Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Preparations
2.3. Experimental Design
2.4. Behavioural Tests
2.4.1. Grip Strength Test
2.4.2. Y-Maze Spontaneous Alternation Task
2.4.3. Novel Object Recognition Test (NORT)
2.4.4. Novel Arm Discrimination Task (NADT)
2.5. Histological Examination
2.6. Statistical Analysis
3. Results
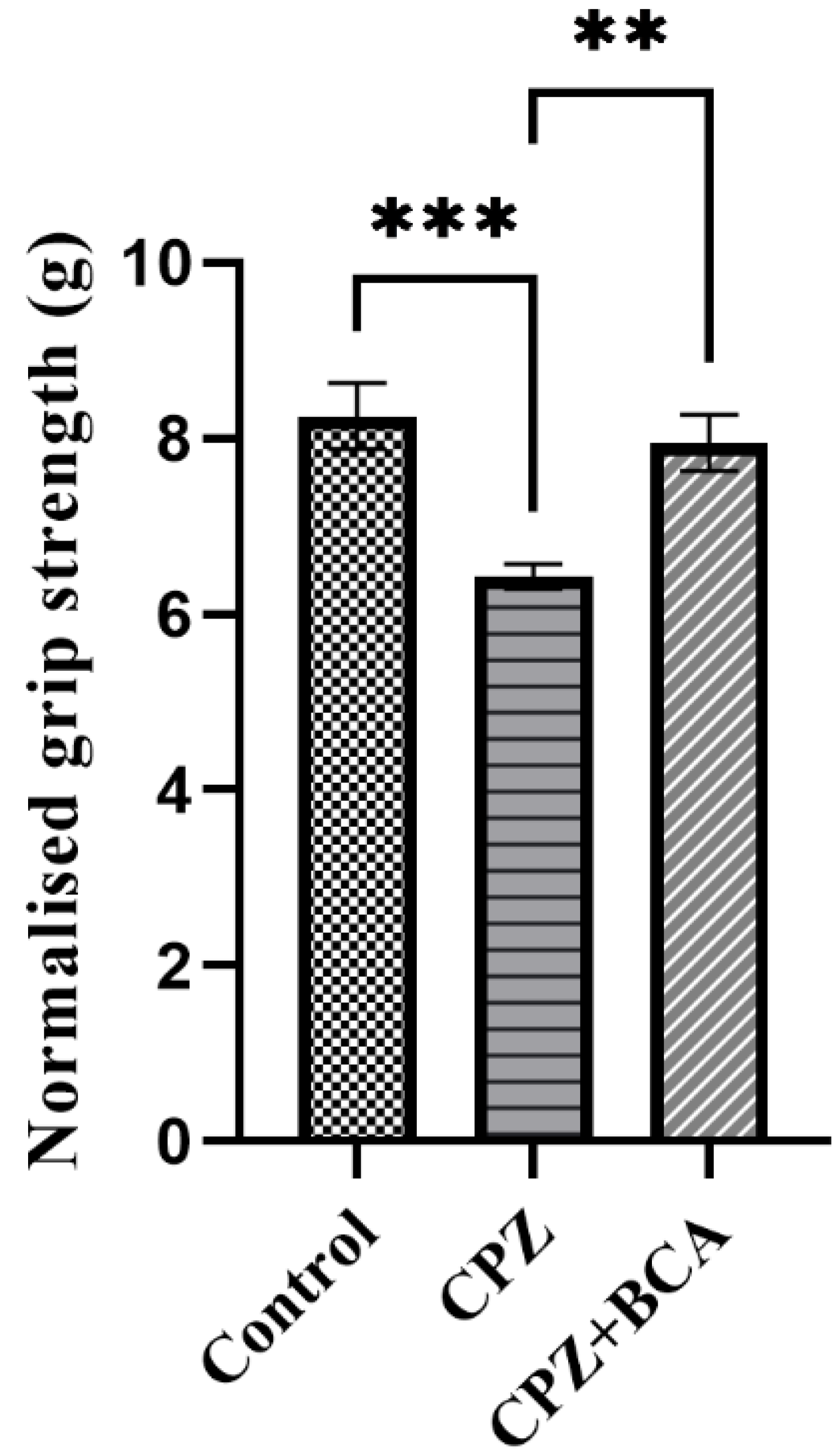
3.1. BCA Improved the Grip Strength in the CPZ Model
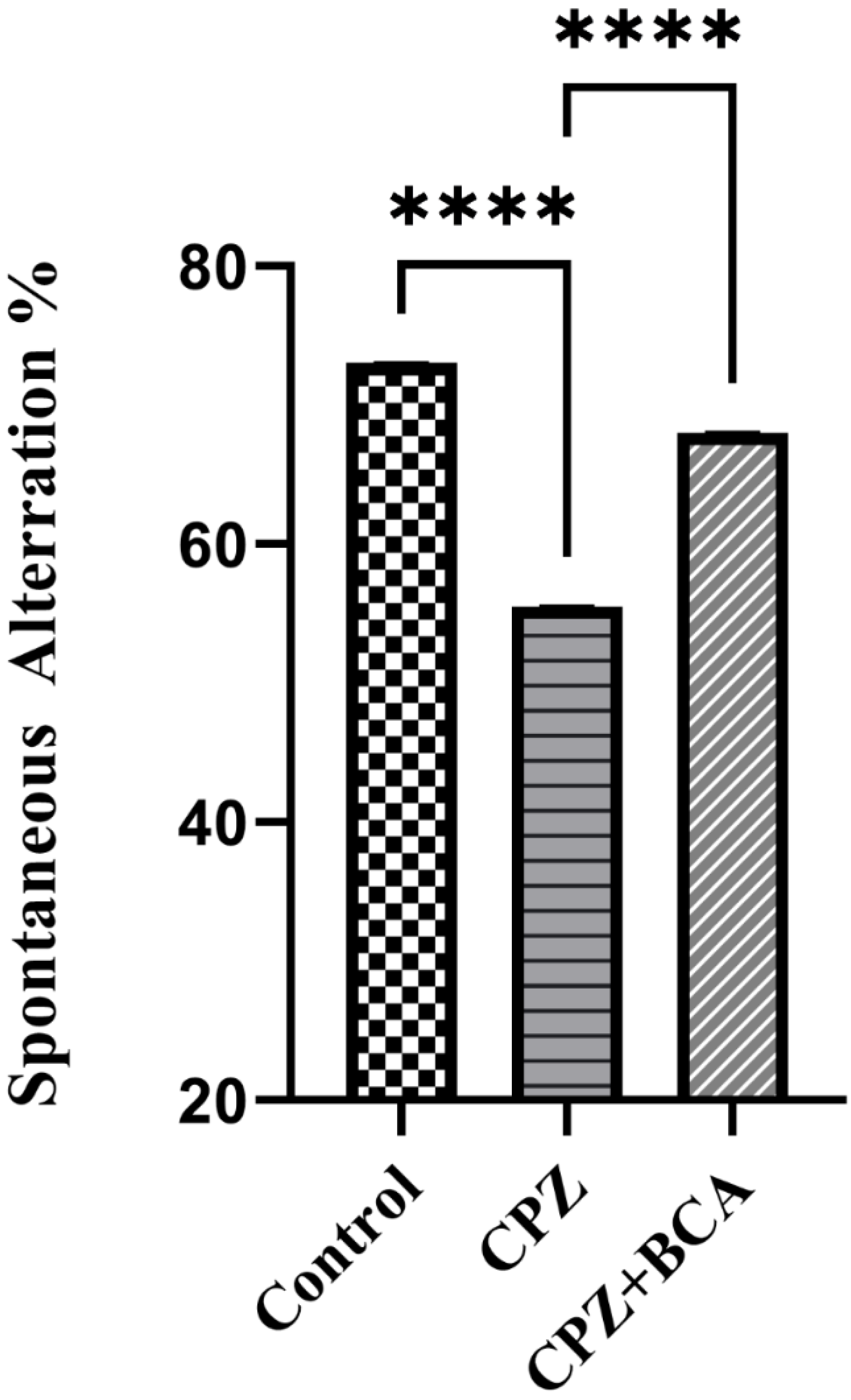
3.2. BCA Improved the Spontaneous Alteration in the CPZ Model
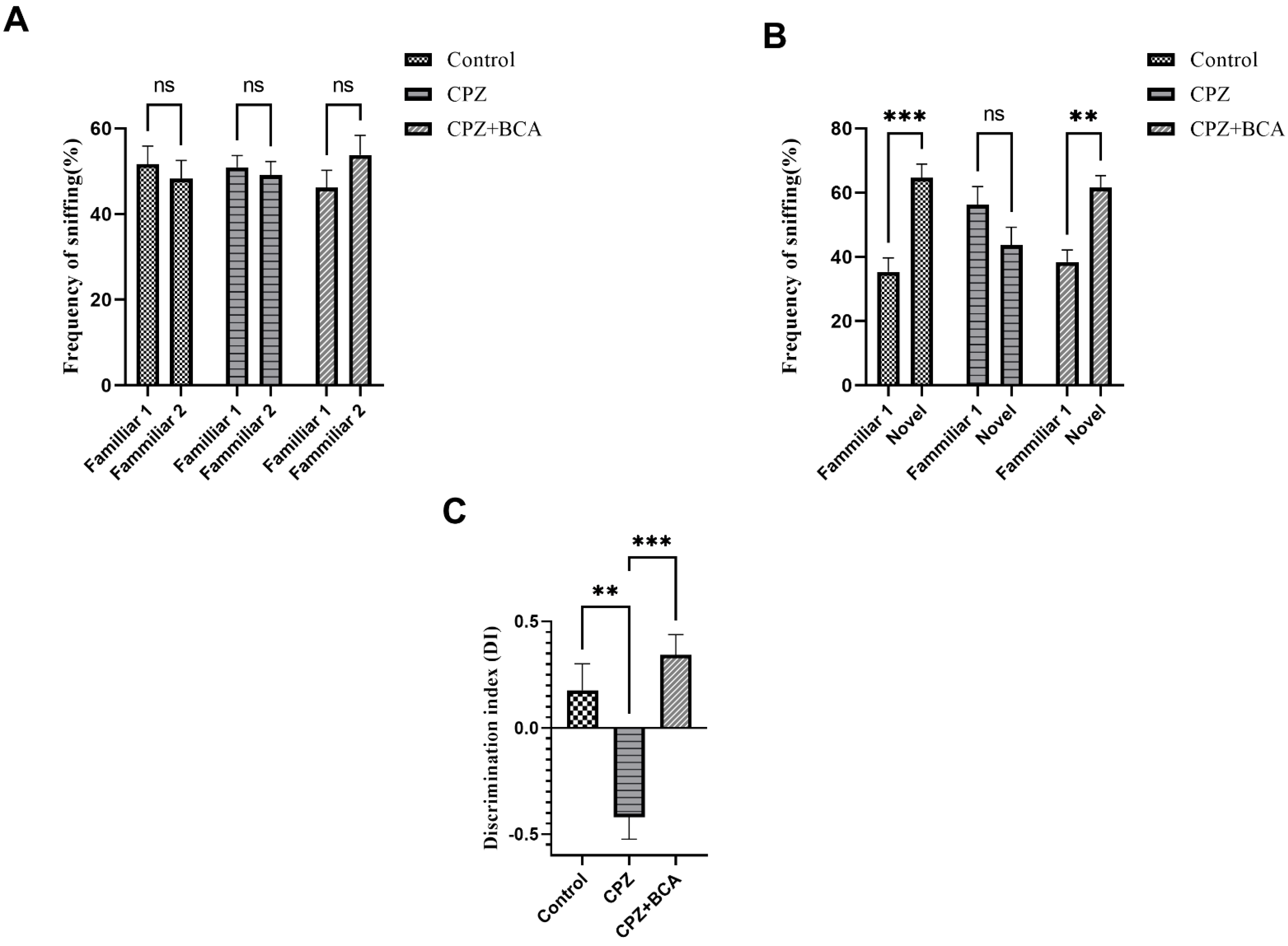
3.3. BCA Improved the DI and the Frequency of Sniffing in CPZ Model
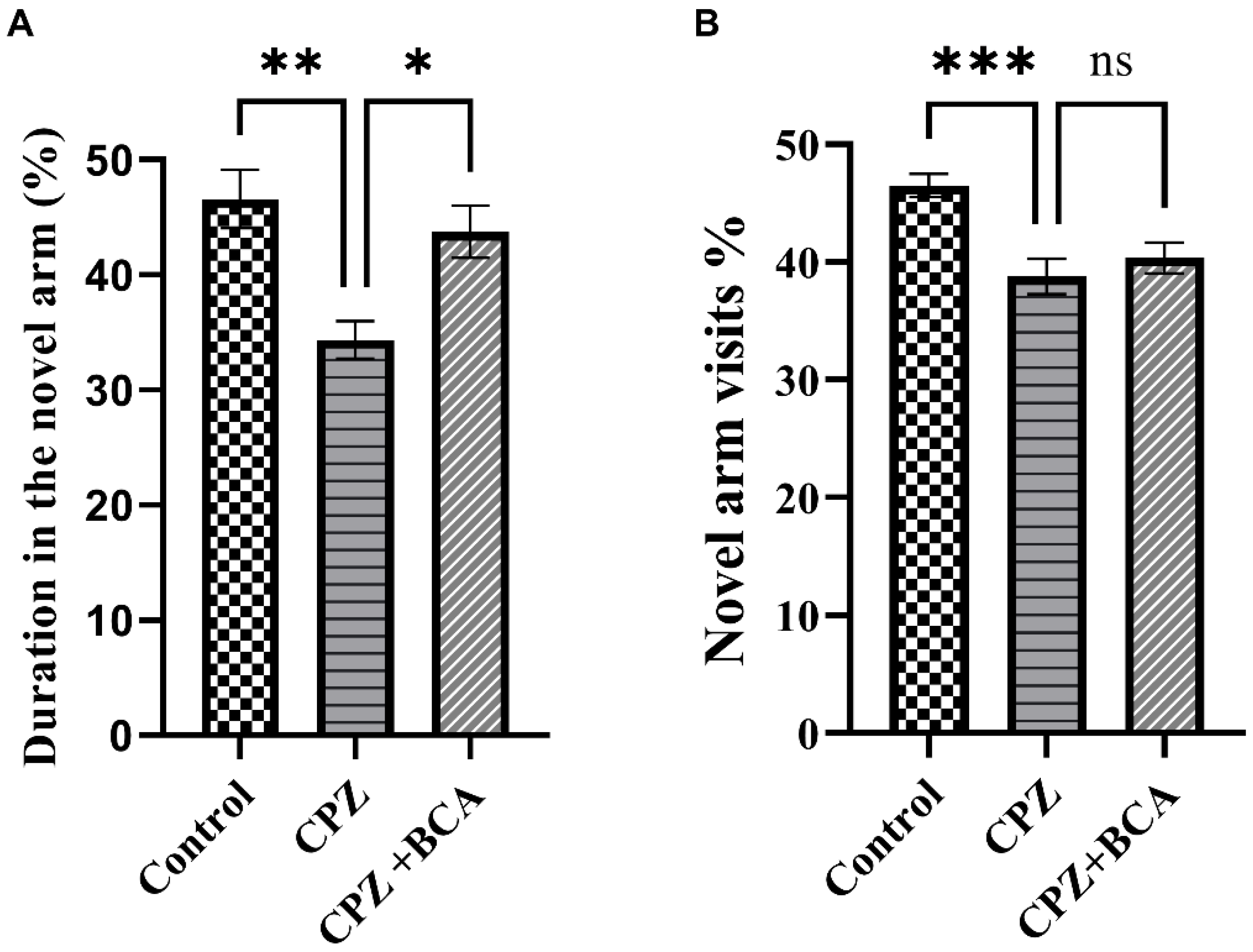
3.4. BCA Improved the Duration in the Novel Arm in the CPZ Model
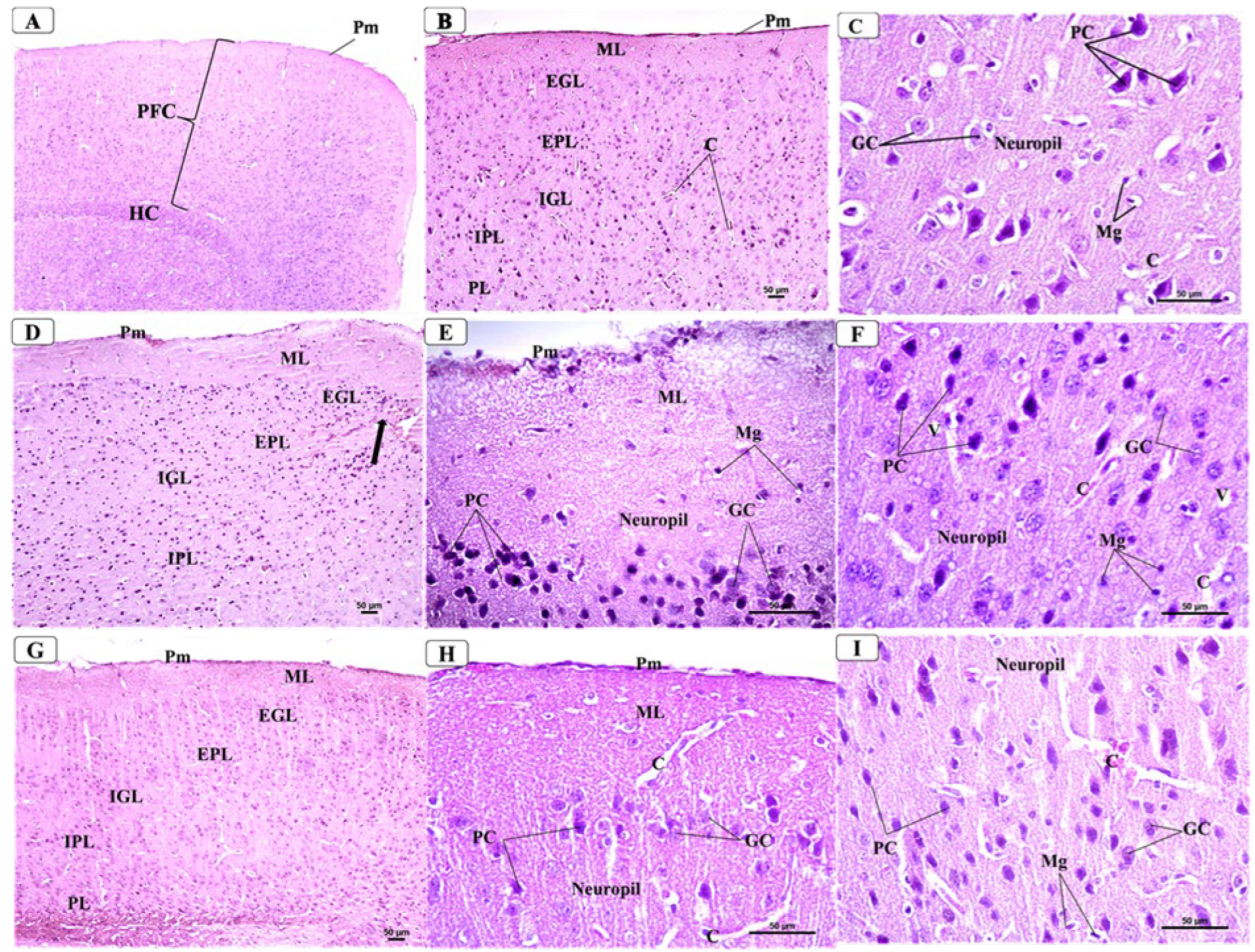
3.5. BCA Mitigated Neuronal Damage in the PFC in the CPZ Model
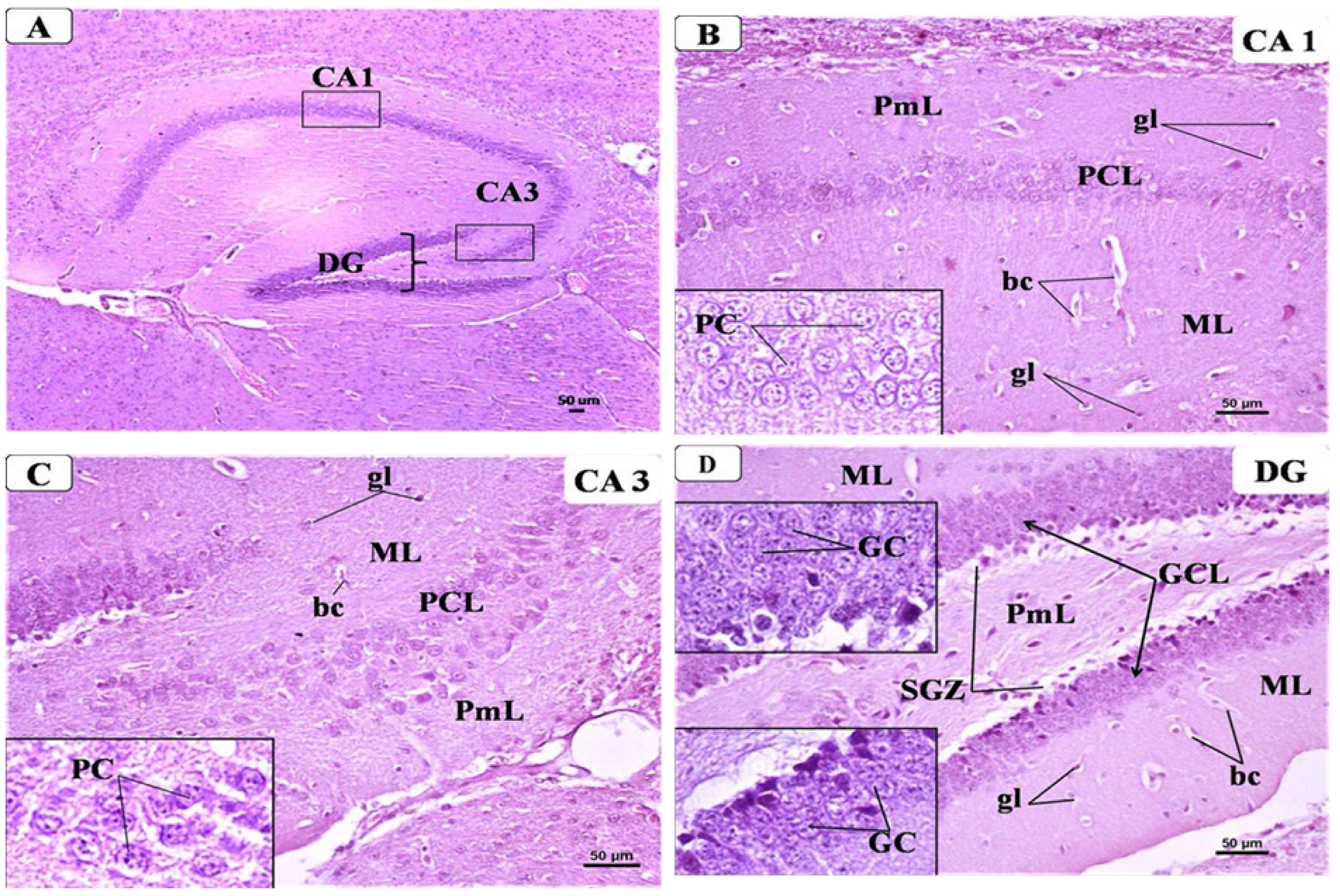
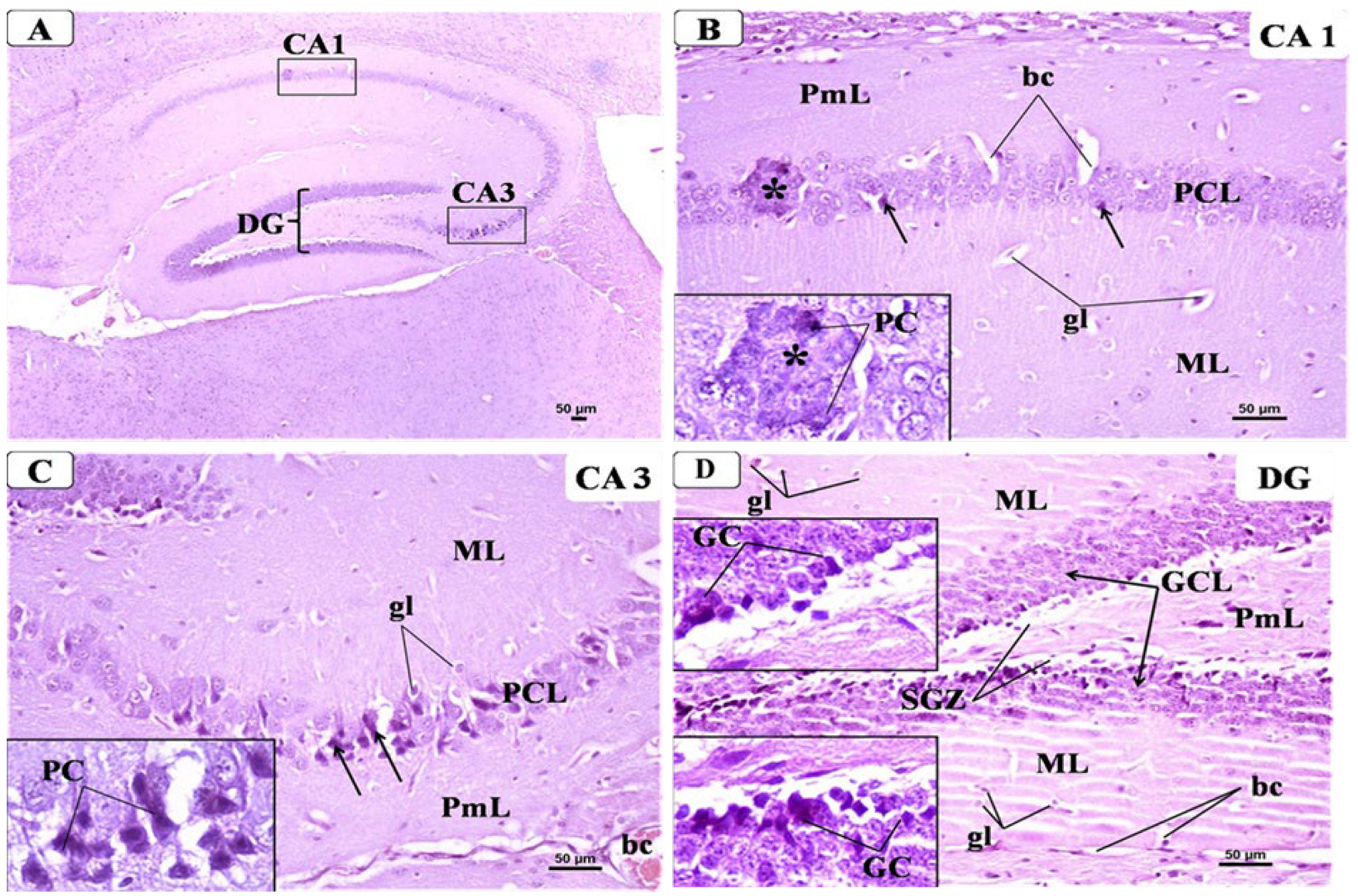
3.6. BCA Mitigated Neuronal Damage in the Hippocampus in the CPZ Model
3.6.1. Control Group
3.6.2. CPZ Group
3.6.3. CPZ + BCA Group
4. Discussion
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, A.M. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Salvadori, N.; Chipi, E.; Gentili, L.; Borrelli, A.; Parnetti, L.; Di Filippo, M. Cognitive impairment in multiple sclerosis: Lessons from cerebrospinal fluid biomarkers. Neural Regen. Res. 2021, 16, 36. [Google Scholar]
- Grzegorski, T.; Losy, J. Cognitive impairment in multiple sclerosis—A review of current knowledge and recent research. Rev. Neurosci. 2017, 28, 845–860. [Google Scholar] [CrossRef]
- Meca-Lallana, V.; Gascón-Giménez, F.; Ginestal-López, R.C.; Higueras, Y.; Téllez-Lara, N.; Carreres-Polo, J.; Eichau-Madueño, S.; Romero-Imbroda, J.; Vidal-Jordana, Á.; Pérez-Miralles, F. Cognitive impairment in multiple sclerosis: Diagnosis and monitoring. Neurol. Sci. 2021, 42, 5183–5193. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Nickel, M.; Gu, C. Regulation of Central Nervous System Myelination in Higher Brain Functions. Neural Plast. 2018, 2018, 6436453. [Google Scholar] [CrossRef]
- Alghamdi, B. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J. Integr. Neurosci. 2020, 19, 229–237. [Google Scholar] [CrossRef]
- Barros, C.; Fernandes, A. Linking Cognitive Impairment to Neuroinflammation in Multiple Sclerosis using neuroimaging tools. Mult. Scler. Relat. Disord. 2020, 47, 102622. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, X.; Adebiyi, O.; Wang, J.; Mooshekhian, A.; Cohen, J.; Wei, Z.; Wang, F.; Li, X.-M. Venlafaxine Improves the Cognitive Impairment and Depression-Like Behaviors in a Cuprizone Mouse Model by Alleviating Demyelination and Neuroinflammation in the Brain. Front. Pharmacol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Torkildsen, Ø.; Brunborg, L.A.; Myhr, K.-M.; Bø, L. The cuprizone model for demyelination. Acta Neurol. Scand. 2008, 117, 72–76. [Google Scholar] [CrossRef]
- Kipp, M.; Clarner, T.; Dang, J.; Copray, S.; Beyer, C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009, 118, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, E.; Senousy, M.; El-Tanbouly, D.M.; Sayed, R.H. Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol. Appl. Pharmacol. 2018, 352, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Vakilzadeh, G.; Khodagholi, F.; Ghadiri, T.; Ghaemi, A.; Noorbakhsh, F.; Sharifzadeh, M.; Gorji, A. The Effect of Melatonin on Behavioral, Molecular, and Histopathological Changes in Cuprizone Model of Demyelination. Mol. Neurobiol. 2015, 53, 4675–4684. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.A.; Fukudome, D.; Smith, D.R.; Gallagher, M.; Kamiya, A.; Sawa, A. Dimensional assessment of behavioral changes in the cuprizone short-term exposure model for psychosis. Neurosci. Res. 2016, 107, 70–74. [Google Scholar] [CrossRef][Green Version]
- Murakami, M.; Nagahama, M.; Abe, Y.; Niikura, T. Humanin affects object recognition and gliosis in short-term cuprizone-treated mice. Neuropeptides 2017, 66, 90–96. [Google Scholar] [CrossRef]
- Nickel, M.; Eid, F.; Jukkola, P.; Gu, C. Copper chelation and autoimmunity differentially impact myelin in the hippocampal-prefrontal circuit. J. Neuroimmunol. 2019, 334, 576998. [Google Scholar] [CrossRef]
- Omotoso, G.; Olajide, O.; Gbadamosi, I.; Adebayo, J.; Enaibe, B.; Akinola, O.; Owoyele, B. Cuprizone toxicity and Garcinia kola biflavonoid complex activity on hippocampal morphology and neurobehaviour. Heliyon 2019, 5, e02102. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Gbadamosi, I.; Afolabi, T.T.; Abdulwahab, A.B.; Akinlolu, A.A. Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis. Anat. Cell Biol. 2018, 51, 119–127. [Google Scholar] [CrossRef]
- Chang, H.; Liu, J.; Zhang, Y.; Wang, F.; Wu, Y.; Zhang, L.; Ai, H.; Chen, G.; Yin, L. Increased central dopaminergic activity might be involved in the behavioral abnormality of cuprizone exposure mice. Behav. Brain Res. 2017, 331, 143–150. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Ukwubile, I.I.; Arietarhire, L.; Sulaimon, F.; Gbadamosi, I. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms: Possible therapeutic applications? Pathophysiology 2018, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and Cognitive Functioning in Women. Exp. Biol. Med. 1998, 217, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Haan, M.; Byers, A.; Tangen, C.; Kuller, L.M.D.D. Estrogen use, APOE, and cognitive decline: Evidence of gene–environment interaction. Neurology 2000, 54, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R. Estrogen's Impact on Cognitive Functions in Multiple Sclerosis. Int. J. Neurosci. 1996, 86, 23–31. [Google Scholar] [CrossRef]
- Gold, S.M.; Sasidhar, M.V.; Morales, L.B.; Du, S.; Sicotte, N.L.; Tiwari-Woodruff, S.K.; Voskuhl, R.R. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERα). Lab. Investig. 2009, 89, 1076–1083. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Wang, H.; Wu, T.C.J.; Sicotte, N.L.; Nakamura, K.; Kurth, F.; Itoh, N.; Bardens, J.; Bernard, J.T.; Corboy, J.R.; et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2015, 15, 35–46. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Liva, S.M.; Klutch, R.; Pfeiffer, P.; Bouvier, S.; Odesa, S.; Wu, T.C.J.; Voskuhl, R.R. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 2002, 52, 421–428. [Google Scholar] [CrossRef]
- Seifert, H.; Benedek, G.; Nguyen, H.; Kent, G.; Vandenbark, A.A.; Offner, H. Estrogen protects both sexes against EAE by promoting common regulatory cell subtypes independent of endogenous estrogen. Metab. Brain Dis. 2017, 32, 1747–1754. [Google Scholar] [CrossRef]
- Lélu, K.; Laffont, S.; Delpy, L.; Paulet, P.-E.; Périnat, T.; Tschanz, S.A.; Pelletier, L.; Engelhardt, B.; Guéry, J.-C. Estrogen Receptor α Signaling in T Lymphocytes Is Required for Estradiol-Mediated Inhibition of Th1 and Th17 Cell Differentiation and Protection against Experimental Autoimmune Encephalomyelitis. J. Immunol. 2011, 187, 2386–2393. [Google Scholar] [CrossRef]
- Acs, P.; Kipp, M.; Norkute, A.; Johann, S.; Clarner, T.; Braun, A.; Berente, Z.; Komoly, S.; Beyer, C. 17β-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia 2008, 57, 807–814. [Google Scholar] [CrossRef]
- Liu, Z.; Kanjo, Y.; Mizutani, S. A review of phytoestrogens: Their occurrence and fate in the environment. Water Res. 2010, 44, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. J. Cereb. Blood Flow Metab. 2016, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- De Paula, M.L.; Rodrigues, D.H.; Teixeira, H.C.; Barsante, M.M.; Souza, M.A.; Ferreira, A.P. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ohgomori, T.; Jinno, S. Cuprizone-induced demyelination in the mouse hippocampus is alleviated by phytoestrogen genistein. Toxicol. Appl. Pharmacol. 2018, 363, 98–110. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Chen, J.; Ge, B.; Wang, Y.; Ye, Y.; Zeng, S.; Huang, Z. Biochanin A Promotes Proliferation that Involves a Feedback Loop of MicroRNA-375 and Estrogen Receptor Alpha in Breast Cancer Cells. Cell. Physiol. Biochem. 2015, 35, 639–646. [Google Scholar] [CrossRef]
- Jalaludeen, A.M.; Ha, W.T.; Lee, R.; Kim, J.H.; Do, J.T.; Park, C.; Heo, Y.T.; Lee, W.Y.; Song, H. Biochanin A Ameliorates Arsenic-Induced Hepato- and Hematotoxicity in Rats. Molecules 2016, 21, 69. [Google Scholar] [CrossRef]
- Jin, H.; Qi, C.; Zou, Y.; Kong, Y.; Ruan, Z.; Ding, H.; Xie, X.; Zhang, J. Biochanin A partially restores the activity of ofloxacin and ciprofloxacin against topoisomerase IV mutation-associated fluoroquinolone-resistant Ureaplasma species. J. Med. Microbiol. 2017, 66, 1545–1553. [Google Scholar] [CrossRef]
- Liang, F.; Cao, W.; Huang, Y.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. BioFactors 2019, 45, 563–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.-A. Biochanin A Inhibits Lipopolysaccharide-Induced Inflammatory Cytokines and Mediators Production in BV2 Microglia. Neurochem. Res. 2014, 40, 165–171. [Google Scholar] [CrossRef]
- Biradar, S.M.; Joshi, H.; Chheda, T.K. Biochanin-A ameliorates behavioural and neurochemical derangements in cognitive-deficit mice for the betterment of Alzheimer’s disease. Hum. Exp. Toxicol. 2013, 33, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, C.; Wu, W.-Y.; Chen, F.; Li, W.-Z.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson's disease. Pharmacol. Biochem. Behav. 2015, 138, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Ye, Z.-N.; Zhuang, Z.; Gao, Y.; Tang, C.; Zhou, C.-H.; Wang, C.-X.; Zhang, X.-S.; Xie, G.-B.; Liu, J.-P.; et al. Biochanin A Reduces Inflammatory Injury and Neuronal Apoptosis following Subarachnoid Hemorrhage via Suppression of the TLRs/TIRAP/MyD88/NF-κB Pathway. Behav. Neurol. 2018, 2018, 1960106. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, S. Beneficial Effect of Protein Tyrosine Phosphatase Inhibitor and Phytoestrogen in Dyslipidemia-Induced Vascular Dementia in Ovariectomized Rats. J. Stroke Cerebrovasc. Dis. 2015, 24, 2434–2446. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, B.; Yu, H.; Zhao, W.; Song, X.; Liu, Y.; Wang, K.; Peacher, N.; Zhao, X.; Zhang, H.-T. Biochanin A Attenuates Ovariectomy-Induced Cognition Deficit via Antioxidant Effects in Female Rats. Front. Pharmacol. 2021, 12, 603316. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.; Mullin, A.P.; Caggiano, A.O.; Parry, T.J.; Colburn, R.W.; Pavlopoulos, E. The antibody rHIgM22 facilitates hippocampal remyelination and ameliorates memory deficits in the cuprizone mouse model of demyelination. Brain Res. 2018, 1694, 73–86. [Google Scholar] [CrossRef]
- Gingele, S.; Henkel, F.; Heckers, S.; Moellenkamp, T.M.; Hümmert, M.W.; Skripuletz, T.; Stangel, M.; Gudi, V. Delayed Demyelination and Impaired Remyelination in Aged Mice in the Cuprizone Model. Cells 2020, 9, 945. [Google Scholar] [CrossRef]
- Ai, R.-S.; Xing, K.; Deng, X.; Han, J.-J.; Hao, D.-X.; Qi, W.-H.; Han, B.; Yang, Y.-N.; Li, X.; Zhang, Y. Baicalin Promotes CNS Remyelination via PPARγ Signal Pathway. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1142. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, J.; Tang, X.; Zhao, Y.; Zhu, S.; Liu, Q. Clemastine Rescues Chemotherapy-Induced Cognitive Impairment by Improving White Matter Integrity. Neuroscience 2022, 484, 66–79. [Google Scholar] [CrossRef]
- Vega-Riquer, J.M.; Mendez-Victoriano, G.; Morales-Luckie, R.A.; Gonzalez-Perez, O. Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases. Curr. Neuropharmacol. 2019, 17, 129–141. [Google Scholar] [CrossRef]
- Bai, Y.; Li, Z.; Liu, W.; Gao, D.; Liu, M.; Zhang, P. Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir. Bras. 2019, 34, e201901104. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, S.; Thangaiyan, R.; Balaraman, D. Biochanin A, a soy isoflavone, diminishes insulin resistance by modulating insulin-signalling pathway in high-fat diet-induced diabetic mice. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Govindasami, S.; Uddandrao, V.V.S.; Raveendran, N.; Sasikumar, V. Therapeutic Potential of Biochanin-A Against Isoproterenol-Induced Myocardial Infarction in Rats. Cardiovasc. Hematol. Agents Med. Chem. 2020, 18, 31–36. [Google Scholar] [CrossRef]
- Derangula, M.; Panati, K.; Narala, V.R. Biochanin A Ameliorates Ovalbumin-induced Airway Inflammation through Peroxisome Proliferator-Activated Receptor-Gamma in a Mouse Model. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 145–155. [Google Scholar] [CrossRef]
- Wang, W.; Tang, L.; Li, Y.; Wang, Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 2015, 348, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Exner, G.L.; Nyamoya, S.; Schmitz, C.; Kipp, M. Cuprizone-Containing Pellets Are Less Potent to Induce Consistent Demyelination in the Corpus Callosum of C57BL/6 Mice. J. Mol. Neurosci. 2017, 61, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Suliman, F.A.; Khodeer, D.M.; Ibrahiem, A.; Mehanna, E.T.; El-Kherbetawy, M.K.; Mohammad, H.M.; Zaitone, S.A.; Moustafa, Y.M. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: Effect on inflammatory burden and p53 apoptosis. Int. Immunopharmacol. 2018, 61, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols, Methods in Molecular Biology; Guest, P.C., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 105–111. [Google Scholar] [CrossRef]
- Adilijiang, A.; Guan, T.; He, J.; Hartle, K.; Wang, W.; Li, X. The Protective Effects ofAreca catechuExtract on Cognition and Social Interaction Deficits in a Cuprizone-Induced Demyelination Model. Evid. Based Complement. Altern. Med. 2015, 2015, 426092. [Google Scholar] [CrossRef]
- Labban, S.; Alghamdi, B.S.; Alshehri, F.S.; Kurdi, M. Effects of melatonin and resveratrol on recognition memory and passive avoidance performance in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2021, 402, 113100. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Hou, X.; Zheng, B.; Stockmeier, C.; Ou, X.; Paul, I.; Mosley, T.; Weisgraber, K.; Wang, J.M. Cognitive Deficits and Disruption of Neurogenesis in a Mouse Model of Apolipoprotein E4 Domain Interaction. J. Biol. Chem. 2014, 289, 2946–2959. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Christogianni, A.; Bibb, R.; Davis, S.L.; Jay, O.; Barnett, M.; Evangelou, N.; Filingeri, D. Temperature sensitivity in multiple sclerosis: An overview of its impact on sensory and cognitive symptoms. Temperature 2018, 5, 208–223. [Google Scholar] [CrossRef]
- Han, S.R.; Kang, Y.H.; Jeon, H.; Lee, S.; Park, S.J.; Song, D.Y.; Min, S.S.; Yoo, S.M.; Lee, M.S.; Lee, S.H. Differential Expression of miRNAs and Behavioral Change in the Cuprizone-Induced Demyelination Mouse Model. Int. J. Mol. Sci. 2020, 21, 646. [Google Scholar] [CrossRef]
- Soundarapandian, M.M.; Selvaraj, V.; Lo, U.-G.; Golub, M.S.; Feldman, D.H.; Pleasure, D.E.; Deng, W. Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci. Rep. 2011, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2011, 13, 93–110. [Google Scholar] [CrossRef]
- Honarvar, F.; Hojati, V.; Bakhtiari, N.; Vaezi, G.; Javan, M. Myelin Protection by Ursolic Acid in Cuprizone-Induced Demyelination in Mice. Iran. J. Pharm. Res. IJPR 2019, 18, 1978–1988. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Gu, H.-S.; Chen, X.; Zhang, L.; Li, X.-M.; Zhang, J.-W.; Li, L. A novel flavanone derivative ameliorates cuprizone-induced behavioral changes and white matter pathology in the brain of mice. Psychiatry Res. 2017, 257, 249–259. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Fang, Z.; Kong, J.; Huang, Q.; Xu, H. Adenosine Promotes the Recovery of Mice from the Cuprizone-Induced Behavioral and Morphological Changes while Effecting on Microglia and Inflammatory Cytokines in the Brain. J. Neuroimmune Pharmacol. 2018, 13, 412–425. [Google Scholar] [CrossRef]
- Zhou, C.; Cai, M.; Wang, Y.; Wu, W.; Yin, Y.; Wang, X.; Hu, G.; Wang, H.; Tan, Q.; Peng, Z. The Effects of Repetitive Transcranial Magnetic Stimulation on Cognitive Impairment and the Brain Lipidome in a Cuprizone-Induced Mouse Model of Demyelination. Front. Neurosci. 2021, 15, 706786. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Gu, L.-H.; Ma, D.-L.; Wang, M.-Y.; Yang, C.-C.; Zhang, L.; Li, X.-M.; Zhang, J.-W.; Li, L. Behavioral and neurobiological changes in a novel mouse model of schizophrenia induced by the combination of cuprizone and MK-801. Brain Res. Bull. 2021, 174, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Tamura, M.; Kondo, M.A.; Sakaue, M.; Okada, K.; Takemoto, K.; Fukunari, A.; Miwa, K.; Ohzeki, H.; Kano, S.-I.; et al. Cuprizone short-term exposure: Astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol. Dis. 2013, 59, 63–68. [Google Scholar] [CrossRef]
- Meknatkhah, S.; Mousavi, M.-S.; Dashti, P.S.; Pormehr, L.A.; Riazi, G.H. The brain 3β-HSD up-regulation in response to deteriorating effects of background emotional stress: An animal model of multiple sclerosis. Metab. Brain Dis. 2021, 36, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Bebo, B.F.; Fyfe-Johnson, A.; Adlard, K.; Beam, A.G.; Vandenbark, A.A.; Offner, H. Low-Dose Estrogen Therapy Ameliorates Experimental Autoimmune Encephalomyelitis in Two Different Inbred Mouse Strains. J. Immunol. 2001, 166, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, A.J.; Yoon, J.; Nakai, J.; Winchester, Z.; Moore, S.M.; Yoo, T.; Martinez-Torres, L.; Kumar, S.; Itoh, N.; Tiwari-Woodruff, S.K. Estrogen receptor (ER) expression in oligodendrocytes is required for attenuation of clinical disease by an ER ligand. Proc. Natl. Acad. Sci. USA 2013, 110, 19125–19130. [Google Scholar] [CrossRef]
- Moore, S.M.; Khalaj, A.J.; Kumar, S.; Winchester, Z.; Yoon, J.; Yoo, T.; Martinez-Torres, L.; Yasui, N.; Katzenellenbogen, J.A.; Tiwari-Woodruff, S.K. Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2014, 111, 18061–18066. [Google Scholar] [CrossRef]
- Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of Phytoestrogens with Estrogen Receptors ALPHA and BETA (II). Biol. Pharm. Bull. 2002, 25, 48–52. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhahri, R.S.; Alghamdi, B.S.; Alzahrani, N.A.; Bahaidrah, K.A.; Alsufiani, H.M.; Mansouri, R.A.; Ashraf, G.M. Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis. Behav. Sci. 2022, 12, 70. https://doi.org/10.3390/bs12030070
Aldhahri RS, Alghamdi BS, Alzahrani NA, Bahaidrah KA, Alsufiani HM, Mansouri RA, Ashraf GM. Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis. Behavioral Sciences. 2022; 12(3):70. https://doi.org/10.3390/bs12030070
Chicago/Turabian StyleAldhahri, Rahaf Saeed, Badrah Saeed Alghamdi, Noor Ahmed Alzahrani, Khulud Abdullah Bahaidrah, Hadeil Muhanna Alsufiani, Rasha Abdulrashed Mansouri, and Ghulam Md Ashraf. 2022. "Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis" Behavioral Sciences 12, no. 3: 70. https://doi.org/10.3390/bs12030070
APA StyleAldhahri, R. S., Alghamdi, B. S., Alzahrani, N. A., Bahaidrah, K. A., Alsufiani, H. M., Mansouri, R. A., & Ashraf, G. M. (2022). Biochanin A Improves Memory Decline and Brain Pathology in Cuprizone-Induced Mouse Model of Multiple Sclerosis. Behavioral Sciences, 12(3), 70. https://doi.org/10.3390/bs12030070





