The Interplay between Chronotype and Emotion Regulation in the Recognition of Facial Expressions of Emotion
Abstract
1. Introduction
1.1. Chronotype and Emotional Face Recognition
1.2. Chronotype, Emotion Regulation Strategy and Emotional Processing
1.3. The Present Study
2. Method
2.1. Participants and Procedure
2.2. Materials
2.2.1. Chronotype
2.2.2. Emotion Regulation
2.2.3. Facial Emotion Recognition Task
2.3. Data Analysis
3. Results
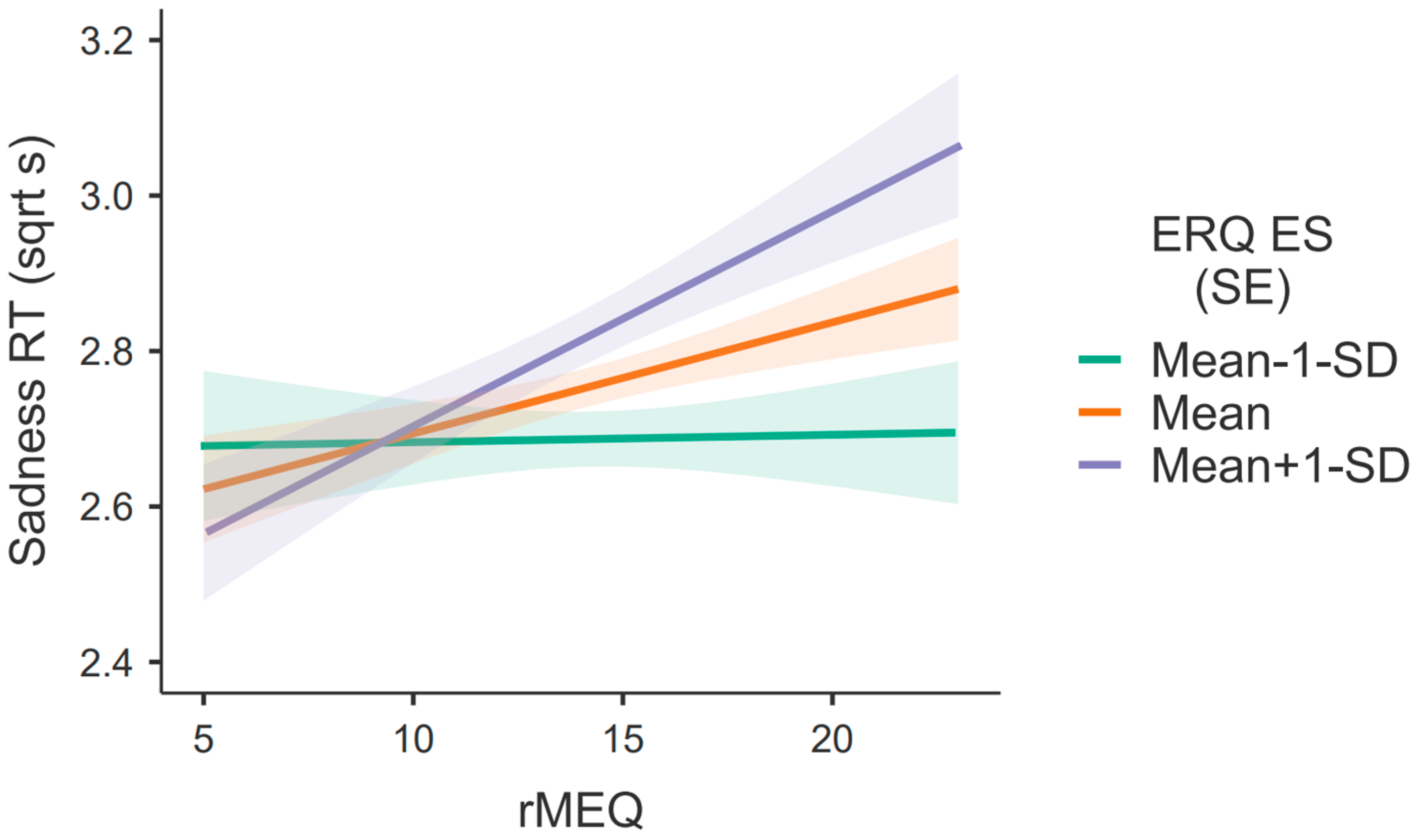
3.1. Models with Expressive Suppression
3.2. Models with Cognitive Reappraisal
4. Discussion
4.1. Chronotype and Emotional Facial Expressions
4.2. Emotion Regulation and Emotional Facial Expressions
4.3. Moderating Role of Emotion Regulation on the Relationship between Chronotype and Facial Expression Recognition
4.4. Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bach, D.R.; Dayan, P. Algorithms for Survival: A Comparative Perspective on Emotions. Nat. Rev. Neurosci. 2017, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, V.; Papaleo, F. Understanding Others: Emotion Recognition in Humans and Other Animals. Genes Brain Behav. 2019, 18, e12544. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.A.; Kozak, M.N.; Ambady, N. Accurate Identification of Fear Facial Expressions Predicts Prosocial Behavior. Emotion 2007, 7, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Ekman, P. An Argument for Basic Emotions. Cogn. Emot. 1992, 6, 169–200. [Google Scholar] [CrossRef]
- Theurel, A.; Witt, A.; Malsert, J.; Lejeune, F.; Fiorentini, C.; Barisnikov, K.; Gentaz, E. The Integration of Visual Context Information in Facial Emotion Recognition in 5-to 15-Year-Olds. J. Exp. Child Psychol. 2016, 150, 252–271. [Google Scholar] [CrossRef]
- Wells, L.J.; Gillespie, S.M.; Rotshtein, P. Identification of Emotional Facial Expressions: Effects of Expression, Intensity, and Sex on Eye Gaze. PLoS ONE 2016, 11, e0168307. [Google Scholar] [CrossRef]
- Elfenbein, H.A.; Mandal, M.K.; Ambady, N.; Harizuka, S.; Kumar, S. Cross-Cultural Patterns in Emotion Recognition: Highlighting Design and Analytical Techniques. Emotion 2002, 2, 75–84. [Google Scholar] [CrossRef]
- Cooper, H.; Brar, A.; Beyaztas, H.; Jennings, B.J.; Bennetts, R.J. The Effects of Face Coverings, Own-Ethnicity Biases, and Attitudes on Emotion Recognition. Cogn. Res. Princ. Implic. 2022, 7, 57. [Google Scholar] [CrossRef]
- Lunn, J.; Chen, J.-Y. Chronotype and Time of Day Effects on Verbal and Facial Emotional Stroop Task Performance in Adolescents. Chronobiol. Int. 2022, 39, 323–332. [Google Scholar] [CrossRef]
- Horne, C.M.; Marr-Phillips, S.D.M.; Jawaid, R.; Gibson, E.L.; Norbury, R. Negative Emotional Biases in Late Chronotypes. Biol. Rhythm Res. 2017, 48, 151–155. [Google Scholar] [CrossRef]
- Itzek-Greulich, H.; Randler, C.; Vollmer, C. The Interaction of Chronotype and Time of Day in a Science Course: Adolescent Evening Types Learn More and Are More Motivated in the Afternoon. Learn. Individ. Differ. 2016, 51, 189–198. [Google Scholar] [CrossRef]
- Schneider, J.; Fárková, E.; Bakštein, E. Human Chronotype: Comparison of Questionnaires and Wrist-Worn Actigraphy. Chronobiol. Int. 2022, 39, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Schmidt, C.; Collette, F.; Cajochen, C.; Peigneux, P. A Time to Think: Circadian Rhythms in Human Cognition. Cogn. Neuropsychol. 2007, 24, 755–789. [Google Scholar] [CrossRef] [PubMed]
- Berdynaj, D.; Boudissa, S.N.; Grieg, M.S.; Hope, C.; Mahamed, S.H.; Norbury, R. Effect of Chronotype on Emotional Processing and Risk Taking. Chronobiol. Int. 2016, 33, 406–418. [Google Scholar] [CrossRef]
- Randler, C.; Weber, V. Positive and Negative Affect during the School Day and Its Relationship to Morningness–Eveningness. Biol. Rhythm Res. 2015, 46, 683–690. [Google Scholar] [CrossRef]
- Au, J.; Reece, J. The Relationship between Chronotype and Depressive Symptoms: A Meta-Analysis. J. Affect. Disord. 2017, 218, 93–104. [Google Scholar] [CrossRef]
- Nielsen, T. Nightmares Associated with the Eveningness Chronotype. J. Biol. Rhythms 2010, 25, 53–62. [Google Scholar] [CrossRef]
- Murray, G.; Allen, N.B.; Trinder, J. Seasonality and Circadian Phase Delay: Prospective Evidence That Winter Lowering of Mood Is Associated with a Shift towards Eveningness. J. Affect. Disord. 2003, 76, 15–22. [Google Scholar] [CrossRef]
- Park, C.I.; An, S.K.; Kim, H.W.; Koh, M.J.; Namkoong, K.; Kang, J.I.; Kim, S.J. Relationships between Chronotypes and Affective Temperaments in Healthy Young Adults. J. Affect. Disord. 2015, 175, 256–259. [Google Scholar] [CrossRef]
- Chrobak, A.A.; Tereszko, A.; Dembinska-Krajewska, D.; Arciszewska, A.; Siwek, M.; Dudek, D.; Rybakowski, J. Morningness–Eveningness and Affective Temperaments Assessed by the Temperament Evaluation of Memphis, Pisa and San Diego-Autoquestionnaire (TEMPS-A). Chronobiol. Int. 2017, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Horne, C.M.; Norbury, R. Late Chronotype Is Associated with Enhanced Amygdala Reactivity and Reduced Fronto-Limbic Functional Connectivity to Fearful versus Happy Facial Expressions. NeuroImage 2018, 171, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.; Hasler, B.P. Chronotype and Mental Health: Recent Advances. Curr. Psychiatry Rep. 2018, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Toomey, R.; Panizzon, M.S.; Kremen, W.S.; Franz, C.E.; Lyons, M.J. A Twin-Study of Genetic Contributions to Morningness–Eveningness and Depression. Chronobiol. Int. 2015, 32, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Vlasac, I.; Anderson, S.G.; Kyle, S.D.; Dixon, W.G.; Bechtold, D.A.; Gill, S.; Little, M.A.; Luik, A.; Loudon, A.; et al. Genome-Wide Association Analysis Identifies Novel Loci for Chronotype in 100,420 Individuals from the UK Biobank. Nat. Commun. 2016, 7, 10889. [Google Scholar] [CrossRef]
- Merikanto, I.; Kronholm, E.; Peltonen, M.; Laatikainen, T.; Vartiainen, E.; Partonen, T. Circadian Preference Links to Depression in General Adult Population. J. Affect. Disord. 2015, 188, 143–148. [Google Scholar] [CrossRef]
- Gross, J.J. The Emerging Field of Emotion Regulation: An Integrative Review. Rev. Gen. Psychol. 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Gross, J.J. Emotion Regulation: Conceptual and Empirial Foundations. In Handbook of Emotion Regulation; The Guilford Press: New York, NY, USA, 2014; pp. 3–20. [Google Scholar]
- De France, K.; Hollenstein, T. Emotion Regulation and Relations to Well-Being across the Lifespan. Dev. Psychol. 2019, 55, 1768–1774. [Google Scholar] [CrossRef]
- Sheppes, G.; Suri, G.; Gross, J.J. Emotion Regulation and Psychopathology. Annu. Rev. Clin. Psychol. 2015, 11, 379–405. [Google Scholar] [CrossRef]
- Waugh, C.E.; Zarolia, P.; Mauss, I.B.; Lumian, D.S.; Ford, B.Q.; Davis, T.S.; Ciesielski, B.G.; Sams, K.V.; McRae, K. Emotion Regulation Changes the Duration of the BOLD Response to Emotional Stimuli. Soc. Cogn. Affect. Neurosci. 2016, 11, 1550–1559. [Google Scholar] [CrossRef][Green Version]
- Harrison, A.; Sullivan, S.; Tchanturia, K.; Treasure, J. Emotion Recognition and Regulation in Anorexia Nervosa. Clin. Psychol. Psychother. 2009, 16, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Percinel, I.; Ozbaran, B.; Kose, S.; Simsek, D.G.; Darcan, S. Increased Deficits in Emotion Recognition and Regulation in Children and Adolescents with Exogenous Obesity. World J. Biol. Psychiatry 2018, 19, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.L.; Norbury, R. Reduced Effective Emotion Regulation in Night Owls. J. Biol. Rhythms 2017, 32, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, J.M. Circadian Typology Is Related to Emotion Regulation, Metacognitive Beliefs and Assertiveness in Healthy Adults. PLoS ONE 2020, 15, e0230169. [Google Scholar] [CrossRef] [PubMed]
- Della Longa, L.; Nosarti, C.; Farroni, T. Emotion Recognition in Preterm and Full-Term School-Age Children. Int. J. Environ. Res. Public. Health 2022, 19, 6507. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; Baeken, C.; Van Schuerbeek, P.; Luypaert, R.; De Raedt, R. Inter-Individual Differences in the Habitual Use of Cognitive Reappraisal and Expressive Suppression Are Associated with Variations in Prefrontal Cognitive Control for Emotional Information: An Event Related FMRI Study. Biol. Psychol. 2013, 92, 433–439. [Google Scholar] [CrossRef]
- Harrison, A.; Sullivan, S.; Tchanturia, K.; Treasure, J. Emotional Functioning in Eating Disorders: Attentional Bias, Emotion Recognition and Emotion Regulation. Psychol. Med. 2010, 40, 1887–1897. [Google Scholar] [CrossRef]
- Aldinger, M.; Stopsack, M.; Barnow, S.; Rambau, S.; Spitzer, C.; Schnell, K.; Ulrich, I. The Association between Depressive Symptoms and Emotion Recognition Is Moderated by Emotion Regulation. Psychiatry Res. 2013, 205, 59–66. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, D.; Wang, J.; Mistry, R.; Ran, G.; Wang, X. Relation between Emotion Regulation and Mental Health: A Meta-Analysis Review. Psychol. Rep. 2014, 114, 341–362. [Google Scholar] [CrossRef]
- Schunk, F.; Trommsdorff, G.; König-Teshnizi, D. Regulation of Positive and Negative Emotions across Cultures: Does Culture Moderate Associations between Emotion Regulation and Mental Health? Cogn. Emot. 2022, 36, 352–363. [Google Scholar] [CrossRef]
- Berking, M.; Wupperman, P. Emotion Regulation and Mental Health: Recent Findings, Current Challenges, and Future Directions. Curr. Opin. Psychiatry 2012, 25, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J.; Muñoz, R.F. Emotion Regulation and Mental Health. Clin. Psychol. Sci. Pract. 1995, 2, 151–164. [Google Scholar] [CrossRef]
- Joormann, J.; Stanton, C.H. Examining Emotion Regulation in Depression: A Review and Future Directions. Behav. Res. Ther. 2016, 86, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Ambadar, Z.; Schooler, J.W.; Cohn, J.F. Deciphering the Enigmatic Face: The Importance of Facial Dynamics in Interpreting Subtle Facial Expressions. Psychol. Sci. 2005, 16, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Darke, H.; Cropper, S.J.; Carter, O. A Novel Dynamic Morphed Stimuli Set to Assess Sensitivity to Identity and Emotion Attributes in Faces. Front. Psychol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Yitzhak, N.; Gilaie-Dotan, S.; Aviezer, H. The Contribution of Facial Dynamics to Subtle Expression Recognition in Typical Viewers and Developmental Visual Agnosia. Neuropsychologia 2018, 117, 26–35. [Google Scholar] [CrossRef]
- Kosonogov, V.; Titova, A. Recognition of All Basic Emotions Varies in Accuracy and Reaction Time: A New Verbal Method of Measurement. Int. J. Psychol. 2019, 54, 582–588. [Google Scholar] [CrossRef]
- Bridges, D.; Pitiot, A.; MacAskill, M.R.; Peirce, J.W. The Timing Mega-Study: Comparing a Range of Experiment Generators, Both Lab-Based and Online. PeerJ 2020, 8, e9414. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in Behavior Made Easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Adan, A.; Almirall, H. Horne & Östberg Morningness-Eveningness Questionnaire: A Reduced Scale. Personal. Individ. Differ. 1991, 12, 241–253. [Google Scholar] [CrossRef]
- Loureiro, F.; Garcia-Marques, T. Morning or Evening Person? Which Type Are You? Self-Assessment of Chronotype. Personal. Individ. Differ. 2015, 86, 168–171. [Google Scholar] [CrossRef]
- Gross, J.J.; John, O.P. Individual Differences in Two Emotion Regulation Processes: Implications for Affect, Relationships, and Well-Being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.J.d.S.M. Diferenciação e Regulação Emocional na Idade Adulta: Tradução e Validação de dois Instrumentos de Avaliação para a População Portuguesa. Master’s Thesis, Instituto de Educação e Psicologia Universidade do Minho, Braga, Portugal, 2009. [Google Scholar]
- Goeleven, E.; De Raedt, R.; Leyman, L.; Verschuere, B. The Karolinska Directed Emotional Faces: A Validation Study. Cogn. Emot. 2008, 22, 1094–1118. [Google Scholar] [CrossRef]
- Kivelä, L.; Papadopoulos, M.R.; Antypa, N. Chronotype and Psychiatric Disorders. Curr. Sleep Med. Rep. 2018, 4, 94–103. [Google Scholar] [CrossRef]
- Clark, L.; Chamberlain, S.R.; Sahakian, B.J. Neurocognitive Mechanisms in Depression: Implications for Treatment. Annu. Rev. Neurosci. 2009, 32, 57–74. [Google Scholar] [CrossRef]
- Milders, M.; Bell, S.; Platt, J.; Serrano, R.; Runcie, O. Stable Expression Recognition Abnormalities in Unipolar Depression. Psychiatry Res. 2010, 179, 38–42. [Google Scholar] [CrossRef]
- Antypa, N.; Verkuil, B.; Molendijk, M.; Schoevers, R.; Penninx, B.W.J.H.; Van Der Does, W. Associations between Chronotypes and Psychological Vulnerability Factors of Depression. Chronobiol. Int. 2017, 34, 1125–1135. [Google Scholar] [CrossRef]
- Ottoni, G.L.; Antoniolli, E.; Lara, D.R. Circadian Preference Is Associated with Emotional and Affective Temperaments. Chronobiol. Int. 2012, 29, 786–793. [Google Scholar] [CrossRef]
- Carciofo, R. Morning Affect, Eveningness, and Amplitude Distinctness: Associations with Negative Emotionality, Including the Mediating Roles of Sleep Quality, Personality, and Metacognitive Beliefs. Chronobiol. Int. 2020, 37, 1565–1579. [Google Scholar] [CrossRef]
- Kivelä, L.; Riese, H.; Fakkel, T.G.; Verkuil, B.; Penninx, B.W.J.H.; Lamers, F.; van der Does, W.; Antypa, N. Chronotype, Daily Affect and Social Contact: An Ecological Momentary Assessment Study. Psychiatry Res. 2022, 309, 114386. [Google Scholar] [CrossRef]
- Miller, M.A.; Rothenberger, S.D.; Hasler, B.P.; Donofry, S.D.; Wong, P.M.; Manuck, S.B.; Kamarck, T.W.; Roecklein, K.A. Chronotype Predicts Positive Affect Rhythms Measured by Ecological Momentary Assessment. Chronobiol. Int. 2015, 32, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, J.M.; Hietanen, J.K. Positive Facial Expressions Are Recognized Faster than Negative Facial Expressions, but Why? Psychol. Res. 2004, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Surguladze, S.A.; El-Hage, W.; Dalgleish, T.; Radua, J.; Gohier, B.; Phillips, M.L. Depression Is Associated with Increased Sensitivity to Signals of Disgust: A Functional Magnetic Resonance Imaging Study. J. Psychiatr. Res. 2010, 44, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Park, C.I.; Sohn, S.; Kim, H.W.; Namkoong, K.; Kim, S.J. Circadian Preference and Trait Impulsivity, Sensation-Seeking and Response Inhibition in Healthy Young Adults. Chronobiol. Int. 2015, 32, 235–241. [Google Scholar] [CrossRef]
- Mathersul, D.; Palmer, D.M.; Gur, R.C.; Gur, R.E.; Cooper, N.; Gordon, E.; Williams, L.M. Explicit Identification and Implicit Recognition of Facial Emotions: II. Core Domains and Relationships with General Cognition. J. Clin. Exp. Neuropsychol. 2009, 31, 278–291. [Google Scholar] [CrossRef]
- Calvo, M.G.; Beltrán, D. Recognition Advantage of Happy Faces: Tracing the Neurocognitive Processes. Neuropsychologia 2013, 51, 2051–2061. [Google Scholar] [CrossRef]
- Schneider, K.G.; Hempel, R.J.; Lynch, T.R. That “Poker Face” Just Might Lose You the Game! The Impact of Expressive Suppression and Mimicry on Sensitivity to Facial Expressions of Emotion. Emotion 2013, 13, 852–866. [Google Scholar] [CrossRef]
- Ortner, C.N.M.; Zelazo, P.D.; Anderson, A.K. Effects of Emotion Regulation on Concurrent Attentional Performance. Motiv. Emot. 2013, 37, 346–354. [Google Scholar] [CrossRef]
- Joormann, J.; Gotlib, I.H. Emotion Regulation in Depression: Relation to Cognitive Inhibition. Cogn. Emot. 2010, 24, 281–298. [Google Scholar] [CrossRef]
- Neta, M.; Kelley, W.M.; Whalen, P.J. Neural Responses to Ambiguity Involve Domain-General and Domain-Specific Emotion Processing Systems. J. Cogn. Neurosci. 2013, 25, 547–557. [Google Scholar] [CrossRef]
- Neta, M.; Norris, C.J.; Whalen, P.J. Corrugator Muscle Responses Are Associated with Individual Differences in Positivity-Negativity Bias. Emotion 2009, 9, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ding, Q.; Wang, Y.; Wu, M.; Gao, T.; Liu, X. The Effect of Cognitive Reappraisal and Expression Suppression on Sadness and the Recognition of Sad Scenes: An Event-Related Potential Study. Front. Psychol. 2022, 13, 5851. [Google Scholar] [CrossRef] [PubMed]
- Randler, C.; Díaz-Morales, J.F.; Rahafar, A.; Vollmer, C. Morningness–Eveningness and Amplitude–Development and Validation of an Improved Composite Scale to Measure Circadian Preference and Stability (MESSi). Chronobiol. Int. 2016, 33, 832–848. [Google Scholar] [CrossRef] [PubMed]


| Emotion | Main Effect rMEQ | Main Effect ES | rMEQ × ES | Moderating Effect of ES? |
|---|---|---|---|---|
| Happiness | p = 0.037, | p = 0.186, | p = 0.416, | NO |
| η2p = 0.017 | η2p = 0.007 | η2p = 0.003 | ||
| Sadness | p = 0.041, | p = 0.008, | p = 0.038, | YES |
| η2p = 0.017 | η2p = 0.007 | η2p = 0.017 | ||
| Fear | p = 0.110, | p = 0.806, | p = 0.749, | NO |
| η2p = 0.010 | η2p < 0.001 | η2p < 0.001 | ||
| Disgust | p = 0.022, | p = 0.043, | p = 0.478, | NO |
| η2p = 0.021 | η2p = 0.016 | η2p = 0.002 | ||
| Anger | p = 0.249, | p = 0.006, | p = 0.023, | YES |
| η2p = 0.005 | η2p = 0.029 | η2p = 0.020 | ||
| Surprise | p = 0.054, | p = 0.014, | p = 0.090, | NO |
| η2p = 0.015 | η2p = 0.024 | η2p = 0.011 |
| Emotion | Main Effect rMEQ | Main Effect CR | rMEQ × CR | Moderating Effect of CR? |
|---|---|---|---|---|
| Happiness | p = 0.034, | p = 0.453, | p = 0.255, | NO |
| η2p = 0.018 | η2p = 0.002 | η2p = 0.005 | ||
| Sadness | p = 0.037, | p = 0.632, | p = 0.208, | NO |
| η2p = 0.017 | η2p = 0.001 | η2p = 0.006 | ||
| Fear | p = 0.086, | p = 0.626, | p = 0.323, | NO |
| η2p = 0.012 | η2p = 0.001 | η2p = 0.004 | ||
| Disgust | p = 0.029, | p = 0.889, | p = 0.823, | NO |
| η2p = 0.019 | η2p < 0.001 | η2p = 0.002 | ||
| Anger | p = 0.240, | p = 0.658, | p = 0.203, | NO |
| η2p = 0.005 | η2p = 0.001 | η2p = 0.006 | ||
| Surprise | p = 0.070, | p = 0.403, | p = 0.869, | NO |
| η2p = 0.013 | η2p = 0.003 | η2p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.M.; Bem-Haja, P.; Silva, A.; Rosa, C.; Queiroz, D.F.; Alves, M.F.; Barroso, T.; Cerri, L.; Silva, C.F. The Interplay between Chronotype and Emotion Regulation in the Recognition of Facial Expressions of Emotion. Behav. Sci. 2023, 13, 38. https://doi.org/10.3390/bs13010038
Santos IM, Bem-Haja P, Silva A, Rosa C, Queiroz DF, Alves MF, Barroso T, Cerri L, Silva CF. The Interplay between Chronotype and Emotion Regulation in the Recognition of Facial Expressions of Emotion. Behavioral Sciences. 2023; 13(1):38. https://doi.org/10.3390/bs13010038
Chicago/Turabian StyleSantos, Isabel M., Pedro Bem-Haja, André Silva, Catarina Rosa, Diâner F. Queiroz, Miguel F. Alves, Talles Barroso, Luíza Cerri, and Carlos F. Silva. 2023. "The Interplay between Chronotype and Emotion Regulation in the Recognition of Facial Expressions of Emotion" Behavioral Sciences 13, no. 1: 38. https://doi.org/10.3390/bs13010038
APA StyleSantos, I. M., Bem-Haja, P., Silva, A., Rosa, C., Queiroz, D. F., Alves, M. F., Barroso, T., Cerri, L., & Silva, C. F. (2023). The Interplay between Chronotype and Emotion Regulation in the Recognition of Facial Expressions of Emotion. Behavioral Sciences, 13(1), 38. https://doi.org/10.3390/bs13010038








