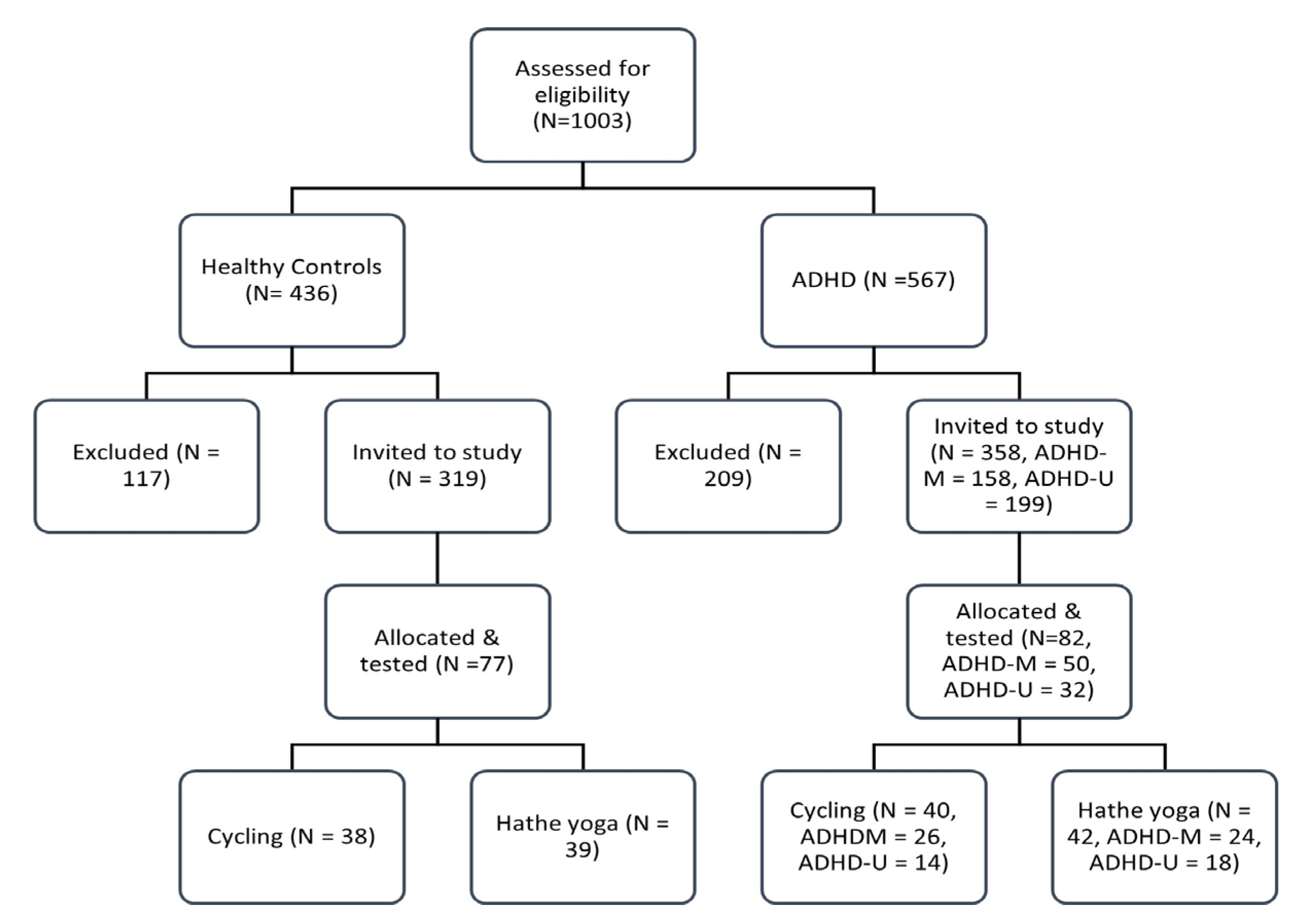
3.1. Study Population
3.2. Exercise Fidelity
3.3. Treatment Outcomes
3.3.1. Inattention
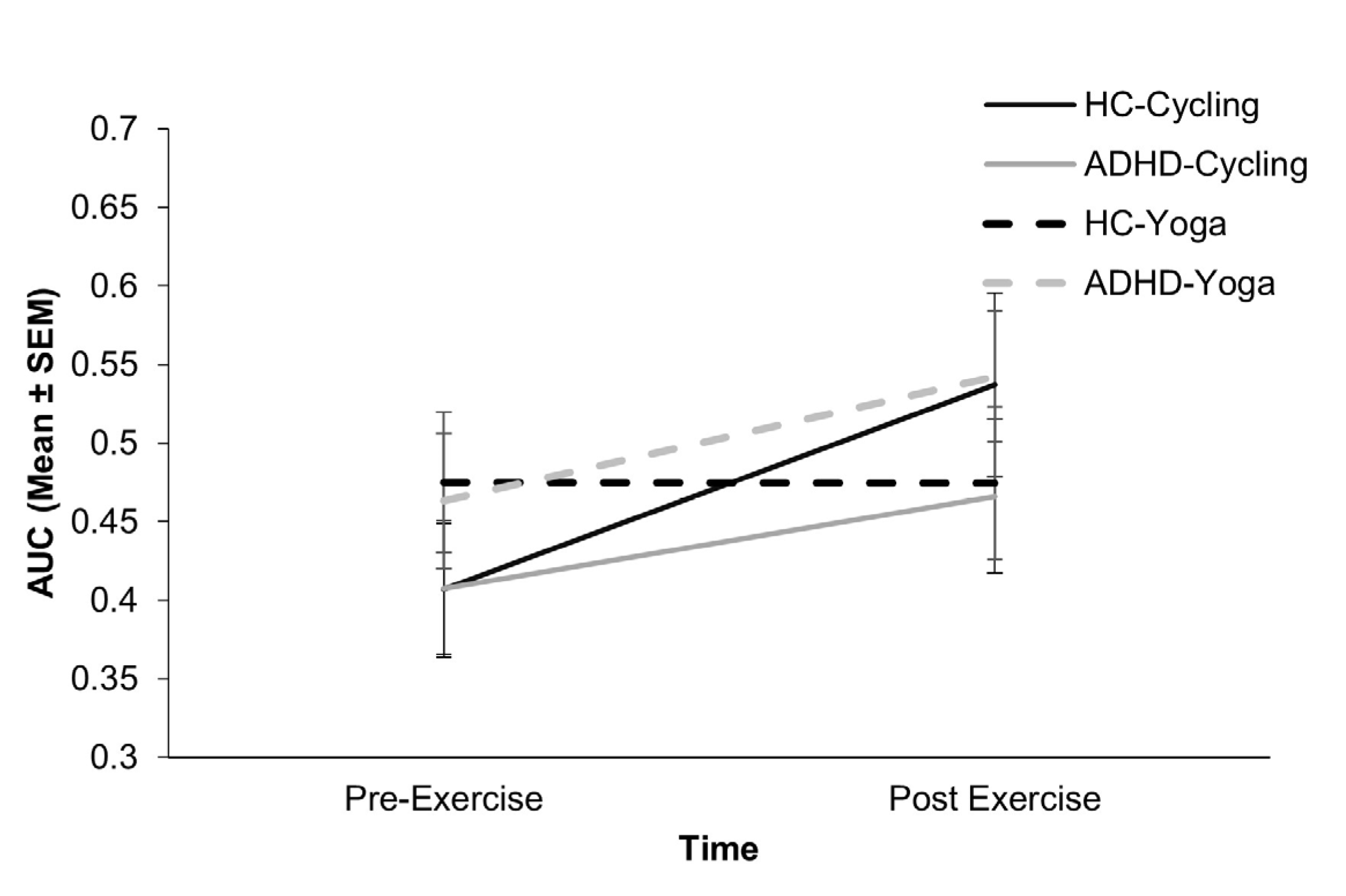
3.3.2. Temporal Impulsivity
3.3.3. Motor Impulsivity
3.3.4. Cognitive Impulsivity
3.3.5. Motor Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Zalsman, G.; Shilton, T. Adult ADHD: A new disease? Int. J. Psychiatry Clin. Pr. 2016, 20, 70–76. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef]
- Caye, A.; Rocha, T.B.-M.; Anselmi, L.; Murray, J.; Menezes, A.M.; Barros, F.C.; Gonçalves, H.; Wehrmeister, F.; Jensen, C.M.; Steinhausen, H.-C. Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: Evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry 2016, 73, 705–712. [Google Scholar] [CrossRef]
- Moffitt, T.E.; Houts, R.; Asherson, P.; Belsky, D.W.; Corcoran, D.L.; Hammerle, M.; Harrington, H.; Hogan, S.; Meier, M.H.; Polanczyk, G.V. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am. J. Psychiatry 2015, 172, 967–977. [Google Scholar] [CrossRef]
- Simon, V.; Czobor, P.; Balint, S.; Meszaros, A.; Bitter, I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br. J. Psychiatry 2009, 194, 204–211. [Google Scholar] [CrossRef]
- Doggett, A.M. ADHD and drug therapy: Is it still a valid treatment? J. Child Health Care 2004, 8, 69–81. [Google Scholar] [CrossRef]
- MacLean, L.; Prabhakar, D. Attention-Deficit/Hyperactivity Disorder and Sports: A Lifespan Perspective. Psychiatr. Clin. 2021, 44, 419–430. [Google Scholar]
- NICE. Diagnosis and Management of ADHD in Children, Young People and Adults; British Psychological Society: Leicester, UK, 2009. [Google Scholar]
- Danckaerts, M.; Sonuga-Barke, E.J.; Banaschewski, T.; Buitelaar, J.; Döpfner, M.; Hollis, C.; Santosh, P.; Rothenberger, A.; Sergeant, J.; Steinhausen, H.-C. The quality of life of children with attention deficit/hyperactivity disorder: A systematic review. Eur. Child Adolesc. Psychiatry 2010, 19, 83–105. [Google Scholar] [CrossRef]
- Barkley, R.A.; DuPaul, G.J.; McMurray, M.B. Attention deficit disorder with and without hyperactivity: Clinical response to three dose levels of methylphenidate. Pediatrics 1991, 87, 519–531. [Google Scholar] [CrossRef]
- Dittmann, R.W.; Cardo, E.; Nagy, P.; Anderson, C.S.; Adeyi, B.; Caballero, B.; Hodgkins, P.; Civil, R.; Coghill, D.R. Treatment response and remission in a double-blind, randomized, head-to-head study of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs 2014, 28, 1059–1069. [Google Scholar] [CrossRef]
- Milich, R.; Balentine, A.C.; Lynam, D.R. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clin. Psychol. Sci. Pract. 2001, 8, 463–488. [Google Scholar] [CrossRef]
- Smith, T.E.; Martel, M.M.; DeSantis, A.D. Subjective report of side effects of prescribed and nonprescribed psychostimulant use in young adults. Subst. Use Misuse 2017, 52, 548–552. [Google Scholar] [CrossRef]
- Mariani, J.J.; Mariani, J.J.; Levin, F.R. Treatment strategies for co-occurring ADHD and substance use disorders. Am. J. Addict. 2007, 16, 45–56. [Google Scholar] [CrossRef]
- Aadil, M.; Cosme, R.M.; Chernaik, J. Mindfulness-Based Cognitive Behavioral Therapy as an Adjunct Treatment of Attention Deficit Hyperactivity Disorder in Young Adults: A Literature Review. Cureus 2017, 9, e1269. [Google Scholar] [CrossRef]
- Laviola, G.; Adriani, W.; Terranova, M.L.; Gerra, G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci. Biobehav. Rev. 1999, 23, 993–1010. [Google Scholar] [CrossRef]
- Schenk, S.; Davidson, E.S. Stimulant preexposure sensitizes rats and humans to the rewarding effects of cocaine. NIDA Res. Monogr. 1998, 169, 56–82. [Google Scholar]
- Darredeau, C.; Barrett, S.P.; Jardin, B.; Pihl, R.O. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 529–536. [Google Scholar] [CrossRef]
- Dittmann, R.W.; Cardo, E.; Nagy, P.; Anderson, C.S.; Bloomfield, R.; Caballero, B.; Higgins, N.; Hodgkins, P.; Lyne, A.; Civil, R. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: A head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs 2013, 27, 1081–1092. [Google Scholar] [CrossRef]
- Den Heijer, A.E.; Groen, Y.; Tucha, L.; Fuermaier, A.B.; Koerts, J.; Lange, K.W.; Thome, J.; Tucha, O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: A systematic literature review. J. Neural Transm. 2017, 124, 3–26. [Google Scholar] [CrossRef]
- Best, J.R. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Dev. Rev. 2010, 30, 331–351. [Google Scholar] [CrossRef]
- Field, T. Exercise research on children and adolescents. Complement. Ther. Clin. Pract. 2012, 18, 54–59. [Google Scholar] [CrossRef]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef]
- Kim, H.; Heo, H.-I.; Kim, D.-H.; Ko, I.-G.; Lee, S.-S.; Kim, S.-E.; Kim, B.-K.; Kim, T.-W.; Ji, E.-S.; Kim, J.-D. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci. Lett. 2011, 504, 35–39. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Bertelsen, C.; Piccini, P.; Brooks, D.; Alving, J.; Lou, H.C. Increased dopamine tone during meditation-induced change of consciousness. Cogn. Brain Res. 2002, 13, 255–259. [Google Scholar] [CrossRef]
- Pal, R.; Singh, S.N.; Chatterjee, A.; Saha, M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: Role of yogic practice. Age 2014, 36, 9683. [Google Scholar] [CrossRef]
- Abramovitch, A.; Goldzweig, G.; Schweiger, A. Correlates of physical activity with intrusive thoughts, worry and impulsivity in adults with attention deficit/hyperactivity disorder: A cross-sectional pilot study. Isr. J. Psychiatry Relat. Sci. 2013, 50, 47–54. [Google Scholar]
- Berger, N.A.; Müller, A.; Brähler, E.; Philipsen, A.; de Zwaan, M. Association of symptoms of attention-deficit/hyperactivity disorder with symptoms of excessive exercising in an adult general population sample. BMC Psychiatry 2014, 14, 250. [Google Scholar] [CrossRef]
- Chang, S.H.; Shie, J.J.; Yu, N.Y. Enhancing Executive Functions and Handwriting with a Concentrative Coordination Exercise in Children with ADHD: A Randomized Clinical Trial. Percept. Mot. Ski. 2022, 129, 1014–1035. [Google Scholar] [CrossRef]
- Pan, C.Y.; Tsai, C.L.; Chu, C.H.; Sung, M.C.; Huang, C.Y.; Ma, W.Y. Effects of Physical Exercise Intervention on Motor Skills and Executive Functions in Children with ADHD: A Pilot Study. J. Atten. Disord. 2019, 23, 384–397. [Google Scholar] [CrossRef]
- Fritz, K.M.; O’Connor, P.J. Acute Exercise Improves Mood and Motivation in Young Men with ADHD Symptoms. Med. Sci. Sport. Exerc. 2016, 48, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Evenden, J.L. Varieties of impulsivity. Psychopharmacology 1999, 146, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, C.; Mewes, N.; Reimers, A.K.; Woll, A. Exercise Interventions in Children and Adolescents with ADHD: A Systematic Review. J. Atten. Disord. 2019, 23, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.; Beck, M.M.; Bilenberg, N.; Wienecke, J.; Astrup, A.; Lundbye-Jensen, J. Effects of Exercise on Cognitive Performance in Children and Adolescents with ADHD: Potential Mechanisms and Evidence-based Recommendations. J. Clin. Med. 2019, 8, 841. [Google Scholar] [CrossRef]
- Tan, B.W.; Pooley, J.A.; Speelman, C.P. A Meta-Analytic Review of the Efficacy of Physical Exercise Interventions on Cognition in Individuals with Autism Spectrum Disorder and ADHD. J. Autism Dev. Disord. 2016, 46, 3126–3143. [Google Scholar] [CrossRef]
- Sun, W.; Yu, M.; Zhou, X. Effects of physical exercise on attention deficit and other major symptoms in children with ADHD: A meta-analysis. Psychiatry Res. 2022, 311, 114509. [Google Scholar] [CrossRef]
- Doyle, N. Neurodiversity at work: A biopsychosocial model and the impact on working adults. Br. Med. Bull. 2020, 135, 108. [Google Scholar] [CrossRef]
- Hernandez-Reif, M.; Field, T.M.; Thimas, E. Attention deficit hyperactivity disorder: Benefits from Tai Chi. J. Bodyw. Mov. Ther. 2001, 5, 120–123. [Google Scholar] [CrossRef]
- Faber Taylor, A.; Kuo, F.E. Children with attention deficits concentrate better after walk in the park. J. Atten. Disord. 2009, 12, 402–409. [Google Scholar] [CrossRef]
- Haffner, J.; Roos, J.; Goldstein, N.; Parzer, P.; Resch, F. The effectiveness of body-oriented methods of therapy in the treatment of attention-deficit hyperactivity disorder (ADHD): Results of a controlled pilot study. Z. Kinder Jugendpsychiatrie Psychother. 2006, 34, 37–47. [Google Scholar] [CrossRef]
- Jensen, P.S.; Kenny, D.T. The effects of yoga on the attention and behavior of boys with attention-deficit/hyperactivity disorder (ADHD). J. Atten. Disord. 2004, 7, 205–216. [Google Scholar] [CrossRef]
- Converse, A.K.; Barrett, B.P.; Chewning, B.A.; Wayne, P.M. Tai Chi training for attention deficit hyperactivity disorder: A feasibility trial in college students. Complement. Ther. Med. 2020, 53, 102538. [Google Scholar] [CrossRef]
- Fritz, K.; O’Connor, P.J. Effects of a 6 Week Yoga Intervention on Executive Functioning in Women Screening Positive for Adult ADHD: A Pilot Study. Front. Sport. Act. Living 2022, 4, 746409. [Google Scholar] [CrossRef]
- Fuermaier, A.B.; Tucha, L.; Koerts, J.; van Heuvelen, M.J.; van der Zee, E.A.; Lange, K.W.; Tucha, O. Good vibrations--effects of whole body vibration on attention in healthy individuals and individuals with ADHD. PLoS ONE 2014, 9, e90747. [Google Scholar] [CrossRef]
- Firth, J.; Carney, R.; Elliott, R.; French, P.; Parker, S.; McIntyre, R.; McPhee, J.S.; Yung, A.R. Exercise as an intervention for first-episode psychosis: A feasibility study. Early Interv. Psychiatry 2018, 12, 307–315. [Google Scholar] [CrossRef]
- Bustamante, E.E.; Davis, C.L.; Frazier, S.L.; Rusch, D.; Fogg, L.F.; Atkins, M.S.; Marquez, D.X. Randomized Controlled Trial of Exercise for ADHD and Disruptive Behavior Disorders. Med. Sci. Sport. Exerc. 2016, 48, 1397–1407. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, Y.; Li, Y.; Li, L.; Yang, M.; Xue, P. How aerobic exercise improves executive function in ADHD children: A resting-state fMRI study. Int. J. Dev. Neurosci. 2022, 82, 295–302. [Google Scholar] [CrossRef]
- Silva, A.P.; Prado, S.O.; Scardovelli, T.A.; Boschi, S.R.; Campos, L.C.; Frère, A.F. Measurement of the effect of physical exercise on the concentration of individuals with ADHD. PLoS ONE 2015, 10, e0122119. [Google Scholar] [CrossRef]
- Villa-González, R.; Villalba-Heredia, L.; Crespo, I.; Del Valle, M.; Olmedillas, H. A systematic review of acute exercise as a coadjuvant treatment of ADHD in young people. Psicothema 2020, 32, 67–74. [Google Scholar] [CrossRef]
- Bigelow, H.; Gottlieb, M.D.; Ogrodnik, M.; Graham, J.D.; Fenesi, B. The differential impact of acute exercise and mindfulness meditation on executive functioning and psycho-emotional well-being in children and youth with ADHD. Front. Psychol. 2021, 12, 660845. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Mücke, M.; Brand, S.; Weber, P.; Brotzmann, M.; Pühse, U. The Acute Effects of Aerobic Exercise on Cognitive Flexibility and Task-Related Heart Rate Variability in Children with ADHD and Healthy Controls. J. Atten. Disord. 2020, 24, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Vysniauske, R.; Verburgh, L.; Oosterlaan, J.; Molendijk, M.L. The Effects of Physical Exercise on Functional Outcomes in the Treatment of ADHD: A Meta-Analysis. J. Atten. Disord. 2020, 24, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.A.; Netto, T.L.; Muszkat, M.; Medina, A.C.; Botter, D.; Orbetelli, R.; Scaramuzza, L.F.; Sinnes, E.G.; Vilela, M.; Miranda, M.C. Exercise impact on sustained attention of ADHD children, methylphenidate effects. Atten. Defic. Hyperact. Disord. 2010, 2, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, V.; Alves, M.V.; Santos-Galduróz, R.F.; Galduróz, J.C. Possible Cognitive Benefits of Acute Physical Exercise in Children with ADHD. J. Atten. Disord. 2017, 21, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Klil-Drori, S.; Hechtman, L. Potential social and neurocognitive benefits of aerobic exercise as adjunct treatment for patients with ADHD. J. Atten. Disord. 2020, 24, 795–809. [Google Scholar] [CrossRef]
- Mehren, A.; Özyurt, J.; Lam, A.P.; Brandes, M.; Müller, H.H.O.; Thiel, C.M.; Philipsen, A. Acute Effects of Aerobic Exercise on Executive Function and Attention in Adult Patients with ADHD. Front. Psychiatry 2019, 10, 132. [Google Scholar] [CrossRef]
- Mehren, A.; Özyurt, J.; Thiel, C.M.; Brandes, M.; Lam, A.P.; Philipsen, A. Effects of Acute Aerobic Exercise on Response Inhibition in Adult Patients with ADHD. Sci. Rep. 2019, 9, 19884. [Google Scholar] [CrossRef]
- Cochrane, W.G.; Dinu, L.M.; Kika, N.B.; Dommett, E.J. Attitudes and preferences toward exercise interventions in adults with attention deficit hyperactivity disorder: A survey study. Int. J. Ment. Health 2022, 51, 267–285. [Google Scholar] [CrossRef]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Beaulac, J.; Carlson, A.; Boyd, R.J. Counseling on physical activity to promote mental health: Practical guidelines for family physicians. Can. Fam. Physician 2011, 57, 399–401. [Google Scholar]
- Chang, Y.-K.; Hung, C.-L.; Huang, C.-J.; Hatfield, B.D.; Hung, T.-M. Effects of an aquatic exercise program on inhibitory control in children with ADHD: A preliminary study. Arch. Clin. Neuropsychol. 2014, 29, 217–223. [Google Scholar] [CrossRef]
- Fritz, K.; O’Connor, P. Cardiorespiratory Fitness and Leisure Time Physical Activity are Low in Young Men with Elevated Symptoms of Attention Deficit Hyperactivity Disorder. Exerc. Med. 2018, 2, 1. [Google Scholar] [CrossRef]
- Kessler, R.C.; Adler, L.; Ames, M.; Demler, O.; Faraone, S.; Hiripi, E.; Howes, M.J.; Jin, R.; Secnik, K.; Spencer, T. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol. Med. 2005, 35, 245–256. [Google Scholar] [CrossRef]
- Safren, S.A.; Duran, P.; Yovel, I.; Perlman, C.A.; Sprich, S. Medication adherence in psychopharmacologically treated adults with ADHD. J. Atten. Disord. 2007, 10, 257–260. [Google Scholar] [CrossRef]
- Katzman, M.A.; Bilkey, T.S.; Chokka, P.R.; Fallu, A.; Klassen, L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry 2017, 17, 302. [Google Scholar] [CrossRef]
- Shephard, R. Godin leisure-time exercise questionnaire. Med. Sci. Sport. Exerc. 1997, 29, S36–S38. [Google Scholar]
- Deschenes, M.; Garber, C. General principles of exercise prescription. In ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013; pp. 162–193. [Google Scholar]
- She, J.; Nakamura, H.; Makino, K.; Ohyama, Y.; Hashimoto, H. Selection of suitable maximum-heart-rate formulas for use with Karvonen formula to calculate exercise intensity. Int. J. Autom. Comput. 2015, 12, 62–69. [Google Scholar] [CrossRef]
- Williams, N. The Borg rating of perceived exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Huang-Pollock, C.L.; Karalunas, S.L.; Tam, H.; Moore, A.N. Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. J. Abnorm. Psychol. 2012, 121, 360. [Google Scholar] [CrossRef]
- Ossmann, J.M.; Mulligan, N.W. Inhibition and attention deficit hyperactivity disorder in adults. Am. J. Psychol. 2003, 116, 35–50. [Google Scholar] [CrossRef]
- Gansler, D.A.; Fucetola, R.; Krengel, M.; Stetson, S.; Zimering, R.; Makary, C. Are there cognitive subtypes in adult attention deficit/hyperactivity disorder? J. Nerv. Ment. Dis. 1998, 186, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.N.; Johnson, D.E.; Varia, I.M.; Conners, C.K. Neuropsychological assessment of response inhibition in adults with ADHD. J. Clin. Exp. Neuropsyc. 2001, 23, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; Murphy, K.; Kwasnik, D. Psychological adjustment and adaptive impairments in young adults with ADHD. J. Atten. Disord. 1996, 1, 41–54. [Google Scholar] [CrossRef]
- Grane, V.; Endestad, T.; Pinto, A.; Solbakk, A. Attentional control and subjective executive function in treatment-naive adults with Attention Deficit Hyperactivity Disorder. PloS ONE 2014, 9, e115227. [Google Scholar] [CrossRef] [PubMed]
- Vaurio, R.G.; Simmonds, D.J.; Mostofsky, S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia 2009, 47, 2389–2396. [Google Scholar] [CrossRef]
- Adams, Z.W.; Roberts, W.M.; Milich, R.; Fillmore, M.T. Does response variability predict distractibility among adults with attention-deficit/hyperactivity disorder? Psychol. Assess. 2011, 23, 427. [Google Scholar] [CrossRef]
- Hurst, R.M.; Kepley, H.O.; McCalla, M.K.; Livermore, M.K. Internal consistency and discriminant validity of a delay-discounting task with an adult self-reported ADHD sample. J. Atten. Disord. 2011, 15, 412–422. [Google Scholar] [CrossRef]
- Myerson, J.; Green, L.; Warusawitharana, M. Area under the curve as a measure of discounting. J. Exp. Anal. Behav. 2001, 76, 235–243. [Google Scholar] [CrossRef]
- Borges, A.M.; Kuang, J.; Milhorn, H.; Yi, R. An alternative approach to calculating Area-Under-the-Curve (AUC) in delay discounting research. J. Exp. Anal. Behav. 2016, 106, 145–155. [Google Scholar] [CrossRef]
- Kovács, I.; Richman, M.; Janka, Z.; Maraz, A.; Andó, B. Decision making measured by the Iowa Gambling Task in alcohol use disorder and gambling disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2017, 181, 152–161. [Google Scholar] [CrossRef]
- Ernst, M.; Kimes, A.S.; London, E.D.; Matochik, J.A.; Eldreth, D.; Tata, S.; Contoreggi, C.; Leff, M.; Bolla, K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am. J. Psychiatry 2003, 160, 1061–1070. [Google Scholar] [CrossRef]
- Garon, N.; Moore, C.; Waschbusch, D.A. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J. Atten. Disord. 2006, 9, 607–619. [Google Scholar] [CrossRef]
- Toplak, M.E.; Jain, U.; Tannock, R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD). Behav. Brain Funct. 2005, 1, 8. [Google Scholar] [CrossRef]
- Brand, M.; Recknor, E.C.; Grabenhorst, F.; Bechara, A. Decisions under ambiguity and decisions under risk: Correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J. Clin. Exp. Neuropsychol. 2007, 29, 86–99. [Google Scholar] [CrossRef]
- Bolden, J. Hyperactivity in Boys with Attention-Deficit/Hyperactivity Disorder (ADHD): A Ubiquitous Core Symptom or Manifestation of Working Memory Deficits? University of Central Florida: Orlando, FL, USA, 2008. [Google Scholar]
- Dane, A.V.; Schachar, R.J.; Tannock, R. Does actigraphy differentiate ADHD subtypes in a clinical research setting? J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 752–760. [Google Scholar] [CrossRef]
- Hartanto, T.; Krafft, C.; Iosif, A.; Schweitzer, J.B. A trial-by-trial analysis reveals more intense physical activity is associated with better cognitive control performance in attention-deficit/hyperactivity disorder. Child Neuropsychol. 2016, 22, 618–626. [Google Scholar] [CrossRef]
- White, I.R.; Horton, N.J.; Carpenter, J.; Pocock, S.J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011, 342, d40. [Google Scholar] [CrossRef]
- Mick, E.; McManus, D.D.; Goldberg, R.J. Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur. Neuropsychopharmacol. 2013, 23, 534–541. [Google Scholar] [CrossRef]
- Sofis, M.J.; Carrillo, A.; Jarmolowicz, D.P. Maintained Physical Activity Induced Changes in Delay Discounting. Behav. Modif. 2017, 41, 499–528. [Google Scholar] [CrossRef]
- Sharath, S.E.; Lee, M.; Kougias, P.; Taylor, W.C.; Zamani, N.; Barshes, N.R. Delayed gratification and adherence to exercise among patients with claudication. Vasc. Med. 2019, 24, 519–527. [Google Scholar] [CrossRef]
- Tate, L.M.; Tsai, P.F.; Landes, R.D.; Rettiganti, M.; Lefler, L.L. Temporal discounting rates and their relation to exercise behavior in older adults. Physiol. Behav. 2015, 152, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rassovsky, Y.; Alfassi, T. Attention Improves During Physical Exercise in Individuals with ADHD. Front. Psychol. 2018, 9, 2747. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Burne, T.H. Improvement of attention with amphetamine in low- and high-performing rats. Psychopharmacol. 2016, 233, 3383–3394. [Google Scholar] [CrossRef]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Stibbe, T.; Huang, J.; Paucke, M.; Ulke, C.; Strauss, M. Gender differences in adult ADHD: Cognitive function assessed by the test of attentional performance. PLoS ONE 2020, 15, e0240810. [Google Scholar] [CrossRef]
- Rucklidge, J.J. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr. Clin. North Am. 2010, 33, 357–373. [Google Scholar] [CrossRef]
- Dinu, L.M.; Phattharakulnij, N.; Dommett, E.J. Tryptophan modulation in individuals with attention deficit hyperactivity disorder: A systematic review. J. Neural Transm. 2022, 129, 361–377. [Google Scholar] [CrossRef]
| HC (N = 77) | ADHD (N = 82) | t or Χ2 | Significance | |
|---|---|---|---|---|
| Demographic variable; N (%) | ||||
| Female gender | 41 (53) | 68 (83) | 16.23 | <0.001 |
| Right-handed * | 70 (91) | 73 (88) | 0.436 | 0.509 |
| Active LSI | 67 (87) | 70 (85) | 0.090 | 0.764 |
| Age (years) M (SD) | 23.01 (4.29) | 26.62 (5.26) | 3.576 | <0.001 |
| Education (years) M (SD) | 5.36 (3.24) | 5.32 (3.06) | 0.093 | 0.926 |
| Resting Heart Rate (bpm) M (SD) | 67.97 (8.56) | 74.44 (11.57) | 4.03 | <0.001 |
| Clinical Characteristics M (SD) | ||||
| ASRS Total | 21.29 (9.17) | 54.70 (7.68) | 24.96 | <0.001 |
| ASRS-Inattention | 11.97 (5.10) | 29.73 (3.86) | 24.86 | <0.001 |
| ASRS-Hyperactivity-Impulsivity | 9.29 (5.00) | 24.99 (5.56) | 18.67 | <0.001 |
| Aerobic Cycling Exercise | Mind Body Hatha Yoga Exercise | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Controls | ADHD | Healthy Controls | ADHD | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Inattention | ||||||||
| Omission Errors | 2.38 (4.26) | 5.63 (13.38) | 3.91 (7.18) | 5.41 (15.37) | 2.33 (5.43) | 6.01 (12.75) | 6.45 (15.19) | 5.06 (10.49) |
| Hit Reaction Time | 418.56 (59.94) | 437.62 (93.48) | 440.82 (96.07) | 429.63 (83.35) | 404.71 (60.64) | 433.45 (109.80) | 442.15 (101.97) | 439.15 (73.98) |
| d Prime | 0.10 (1.24) | −0.49 (1.38) | −0.11 (1.20) | 0.02 (1.20) | −0.30 (1.35) | −0.02 (1.40) | −0.19 (1.43) | −0.04 (1.32) |
| Motor impulsivity | ||||||||
| Commission Errors | 3.36 (3.07) | 3.42 (2.80) | 4.80 (4.74) | 3.76 (3.77) | 4.73 (10.58) | 2.97 (2.73) | 4.49 (5.66) | 4.28 (5.50) |
| Cognitive Impulsivity | ||||||||
| Net Score (40) | −3.34 (17.41) | −10.21 (20.73) | −6.65 (21.03) | −1.45 (22.09) | −7.95 (20.39) | −8.90 (22.37) | −5.42 (21.95) | −8.38 (23.56) |
| % Risky Decisions | 53.29 (22.26) | 61.58 (27.27) | 58.31 (26.28) | 51.81 (27.61) | 58.40 (26.05) | 61.12 (27.97) | 56.79 (27.44) | 59.98 (29.20) |
| Temporal Impulsivity | ||||||||
| AUC | 0.41 (0.25) | 0.54 (0.35) | 0.41 (0.25) | 0.47 (0.25) | .47 (0.27) | 0.47 (0.27) | 0.46 (0.27) | 0.54 (0.26) |
| Hyperactivity | ||||||||
| Motor frequency | 2.89 (1.64) | 3.17 (1.46) | 3.86 (1.52) | 3.58 (1.75) | 2.76 (1.72) | 3.43 (2.21) | 2.86 (1.60) | 3.19 (1.69) |
| Motor intensity | 27.55 (17.73) | 38.42 (21.69) | 41.25 (21.25) | 47.30 (38.99) | 29.13 (21.40) | 39.09 (26.66) | 33.21 (21.37) | 4.13 (23.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, L.M.; Singh, S.N.; Baker, N.S.; Georgescu, A.L.; Singer, B.F.; Overton, P.G.; Dommett, E.J. The Effects of Different Exercise Approaches on Attention Deficit Hyperactivity Disorder in Adults: A Randomised Controlled Trial. Behav. Sci. 2023, 13, 129. https://doi.org/10.3390/bs13020129
Dinu LM, Singh SN, Baker NS, Georgescu AL, Singer BF, Overton PG, Dommett EJ. The Effects of Different Exercise Approaches on Attention Deficit Hyperactivity Disorder in Adults: A Randomised Controlled Trial. Behavioral Sciences. 2023; 13(2):129. https://doi.org/10.3390/bs13020129
Chicago/Turabian StyleDinu, Larisa M., Samriddhi N. Singh, Neo S. Baker, Alexandra L. Georgescu, Bryan F. Singer, Paul G. Overton, and Eleanor J. Dommett. 2023. "The Effects of Different Exercise Approaches on Attention Deficit Hyperactivity Disorder in Adults: A Randomised Controlled Trial" Behavioral Sciences 13, no. 2: 129. https://doi.org/10.3390/bs13020129
APA StyleDinu, L. M., Singh, S. N., Baker, N. S., Georgescu, A. L., Singer, B. F., Overton, P. G., & Dommett, E. J. (2023). The Effects of Different Exercise Approaches on Attention Deficit Hyperactivity Disorder in Adults: A Randomised Controlled Trial. Behavioral Sciences, 13(2), 129. https://doi.org/10.3390/bs13020129






