The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Randomization
2.3. Cortisol and CRH Assessment
2.4. Therapeutic Techniques of Craniosacral Therapy
2.5. Statistical Analysis
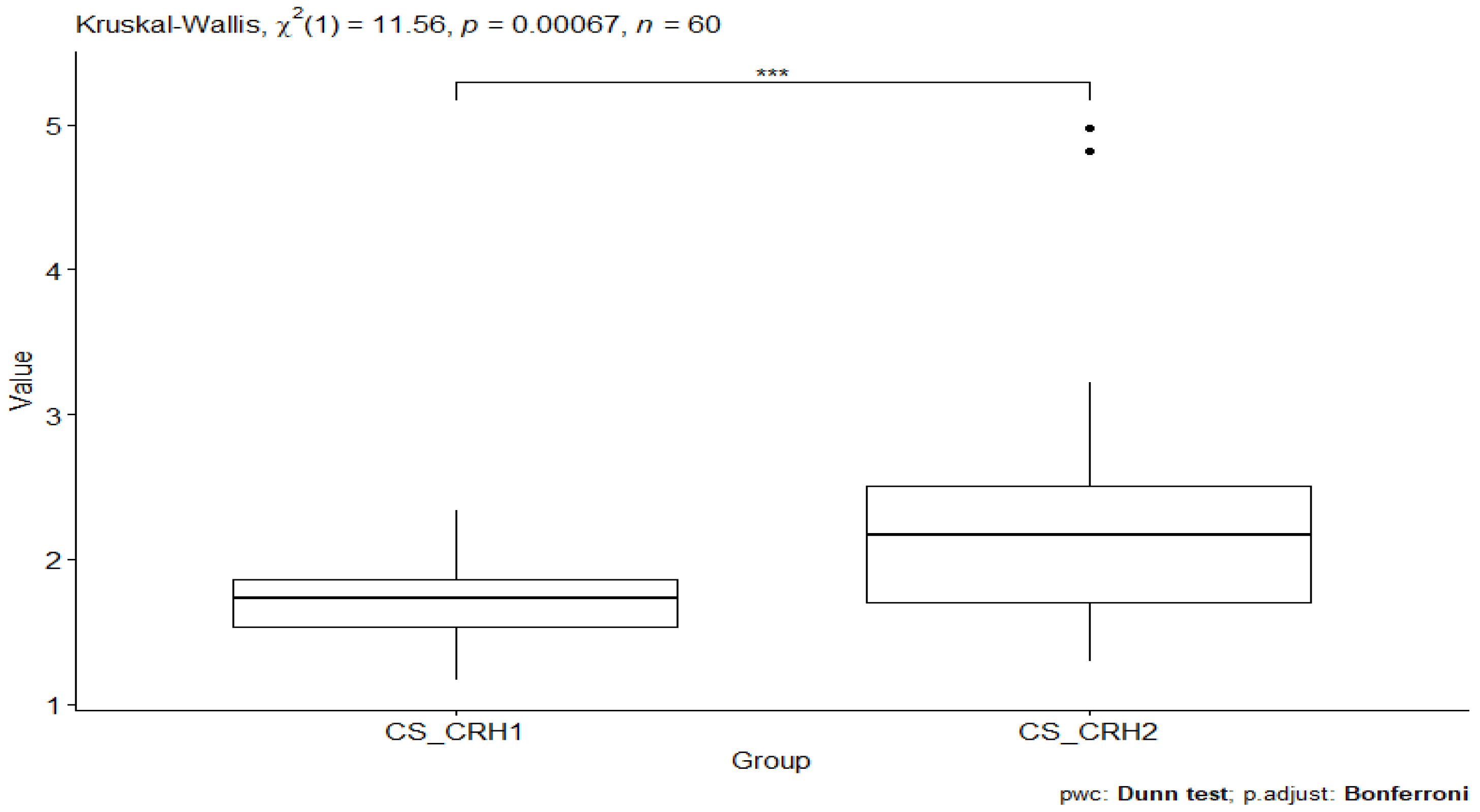
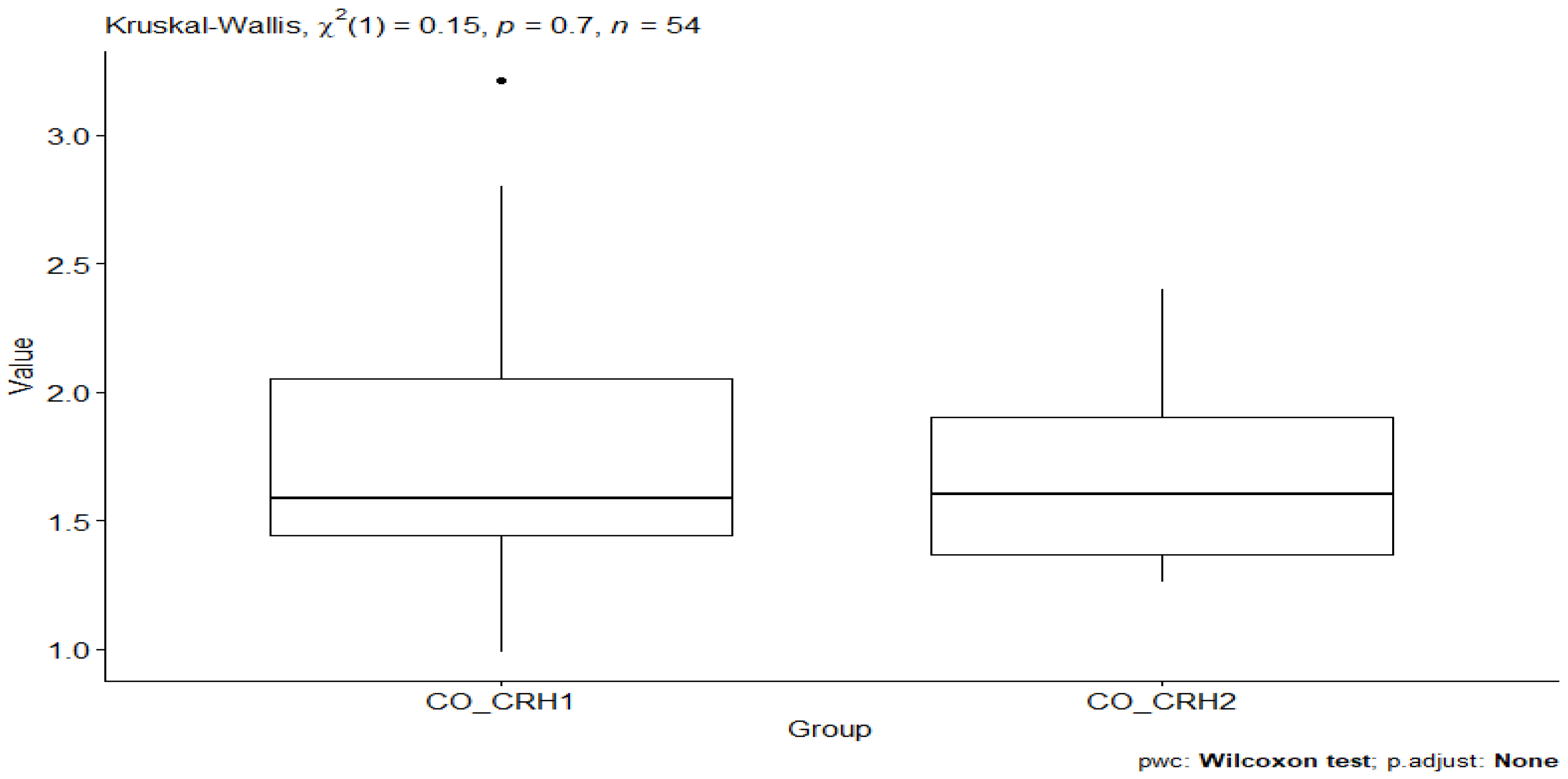
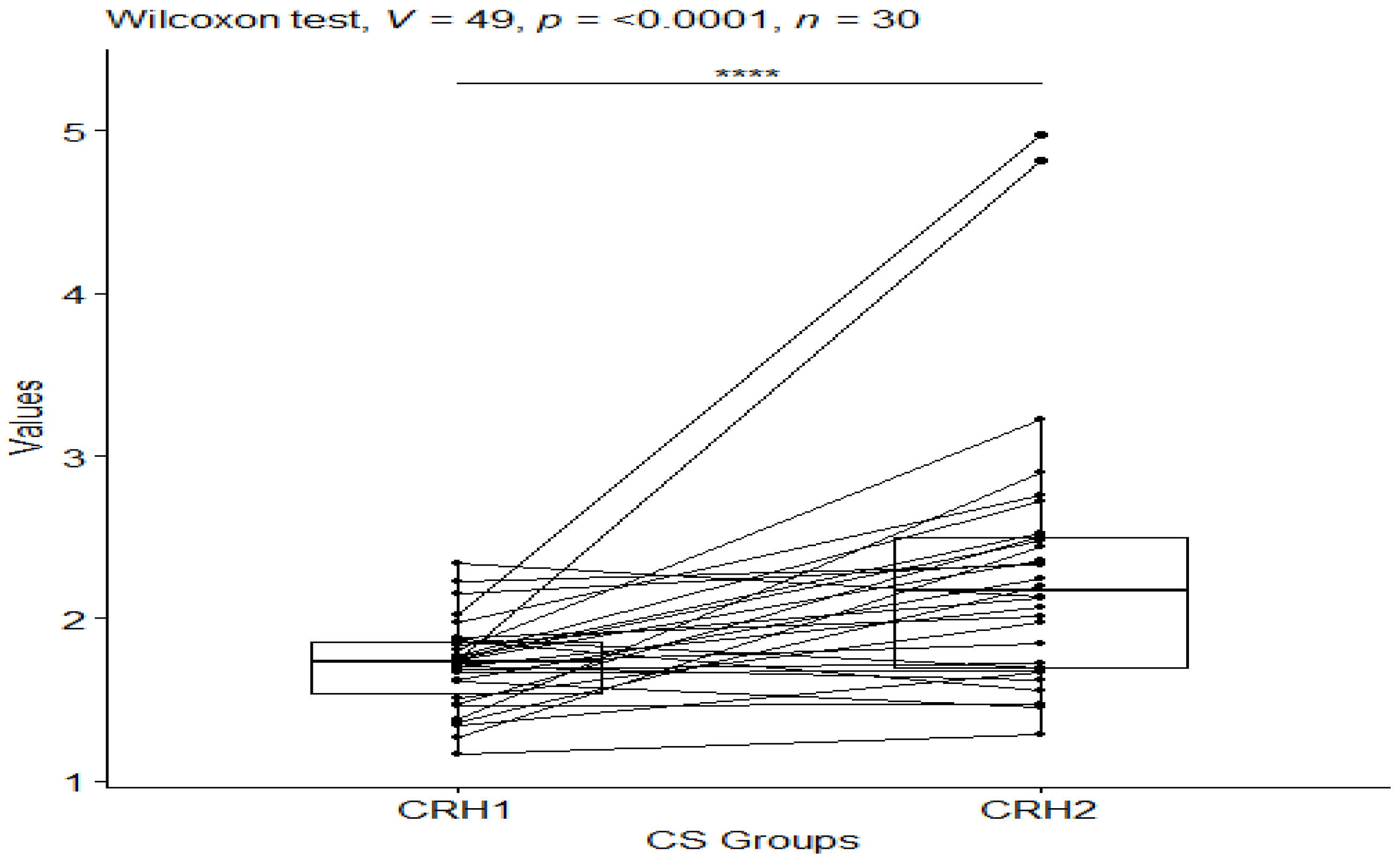
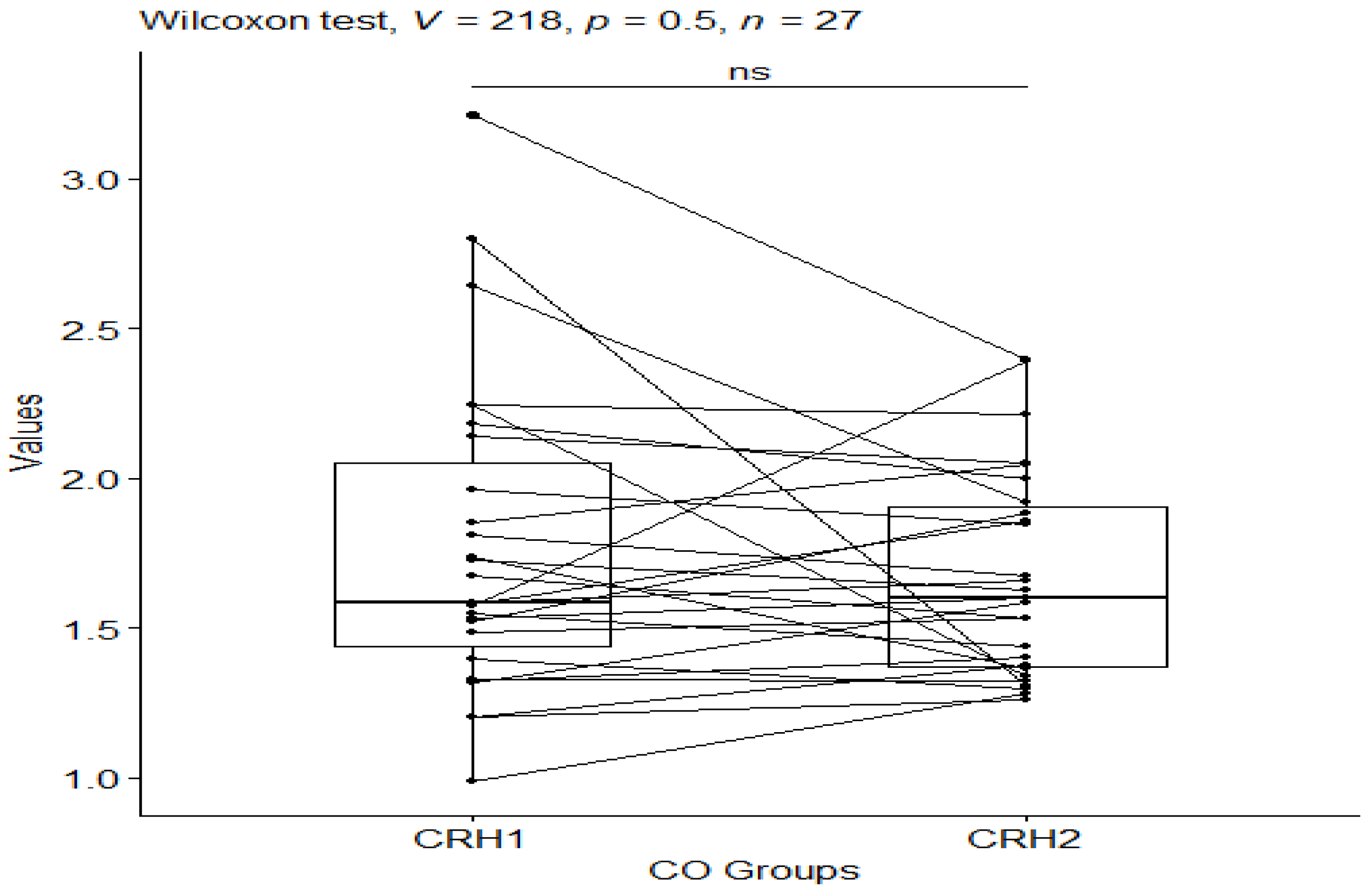
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebeaut, A.; Tran, J.K.; Vujanovic, A.A. Posttraumatic stress, alcohol use severity, and alcohol use motives among firefighters: The role of anxiety sensitivity. Addict. Behav. 2020, 106, 106353. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Tasker, J.G. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Front. Endocrinol. 2016, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Wellman, C.L.; Moench, K.M. Preclinical studies of stress, extinction, and prefrontal cortex: Intriguing leads and pressing questions. Psychopharmacology 2019, 236, 59–72. [Google Scholar] [CrossRef]
- Mayer, S.E.; Lopez-Duran, N.L.; Sen, S.; Abelson, J.L. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology 2018, 92, 57–65. [Google Scholar] [CrossRef]
- Denkova, E.; Zanesco, A.P.; Rogers, S.L.; Jha, A.P. Is resilience trainable? An initial study comparing mindfulness and relaxation training in firefighters. Psychiatry Res. 2020, 285, 112794. [Google Scholar] [CrossRef]
- Stub, T.; Kiil, M.A.; Lie, B.; Kristoffersen, A.E.; Weiss, T.; Hervik, J.B.; Musial, F. Combining psychotherapy with craniosacral therapy for severe traumatised patients: A qualitative study from an outpatient clinic in Norway. Complement. Ther. Med. 2020, 49, 102320. [Google Scholar] [CrossRef]
- Liem, T. Kraniosakrale Osteopathie; Thieme: New York, NY, USA, 2018; Volume 7, pp. 35–39. (In German) [Google Scholar]
- Haller, H.; Lauche, R.; Sundberg, T.; Dobos, G.; Cramer, H. Craniosacral therapy for chronic pain: A systematic review and meta-analysis of randomised controlled trials. BMC Musculoskelet. Disordes 2019, 21, 1. [Google Scholar] [CrossRef]
- Cerritelli, F.; Pizzolorusso, G.; Renzetti, C.; Cozzolino, V.; D’Orazio, M.; Lupacchini, M.; Marinelli, B.; Accorsi, A.; Lucci, C.; Lancellotti, J.; et al. A Multicenter, Randomised, Controlled Trial of Osteopathic Manipulative Treatment on Preterm Infants. PLoS ONE 2015, 10, e0127370. [Google Scholar] [CrossRef]
- Miana, L.; do Vale Bastos, V.H.; Machado, S.; Arias-Carrión, O.; Nardi, E.G.; Almeida, L.; Ribeiro, P.; Machado, D.; King, H.; Silva, J.G. Changes in alpha band activity associated with application of the compression of fourth ventricular (CV-4) osteopathic procedure: A qEEG pilot study. J. Bodyw. Mov. Ther. 2013, 17, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Bignami, E.; Gambetta, S.; Barbetti, M.; Procopio, M.; Freyrie, A.; Carbognani, P.; Ampollini, L.; Sgoifo, A. Cardiac autonomic and cortisol stress responses to real operations in surgeons: Relationship with individual psychobiological characteristics and experience. BioPsychoSoc. Med. 2023, 17, 5. [Google Scholar] [CrossRef]
- Hamidovic, A.; Karapetyan, K.; Serdarević, F.; Choi, S.H.; Eisenlohr-Moul, T.A.; Pinna, G. Higher Circulating Cortisol in the Follicular vs. Luteal Phase of the Menstrual Cycle: A Meta-Analysis. Front. Endocrinol. 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A.C.; Mahon, P.B.; McCaul, M.E.; Wand, G.S. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 2016, 66, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kovanur Sampath, K.; Treffel, L.; P Thomson, O.; Rodi, J.D.; Fleischmann, M.; Tumilty, S. Changes in biochemical markers following a spinal manipulation—A systematic review update. J. Man. Manip. Ther. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, D.; Santarlas, V.; Hoppes, J.; Vásquez-Castillo, A.; Morrow, A.; Oviedo, E.; Toldi, J. Osteopathic Manipulation as a Method of Cortisol Modification: A Systematic Review. Cureus 2023, 15, e36854. [Google Scholar] [CrossRef]
- Liem, T. Praktyka Osteopatii Czaszkowo-Krzyżowej; MedPharm: Guildford, UK, 2022; Volume I, pp. 21, 24, 29, 33–34, 35, 64, 87, 95, 108, 112–114, 129, 134, 137–140, 139, 149, 153–154, 156, 662. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org (accessed on 9 August 2023).
- King, H.H. Manual Craniosacral Therapy May Reduce Symptoms of Migraine Headache. J. Am. Osteopath. Assoc. 2017, 117, 59. [Google Scholar] [CrossRef][Green Version]
- Edwards, D.J.; Toutt, C. An evaluation of osteopathic treatment on psychological outcomes with patients suffering from chronic pain: A prospective observational cohort study collected through a health and well-being academy. Health Psychol. Open 2018, 5, 2055102918774684. [Google Scholar] [CrossRef]
- Girsberger, W.; Bänziger, U.; Lingg, G.; Lothaller, H.; Endler, P.C. Heart rate variability and the influence of craniosacral therapy on autonomous nervous system regulation in persons with subjective discomforts: A pilot study. J. Integr. Med. 2014, 12, 156–161. [Google Scholar] [CrossRef]
- Fornari, M.; Carnevali, L.; Sgoifo, A. Single Osteopathic Manipulative Therapy Session Dampens Acute Autonomic and Neuroendocrine Responses to Mental Stress in Healthy Male Participants. J. Am. Osteopath. Assoc. 2017, 117, 559–567. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hakamata, Y.; Komi, S.; Moriguchi, Y.; Izawa, S.; Motomura, Y.; Sato, E.; Mizukami, S.; Kim, Y.; Hanakawa, T.; Inoue, Y.; et al. Amygdala-centred functional connectivity affects daily cortisol concentrations: A putative link with anxiety. Sci. Rep. 2017, 7, 8313. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical Depression and Non-Atypical Depression: Is HPA axis Function a Biomarker? A Systematic Review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Zaccari, B.; Callahan, M.L.; Storzbach, D.; McFarlane, N.; Hudson, R.; Loftis, J.M. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol. Trauma 2020, 12, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.J.; Gaddy, M.A.; Chen, G.J. Exercise to Reduce Posttraumatic Stress Disorder Symptoms in Veterans. Fed. Pract. 2022, 39, 158–166. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bögels, S.M.; de Bruin, E.I. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef]
- Wójcik, M.; Siatkowski, I. The effect of cranial techniques on the heart rate variability response to psychological stress test in firefighter cadets. Sci. Rep. 2023, 13, 7780. [Google Scholar] [CrossRef]
- de Witte, M.; Pinho, A.D.S.; Stams, G.J.; Moonen, X.; Bos, A.E.R.; van Hooren, S. Music therapy for stress reduction: A systematic review and meta-analysis. Health Psychol. Rev. 2022, 16, 134–159. [Google Scholar] [CrossRef]
- Williams-Southers, T. Relaxation Methods to Reduce Occupational Stress. Workplace Health Saf. 2023, 3, 21650799231184375. [Google Scholar] [CrossRef]
- Kim, J.S.; Han, S.Y.; Iremonger, K.J. Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nat. Commun. 2019, 10, 5696. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Guzmán-Soto, I. Hypothalamic neurohormones and immune responses. Front. Integr. Neurosci. 2013, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Kupcova, I.; Danisovic, L.; Grgac, I.; Harsanyi, S. Anxiety and Depression: What Do We Know of Neuropeptides? Behav. Sci. 2022, 12, 262. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Sarubin, N.; Nothdurfter, C.; Schmotz, C.; Wimmer, A.M.; Trummer, J.; Lieb, M.; Uhr, M.; Baghai, T.C.; Wetter, T.C.; Bühner, M.; et al. Impact on cortisol and antidepressant efficacy of quetiapine and escitalopram in depression. Psychoneuroendocrinology 2014, 39, 141–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, D.; Liu, P.; Zhi, X.Y.; Shi, C.; Zhao, J.; Zhang, H. Study on the mechanism of regulating the hypothalamic cortical hormone releasing hor-mone/corticotropin releasing hormone type I receptor pathway by vibro-annular abdominal massage under the brain-intestine interaction in the treatment of insomnia. Medicine 2021, 100, e25854. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Wang, Y.; Guo, C.; Lu, P.; Mou, F.; Shao, S. Electroacupuncture alleviates anxiety and modulates amygdala CRH/CRHR1 signaling in single prolonged stress mice. Acupunct. Med. 2022, 40, 369–378. [Google Scholar] [CrossRef]
- Mehta, D.; Binder, E.B. Gene environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology 2012, 62, 654–662. [Google Scholar] [CrossRef]
- Coehoorn, C.J.; Neary, J.P.; Krigolson, O.E.; Service, T.W.; Stuart-Hill, L.A. Firefighter salivary cortisol responses following rapid heat stress. J. Therm. Biol. 2022, 108, 103305. [Google Scholar] [CrossRef]
- Van Hasselt, V.B.; Bourke, M.L.; Schuhmann, B.B. Firefighter Stress and Mental Health: Introduction to the Special Issue. Behav. Modif. 2022, 46, 259–266. [Google Scholar] [CrossRef]
- Mathias, K.C.; Bode, E.D.; Stewart, D.F.; Smith, D.L. Changes in Firefighter Weight and Cardiovascular Disease Risk Factors over Five Years. Med. Sci. Sports Exerc. 2020, 52, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Obuobi-Donkor, G.; Oluwasina, F.; Nkire, N.; Agyapong, V.I.O. A Scoping Review on the Prevalence and Determinants of Post-Traumatic Stress Disorder among Military Personnel and Firefighters: Implications for Public Policy and Practice. Int. J. Environ. Res. Public Health 2022, 19, 1565. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic burden of cardiovascular diseases in the European Union: A population-based cost study. Eur. Heart J. 2023, ehad583. [Google Scholar] [CrossRef]
- Mela, M.; Rdzanek, E.; Poniatowski, Ł.A.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Gałązka-Sobotka, M.; Olejniczak, D.; Niewiada, M.; Staniszewska, A. Economic Costs of Cardiovascular Diseases in Poland Estimates for 2015–2017 Years. Front. Pharmacol. 2020, 11, 1231. [Google Scholar] [CrossRef]
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). PharmacoEconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef]
- Available online: https://sciencedaily.com/releases/2017/02/170214162750.htm (accessed on 9 October 2023).
- Available online: https://world-heart-federation.org/wp-content/uploads/2017/05/WHF_Economics_Release_Final.pdf (accessed on 9 October 2023).
- Ras, J.; Smith, D.L.; Soteriades, E.S.; Kengne, A.P.; Leach, L. A Pilot Study on the Relationship between Cardiovascular Health, Musculoskeletal Health, Physical Fitness and Occupational Performance in Firefighters. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 1703–1718. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Negrean, V.; Orășan, O.H.; Vesa, S.C.; Sălăgean, O.; Iluţ, S.; Vlaicu, S.I. Cardiovascular Risk Factors and Physical Activity for the Prevention of Cardiovascular Diseases in the Elderly. Int. J. Environ. Res. Public Health 2021, 19, 207. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef]
- Coehoorn, C.J.; Neary, P.J.; Krigolson, O.E.; Stuart-Hill, L.A. Firefighter pre-frontal cortex oxygenation and hemodynamics during rapid heat stress. Brain Res. 2023, 1798, 148156. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Cardoso, F.; Rios, M.; Vaz, M.; Guedes, J.; Costa, J.T.; Baptista, J.S.; Fernandes, R.J. Machine Learning Approach to Model Physical Fatigue during Incremental Exercise among Firefighters. Sensors 2022, 23, 194. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Buecher, J.; Soujon, M.; Sierro, N.; Weiss, J.; Michel, B. Firefighter Stress Monitoring: Model Quality and Explainability. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 4653–4657. [Google Scholar] [CrossRef]
- Sawhney, G.; Jennings, K.S.; Britt, T.W.; Sliter, M.T. Occupational stress and mental health symptoms: Examining the moderating effect of work recovery strategies in firefighters. J. Occup. Health Psychol. 2018, 23, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Lozano, M.F.; Ramírez-García, C.O. Occupational Hazards in Firefighting: Systematic Literature Review. Saf. Health Work 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Pineles, S.L.; Rasmusson, A.M.; Yehuda, R.; Lasko, N.B.; Macklin, M.L.; Pitman, R.K.; Orr, S.P. Predicting emotional responses to potentially traumatic events from pre-exposure waking cortisol levels: A longitudinal study of police and firefighters anxiety. Stress Coping 2013, 26, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ge, Y.; Tang, B.; Liu, Y.; Kang, P.; Wang, M.; Zhang, L. A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS ONE 2015, 10, e0120270. [Google Scholar] [CrossRef] [PubMed]
- Makara-Studzińska, M.; Golonka, K.; Izydorczyk, B. Self-Efficacy as a Moderator between Stress and Professional Burnout in Firefighters. Int. J. Environ. Res. Public Health 2019, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Vaulerin, J.; Colson, S.S.; Emile, M.; Scoffier-Mériaux, S.; d’Arripe-Longueville, F. The Big Five Personality Traits and French Firefighter Burnout: The Mediating Role of Achievement Goals. J. Occup. Environ. Med. 2016, 58, e128–e132. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ma, Z.; Hou, W.; Zhu, Y.; Zhang, L.; Li, C.; Shi, C. Neuroticism Trait and Mental Health Among Chinese Firefighters: The Moderating Role of Perceived Organizational Support and the Mediating Role of Burnout-A Path Analysis. Front. Public Health 2022, 10, 870772. [Google Scholar] [CrossRef] [PubMed]
- Roșca, A.C.; Burtăverde, V.; Dan, C.I.; Mateizer, A.; Petrancu, C.R.; Iriza, A.I.; Ene, C.A. The Dark Triad Traits of Firefighters and Risk-Taking at Work. The Mediating Role of Altruism, Honesty, and Courage. Int. J. Environ. Res. Public Health 2021, 18, 5983. [Google Scholar] [CrossRef] [PubMed]


| Group | Variable | n | Min | Max | Median | iqr | Mean | sd | se | ci |
|---|---|---|---|---|---|---|---|---|---|---|
| CO1 | C | 27 | 58 | 291 | 136 | 28.2 | 147 | 49.8 | 9.59 | 19.7 |
| CO2 | C | 27 | 91.4 | 286 | 136 | 34.2 | 154 | 45 | 8.66 | 17.8 |
| CS1 | C | 30 | 101 | 512 | 140 | 32.4 | 154 | 71.3 | 13 | 26.6 |
| CS2 | C | 30 | 88.2 | 345 | 127 | 52.4 | 134 | 48.8 | 8.91 | 18.2 |
| Group | Variable | n | Min | Max | Median | iqr | Mean | sd | se | ci |
|---|---|---|---|---|---|---|---|---|---|---|
| CO_CRH1 | CRH | 27 | 0.988 | 3.21 | 1.58 | 0.61 | 1.77 | 0.521 | 0.1 | 0.206 |
| CO_CRH2 | CRH | 27 | 1.26 | 2.40 | 1.60 | 0.533 | 1.67 | 0.345 | 0.066 | 0.136 |
| CS_CRH1 | CRH | 30 | 1.17 | 2.34 | 1.73 | 0.322 | 1.72 | 0.274 | 0.05 | 0.102 |
| CS_CRH2 | CRH | 30 | 1.29 | 4.98 | 2.17 | 0.801 | 2.31 | 0.844 | 0.154 | 0.315 |
| Group 1 | Group 2 | n1 | n2 | Statistic | p p.adj | p.adj.signif |
|---|---|---|---|---|---|---|
| CCO1 | CCO2 | 27 | 27 | 0.675 | 0.500 | 0.500 ns |
| CCO1 | CCS1 | 27 | 30 | 0.432 | 0.665 | 0.665 ns |
| CCO1 | CCS2 | 27 | 30 | −1.38 | 0.166 | 0.166 ns |
| CCO2 | CCS1 | 27 | 30 | −0.260 | 0.795 | 0.795 ns |
| CCO2 | CCS2 | 27 | 30 | −2.08 | 0.0377 | 0.0377 * |
| CCS1 | CCS2 | 30 | 30 | −1.87 | 0.0619 | 0.0619 ns |
| Group 1 | Group 2 | n1 | n2 | Statistic | p p.adj | p.adj.signif |
|---|---|---|---|---|---|---|
| CO_CRH1 | CO_CRH2 | 27 | 27 | −0.506 | 0.613 | 1 ns |
| CO_CRH1 | CS_CRH1 | 27 | 30 | 0.218 | 0.827 | 1 ns |
| CO_CRH1 | CS_CRH2 | 27 | 30 | 3.27 | 0.00106 | 0.00634 ** |
| CO_CRH2 | CS_CRH1 | 27 | 30 | 0.738 | 0.461 | 1 ns |
| CO_CRH2 | CS_CRH2 | 27 | 30 | 3.79 | 0.000148 | 0.000887 *** |
| CS_CRH1 | CS_CRH2 | 30 | 30 | 3.14 | 0.00169 | 0.0101 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Bordoni, B.; Siatkowski, I.; Żekanowska, E. The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial. Behav. Sci. 2023, 13, 914. https://doi.org/10.3390/bs13110914
Wójcik M, Bordoni B, Siatkowski I, Żekanowska E. The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial. Behavioral Sciences. 2023; 13(11):914. https://doi.org/10.3390/bs13110914
Chicago/Turabian StyleWójcik, Małgorzata, Bruno Bordoni, Idzi Siatkowski, and Ewa Żekanowska. 2023. "The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial" Behavioral Sciences 13, no. 11: 914. https://doi.org/10.3390/bs13110914
APA StyleWójcik, M., Bordoni, B., Siatkowski, I., & Żekanowska, E. (2023). The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial. Behavioral Sciences, 13(11), 914. https://doi.org/10.3390/bs13110914








