Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting: An Evolutionary Framework
Abstract
:1. Introduction
1.1. Life History Theory and Individual Differences in Delay Discounting
1.2. The Antagonistic Pleiotropy and Developmental Trajectory of Delay Discounting
1.3. Delay Discounting as the Product of Fundamental and Longitudinal Life History Trade-Offs
1.4. The Current Study
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Age
2.2.2. Subjective Health
2.2.3. Delay Discounting
2.2.4. Future Time Perspective
2.2.5. Anticipatory Time Perception
2.2.6. Life History Strategy
2.2.7. Childhood Socioeconomic Status
2.3. Analytical Strategy
3. Results
3.1. Preliminary Analysis
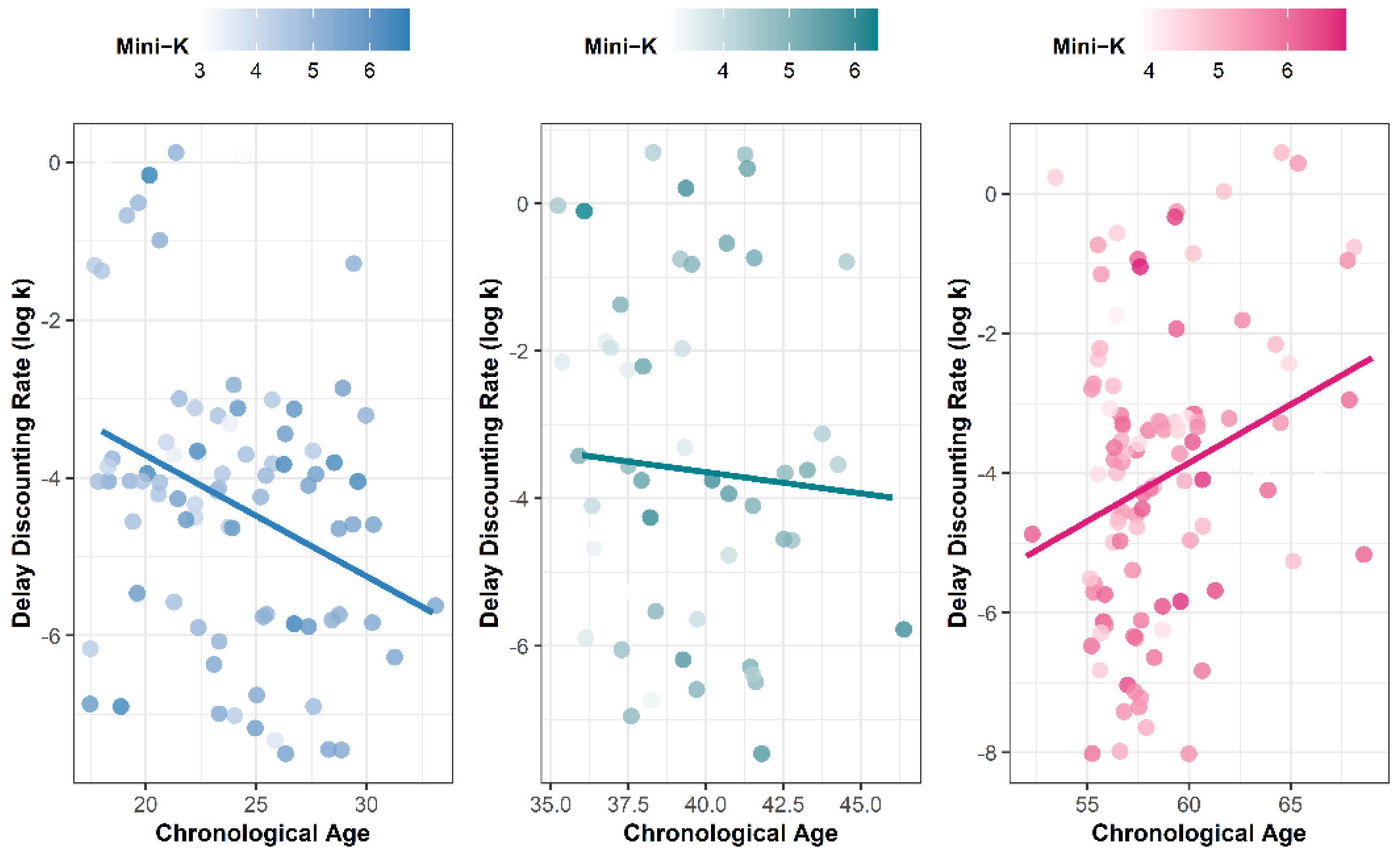
3.1.1. Delay Discounting
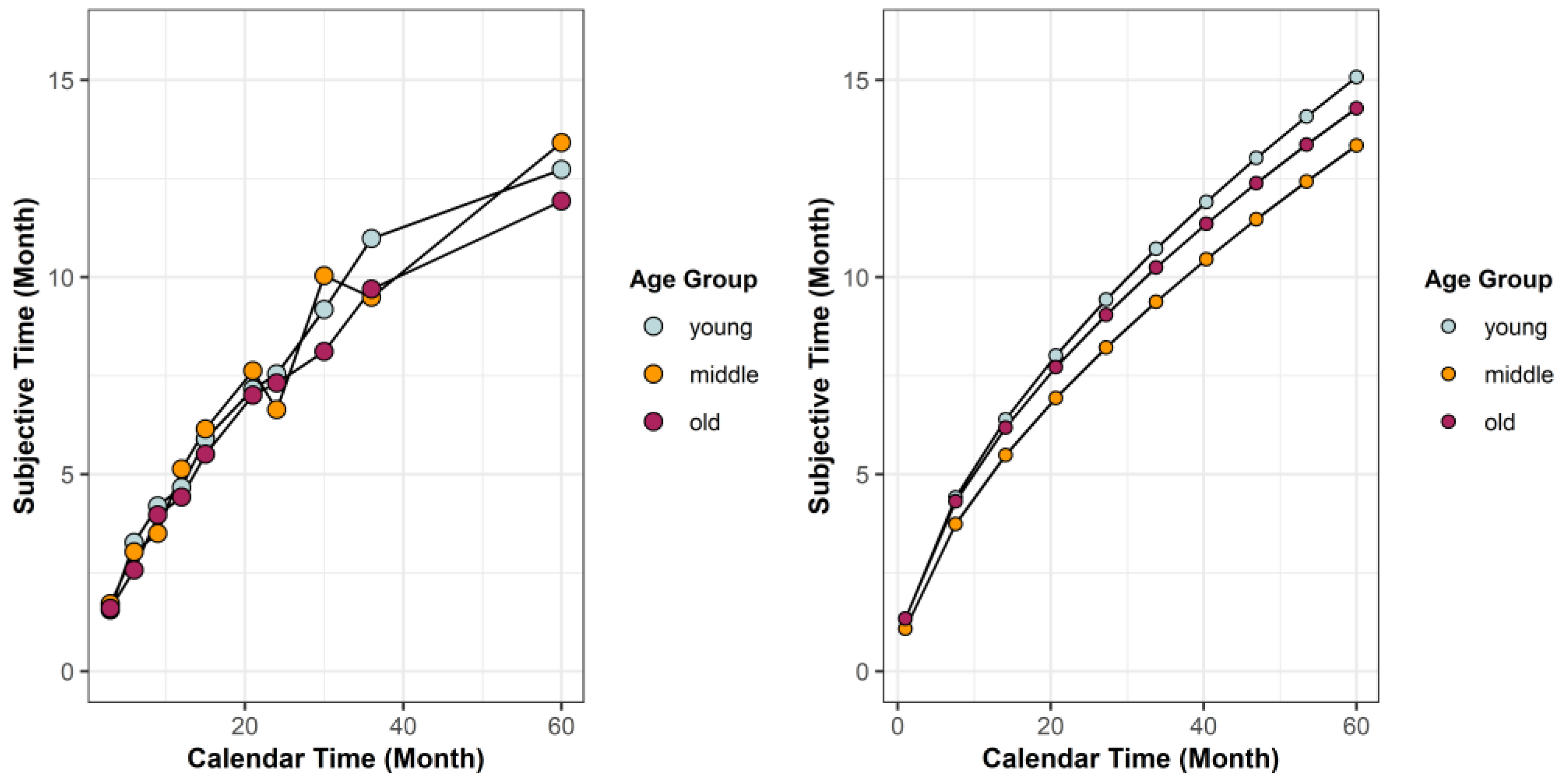
3.1.2. Anticipatory Time Perception
3.2. Delay Discounting as Fundamental and Longitudinal Life History Trade-Offs
3.3. Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting
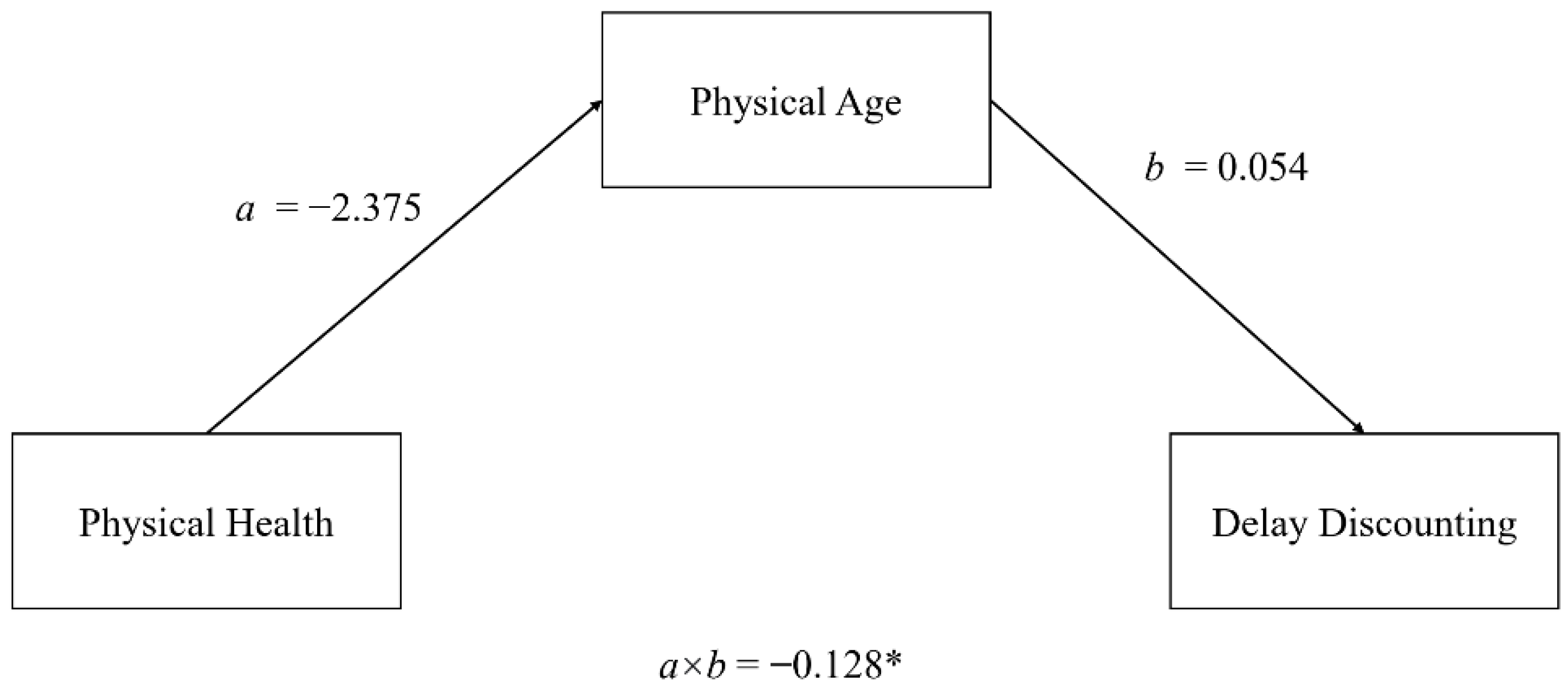
3.4. Mechanisms Underlying Fundamental Life History Trade-Offs
3.5. Mechanisms Underlying Longitudinal Life History Trade-Offs
4. Discussion
4.1. Fundamental and Longitudinal Life History Trade-Offs
4.2. Mechanisms Underlying Fundamental and Longitudinal Life History Trade-Offs
4.3. A Reinterpretation of Relevant Research
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frederick, S.; Loewenstein, G. Time Discounting and Time Preference: A Critical Review. J. Econ. Lit. 2002, 40, 351–401. [Google Scholar] [CrossRef]
- Tzu, C.; Fung, Y. Chuang-Tzu: A New Selected Translation with an Exposition of the Philosophy of Kuo Hsiang; Springer: New York, NY, USA, 2015. [Google Scholar]
- Ainslie, G. Specious Reward: A Behavioral Theory of Impulsiveness and Impulse Control. Psychol. Bull. 1975, 82, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berns, G.S.; Laibson, D.; Loewenstein, G. Intertemporal Choice—Toward an Integrative Framework. Trends Cogn. Sci. 2007, 11, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, K.N. Bidding on the Future: Evidence against Normative Discounting of Delayed Rewards. J. Exp. Psychol. Gen. 1997, 126, 54–70. [Google Scholar] [CrossRef]
- Odum, A.L. Delay Discounting: Trait Variable? Behav. Process. 2011, 87, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamosh, N.A.; DeYoung, C.G.; Green, A.E.; Reis, D.L.; Johnson, M.R.; Conway, A.R.A.; Engle, R.W.; Braver, T.S.; Gray, J.R. Individual Differences in Delay Discounting: Relation to Intelligence, Working Memory, and Anterior Prefrontal Cortex. Psychol. Sci. 2008, 19, 904–911. [Google Scholar] [CrossRef]
- Rogers, A.R. Evolution of Time Preference by Natural Selection. Am. Econ. Rev. 1994, 84, 460–481. [Google Scholar]
- Sozou, P.D.; Seymour, R.M. Augmented Discounting: Interaction between Ageing and Time–Preference Behaviour. Proc. R. Soc. Lond. B 2003, 270, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Trostel, P.A.; Taylor, G.A. A Theory of Time Preference. Econ. Inq. 2001, 39, 379–395. [Google Scholar] [CrossRef]
- Sanchez-Roige, S.; Fontanillas, P.; Elson, S.L.; Pandit, A.; Schmidt, E.M.; Foerster, J.R.; Abecasis, G.R.; Gray, J.C.; de Wit, H.; Davis, L.K.; et al. Genome-Wide Association Study of Delay Discounting in 23,217 Adult Research Participants of European Ancestry. Nat. Neurosci. 2018, 21, 16–18. [Google Scholar] [CrossRef]
- Bickel, W.K.; Athamneh, L.N.; Basso, J.C.; Mellis, A.M.; DeHart, W.B.; Craft, W.H.; Pope, D. Excessive Discounting of Delayed Reinforcers as a Trans-Disease Process: Update on the State of the Science. Curr. Opin. Psychol. 2019, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, J.R.; Brase, G.L. Taking Time to Be Healthy: Predicting Health Behaviors with Delay Discounting and Time Perspective. Personal. Individ. Differ. 2010, 48, 202–207. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Kern, M.L. A Meta-Analysis of the Convergent Validity of Self-Control Measures. J. Res. Personal. 2011, 45, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamosh, N.A.; Gray, J.R. Delay Discounting and Intelligence: A Meta-Analysis. Intelligence 2008, 36, 289–305. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Myerson, J.; Green, L. Delay Discounting, Cognitive Ability, and Personality: What Matters? Psychon. Bull. Rev. 2021, 28, 686–694. [Google Scholar] [CrossRef]
- Chao, L.-W.; Szrek, H.; Pereira, N.S.; Pauly, M.V. Time Preference and Its Relationship with Age, Health, and Survival Probability. Judgm. Decis. Mak. 2009, 4, 1–19. [Google Scholar]
- Green, L.; Fry, A.F.; Myerson, J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychol. Sci. 1994, 5, 33–36. [Google Scholar] [CrossRef]
- Löckenhoff, C.E.; O’Donoghue, T.; Dunning, D. Age Differences in Temporal Discounting: The Role of Dispositional Affect and Anticipated Emotions. Psychol. Aging 2011, 26, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.; Mata, R. Age Differences in Intertemporal Choice: U-Shaped Associations in a Probability Sample of German Households. Psychol. Aging 2018, 33, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Ellis, B.J.; Figueredo, A.J.; Brumbach, B.H.; Schlomer, G.L. Fundamental Dimensions of Environmental Risk: The Impact of Harsh versus Unpredictable Environments on the Evolution and Development of Life History Strategies. Hum. Nat. 2009, 20, 204–268. [Google Scholar] [CrossRef]
- Figueredo, A.; Vasquez, G.; Brumbach, B.; Schneider, S.; Sefcek, J.; Tal, I.; Hill, D.; Wenner, C.; Jacobs, W. Consilience and Life History Theory: From Genes to Brain to Reproductive Strategy. Dev. Rev. 2006, 26, 243–275. [Google Scholar] [CrossRef]
- Griskevicius, V.; Tybur, J.M.; Delton, A.W.; Robertson, T.E. The Influence of Mortality and Socioeconomic Status on Risk and Delayed Rewards: A Life History Theory Approach. J. Personal. Soc. Psychol. 2011, 100, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, J.S.; Ellison, P.T.; Evans, J.; Lee, P.C.; Lieberman, L.S.; Pavlik, Z.; Ryan, A.S.; Salter, E.M.; Stini, W.A.; Worthman, C.M. Death, Hope, and Sex: Life-History Theory and the Development of Reproductive Strategies [and Comments and Reply]. Curr. Anthropol. 1993, 34, 1–24. [Google Scholar] [CrossRef]
- Ellis, B.J. Timing of Pubertal Maturation in Girls: An Integrated Life History Approach. Psychol. Bull. 2004, 130, 920–958. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, W.M. The Application of Optimal Control Theory to the General Life History Problem. Am. Nat. 1983, 121, 418–431. [Google Scholar] [CrossRef]
- Kenrick, D.T.; Luce, C.L. An Evolutionary Life-History Model of Gender Differences and Similarities. In The Developmental Social Psychology of Gender; Psychology Press: New York, NY, USA, 2012; pp. 49–78. [Google Scholar]
- Ellis, B.J.; Del Giudice, M. Developmental Adaptation to Stress: An Evolutionary Perspective. Annu. Rev. Psychol. 2019, 70, 111–139. [Google Scholar] [CrossRef] [Green Version]
- Belsky, J. Early-Life Adversity Accelerates Child and Adolescent Development. Curr. Dir. Psychol. Sci. 2019, 28, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Griskevicius, V.; Ackerman, J.M.; Cantú, S.M.; Delton, A.W.; Robertson, T.E.; Simpson, J.A.; Thompson, M.E.; Tybur, J.M. When the Economy Falters, Do People Spend or Save? Responses to Resource Scarcity Depend on Childhood Environments. Psychol. Sci. 2013, 24, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.C. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Austad, S.N.; Hoffman, J.M. Is Antagonistic Pleiotropy Ubiquitous in Aging Biology? Evol. Med. Public Health 2018, 2018, 287–294. [Google Scholar] [CrossRef]
- Nesse, R.M.; Williams, G.C. Why We Get Sick: The New Science of Darwinian Medicine; Vintage: New York, NY, USA, 2012. [Google Scholar]
- Williams, G.C.; Nesse, R.M. The Dawn of Darwinian Medicine. Q. Rev. Biol. 1991, 66, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, K.; O’Connell, J.F.; Jones, N.B.; Alvarez, H.; Charnov, E.L. Grandmothering, Menopause, and the Evolution of Human Life Histories. Proc. Natl. Acad. Sci. USA 1998, 95, 1336–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eppinger, B.; Nystrom, L.E.; Cohen, J.D. Reduced Sensitivity to Immediate Reward during Decision-Making in Older than Younger Adults. PLoS ONE 2012, 7, e36953. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, K.; Hedgcock, W.; Denburg, N.L. Age-Related Differences in Discounting Future Gains and Losses. J. Neurosci. Psychol. Econ. 2013, 6, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimura, K.; Myerson, J.; Hilgard, J.; Keighley, J.; Braver, T.S.; Green, L. Domain Independence and Stability in Young and Older Adults’ Discounting of Delayed Rewards. Behav. Process. 2011, 87, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Briers, B.; Pandelaere, M.; Dewitte, S.; Warlop, L. Hungry for Money: The Desire for Caloric Resources Increases the Desire for Financial Resources and Vice Versa. Psychol. Sci. 2006, 17, 939–943. [Google Scholar] [CrossRef]
- Wang, X.T.; Dvorak, R.D. Sweet Future: Fluctuating Blood Glucose Levels Affect Future Discounting. Psychol. Sci. 2010, 21, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Charlton, S.R.; Fantino, E. Commodity Specific Rates of Temporal Discounting: Does Metabolic Function Underlie Differences in Rates of Discounting? Behav. Process. 2008, 77, 334–342. [Google Scholar] [CrossRef]
- Friedel, J.E.; DeHart, W.B.; Madden, G.J.; Odum, A.L. Impulsivity and Cigarette Smoking: Discounting of Monetary and Consumable Outcomes in Current and Non-Smokers. Psychopharmacology 2014, 231, 4517–4526. [Google Scholar] [CrossRef] [Green Version]
- Odum, A.L.; Becker, R.J.; Haynes, J.M.; Galizio, A.; Frye, C.C.J.; Downey, H.; Friedel, J.E.; Perez, D.M. Delay Discounting of Different Outcomes: Review and Theory. J. Exp. Anal. Behav. 2020, 113, 657–679. [Google Scholar] [CrossRef]
- Seaman, K.L.; Abiodun, S.J.; Fenn, Z.; Samanez-Larkin, G.R.; Mata, R. Temporal Discounting Across Adulthood: A Systematic Review and Meta-Analysis. Psychol. Aging 2022, 37, 111–124. [Google Scholar] [CrossRef]
- Steinberg, L.; Graham, S.; O’Brien, L.; Woolard, J.; Cauffman, E.; Banich, M. Age Differences in Future Orientation and Delay Discounting. Child Dev. 2009, 80, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Zauberman, G. Perception of Anticipatory Time in Temporal Discounting. J. Neurosci. Psychol. Econ. 2009, 2, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, E.P.; Spaniol, J. Aging and Altruism in Intertemporal Choice. Psychol. Aging 2018, 33, 315–324. [Google Scholar] [CrossRef]
- Voslinsky, A.; Azar, O.H. Incentives in Experimental Economics. J. Behav. Exp. Econ. 2021, 93, 101706. [Google Scholar] [CrossRef]
- Soylu, C.; Ozekes, B.C. Psychometric Properties of the Future Time Perspective Scale for the Turkish Population: Age Differences in Predictors of Time Perspective. Int. J. Aging Hum. Dev. 2020, 91, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, G.J.; Miche, M.; Brothers, A.F.; Barrett, A.E.; Diehl, M.; Montepare, J.M.; Wahl, H.-W.; Wurm, S. The Influence of Subjective Aging on Health and Longevity: A Meta-Analysis of Longitudinal Data. Psychol. Aging 2014, 29, 793. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N.; MarakoviĆ, N.N. Delay-Discounting Probabilistic Rewards: Rates Decrease as Amounts Increase. Psychon. Bull. Rev. 1996, 3, 100–104. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.T.; Gao, J.; Li, X.; Xu, J. Adaptive time management: The effects of death awareness on time perception and intertemporal choice. Acta Psychol. Sin. 2019, 51, 1341–1350. [Google Scholar] [CrossRef]
- Dunn, T.J.; Baguley, T.; Brunsden, V. From Alpha to Omega: A Practical Solution to the Pervasive Problem of Internal Consistency Estimation. Br. J. Psychol. 2014, 105, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, A.; Vásquez, G.; Brumbach, B.H.; Schneider, S.M.R. The Heritability of Life History Strategy: The K-factor, Covitality, and Personality. Biodemog. Soc. Biol. 2004, 51, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Grove, W.M.; Menton, W.H. Hypothetico-Deductive Model. In The Encyclopedia of Clinical Psychology; John Wiley and Sons: Malden, MA, USA, 2014; pp. 1–3. [Google Scholar]
- Buss, D.M. Evolutionary Psychology: The New Science of the Mind, 6th ed.; Routledge: New York, NY, USA, 2019; ISBN 978-0-429-06141-7. [Google Scholar]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of Failure to Meet Assumptions Underlying the Fixed Effects Analyses of Variance and Covariance. Rev. Educ. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Wilcox, R.R. Fundamentals of Modern Statistical Methods: Substantially Improving Power and Accuracy; Springer: New York, NY, USA, 2010. [Google Scholar]
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing, 4th ed.; Elsevier: Waltham, MA, USA, 2016; ISBN 978-0-12-804733-0. [Google Scholar]
- Huber, P.J.; Ronchetti, E. Robust Statistics, 2nd ed.; Wiley series in probability and statistics; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-470-12990-6. [Google Scholar]
- Yohai, V.J. High Breakdown-Point and High Efficiency Robust Estimates for Regression. Ann. Stat. 1987, 15, 642–656. [Google Scholar] [CrossRef]
- Zu, J.; Yuan, K.-H. Local Influence and Robust Procedures for Mediation Analysis. Multivar. Behav. Res. 2010, 45, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Mair, P.; Wilcox, R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Maechler, M.; Rousseeuw, P.; Croux, C.; Todorov, V.; Ruckstuhl, A.; Salibian-Barrera, M.; Verbeke, T.; Koller, M.; Conceicao, E.L.; Di Palma, M.A. Robustbase: Basic Robust Statistics; R Package Version 0.93–6; R Core Team: Murray Hill, NJ, USA, 1975; Available online: http://robustbase.r-forge.r-project.org (accessed on 23 October 2021).
- Mazur, J.E. An Adjusting Procedure for Studying Delayed Reinforcement. Quant. Anal. Behav. 1987, 5, 55–73. [Google Scholar]
- Luce, R.D. Individual Choice Behavior: A Theoretical Analysis; Dover Publications: Mineola, NY, USA, 2005. [Google Scholar]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS Procedures for Estimating Indirect Effects in Simple Mediation Models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Zauberman, G.; Kim, B.K.; Malkoc, S.A.; Bettman, J.R. Discounting Time and Time Discounting: Subjective Time Perception and Intertemporal Preferences. J. Mark. Res. 2009, 46, 15. [Google Scholar] [CrossRef]
- Harrison, G.W.; Lau, M.I.; Williams, M.B. Estimating Individual Discount Rates in Denmark: A Field Experiment. Am. Econ. Rev. 2002, 92, 1606–1617. [Google Scholar] [CrossRef] [Green Version]
- Read, D.; Read, N.L. Time Discounting over the Lifespan. Organ. Behav. Hum. Decis. Process. 2004, 94, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Göllner, L.M.; Ballhausen, N.; Kliegel, M.; Forstmeier, S. Delay of Gratification, Delay Discounting and Their Associations with Age, Episodic Future Thinking, and Future Time Perspective. Front. Psychol. 2018, 8, 2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.; Daly, M. Competitiveness, Risk Taking, and Violence: The Young Male Syndrome. Ethol. Sociobiol. 1985, 6, 59–73. [Google Scholar] [CrossRef]
- Zimbardo, P.G.; Boyd, J.N. Putting Time in Perspective: A Valid, Reliable Individual-Differences Metric. In Time Perspective Theory; Review, Research and Application; Springer: New York, NY, USA, 2015; pp. 17–55. [Google Scholar]
- Dunkel, C.S.; Decker, M. Convergent Validity of Measures of Life-History Strategy. Personal. Individ. Differ. 2010, 48, 681–684. [Google Scholar] [CrossRef]
- Friedman, W.J.; Janssen, S.M.J. Aging and the Speed of Time. Acta Psychol. 2010, 134, 130–141. [Google Scholar] [CrossRef]
- Hancock, P.A.; Rausch, R. The Effects of Sex, Age, and Interval Duration on the Perception of Time. Acta Psychol. 2010, 133, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Rudolph, T.; Linares Gutierrez, D.; Winkler, I. Time Perspective and Emotion Regulation as Predictors of Age-Related Subjective Passage of Time. Int. J. Environ. Res. Public Health 2015, 12, 16027–16042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmann, M.; Lehnhoff, S. Age Effects in Perception of Time. Psychol. Rep. 2005, 97, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, L.L.; Isaacowitz, D.M.; Charles, S.T. Taking Time Seriously: A Theory of Socioemotional Selectivity. Am. Psychol. 1999, 54, 165–181. [Google Scholar] [CrossRef]
- Lang, F.R.; Carstensen, L.L. Time Counts: Future Time Perspective, Goals, and Social Relationships. Psychol. Aging 2002, 17, 125–139. [Google Scholar] [CrossRef]
- Seaman, K.L.; Gorlick, M.A.; Vekaria, K.M.; Hsu, M.; Zald, D.H.; Samanez-Larkin, G.R. Adult Age Differences in Decision Making across Domains: Increased Discounting of Social and Health-Related Rewards. Psychol. Aging 2016, 31, 737–746. [Google Scholar] [CrossRef]
- Figner, B.; Knoch, D.; Johnson, E.J.; Krosch, A.R.; Lisanby, S.H.; Fehr, E.; Weber, E.U. Lateral Prefrontal Cortex and Self-Control in Intertemporal Choice. Nat. Neurosci. 2010, 13, 538–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin Addicts Have Higher Discount Rates for Delayed Rewards than Non-Drug-Using Controls. J. Exp. Psychol. Gen. 1999, 128, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zondervan-Zwijnenburg, M.A.J.; Richards, J.S.; Kevenaar, S.T.; Becht, A.I.; Hoijtink, H.J.A.; Oldehinkel, A.J.; Branje, S.; Meeus, W.; Boomsma, D.I. Robust Longitudinal Multi-Cohort Results: The Development of Self-Control during Adolescence. Dev. Cogn. Neurosci. 2020, 45, 100817. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, G.M.; Hartley, C.A. Developmental Perspectives on Risky and Impulsive Choice. Philos. Trans. R. Soc. B 2019, 374, 20180133. [Google Scholar] [CrossRef] [Green Version]
- Westwater, M.L.; Vilar-López, R.; Ziauddeen, H.; Verdejo-García, A.; Fletcher, P.C. Combined Effects of Age and BMI Are Related to Altered Cortical Thickness in Adolescence and Adulthood. Dev. Cogn. Neurosci. 2019, 40, 100728. [Google Scholar] [CrossRef]
- Rey-Mermet, A.; Gade, M. Inhibition in Aging: What Is Preserved? What Declines? A Meta-Analysis. Psychon. Bull. Rev. 2018, 25, 1695–1716. [Google Scholar] [CrossRef] [Green Version]
- Eysenck, H.J. Systematic Reviews: Meta-Analysis and Its Problems. BMJ 1994, 309, 789–792. [Google Scholar] [CrossRef]
- Hampton, W.H.; Asadi, N.; Olson, I.R. Good Things for Those Who Wait: Predictive Modeling Highlights Importance of Delay Discounting for Income Attainment. Front. Psychol. 2018, 9, 1545. [Google Scholar] [CrossRef]
- Bixter, M.T.; Rogers, W.A. Age-related Differences in Delay Discounting: Immediate Reward, Reward Magnitude, and Social Influence. J. Behav. Decis. Mak. 2019, 32, 471–484. [Google Scholar] [CrossRef]
- McAdams, D.P.; Olson, B.D. Personality Development: Continuity and Change Over the Life Course. Annu. Rev. Psychol. 2010, 61, 517–542. [Google Scholar] [CrossRef]
| Variables | Young Adults | Middle Age | Older Adults |
|---|---|---|---|
| M (SD)/tau | M (SD)/tau | M (SD)/tau | |
| Sample size | 84 | 54 | 104 |
| Gender | 31M/53F | 26M/28F | 61M/43F |
| Chronological age | 24.00 (3.79) | 39.70 (2.71) | 58.80 (3.53) |
| Physical age | 24.20 (4.17) | 37.60 (5.82) | 53.30 (7.97) |
| Psychological age | 25.40 (5.73) | 36.10 (10.3) | 47.70 (11.00) |
| Physical health | 3.92 (0.88) | 3.59 (1.00) | 3.86 (0.81) |
| Psychological health | 3.88 (0.96) | 3.67 (1.03) | 4.25 (0.80) |
| Childhood SES | 3.73 (1.49) | 3.17 (1.40) | 3.59 (1.59) |
| Life history strategy | 5.18 (0.80) | 4.73 (0.83) | 5.40 (0.72) |
| FTP | 0.12 | −0.18 | −0.07 |
| Delay discounting | −0.23 ** | −0.03 | 0.15 |
| alpha | −0.23 ** | 0.08 | 0.02 |
| beta | 0.20 * | −0.25 * | 0.007 |
| Variables | Model 1 | Model 2 |
|---|---|---|
| Intercept | 1.18 (1.39) | 1.82 (1.38) |
| Life history strategy | −0.57 * (0.22) | −0.55 * (0.21) |
| Chronological age | −0.11 (0.06) | −0.14 * (0.06) |
| Age group | −11.56 ** (4.22) | −12.32 ** (4.16) |
| Age *Age group | 0.26 ** (0.09) | 0.30 ** (0.09) |
| Age bias score | −0.11 * (0.06) | |
| Age bias score *Age group | 0.14 * (0.06) | |
| Observations | 185 | 185 |
| R2 | 0.13 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Lu, Q.; Lu, L. Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting: An Evolutionary Framework. Behav. Sci. 2022, 12, 63. https://doi.org/10.3390/bs12030063
Lu J, Lu Q, Lu L. Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting: An Evolutionary Framework. Behavioral Sciences. 2022; 12(3):63. https://doi.org/10.3390/bs12030063
Chicago/Turabian StyleLu, Junsong, Qi’an Lu, and Lin Lu. 2022. "Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting: An Evolutionary Framework" Behavioral Sciences 12, no. 3: 63. https://doi.org/10.3390/bs12030063
APA StyleLu, J., Lu, Q., & Lu, L. (2022). Differential Effects of Fundamental and Longitudinal Life History Trade-Offs on Delay Discounting: An Evolutionary Framework. Behavioral Sciences, 12(3), 63. https://doi.org/10.3390/bs12030063






