Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol
Abstract
1. Introduction
Hypothesis and Objectives
2. Materials and Methods
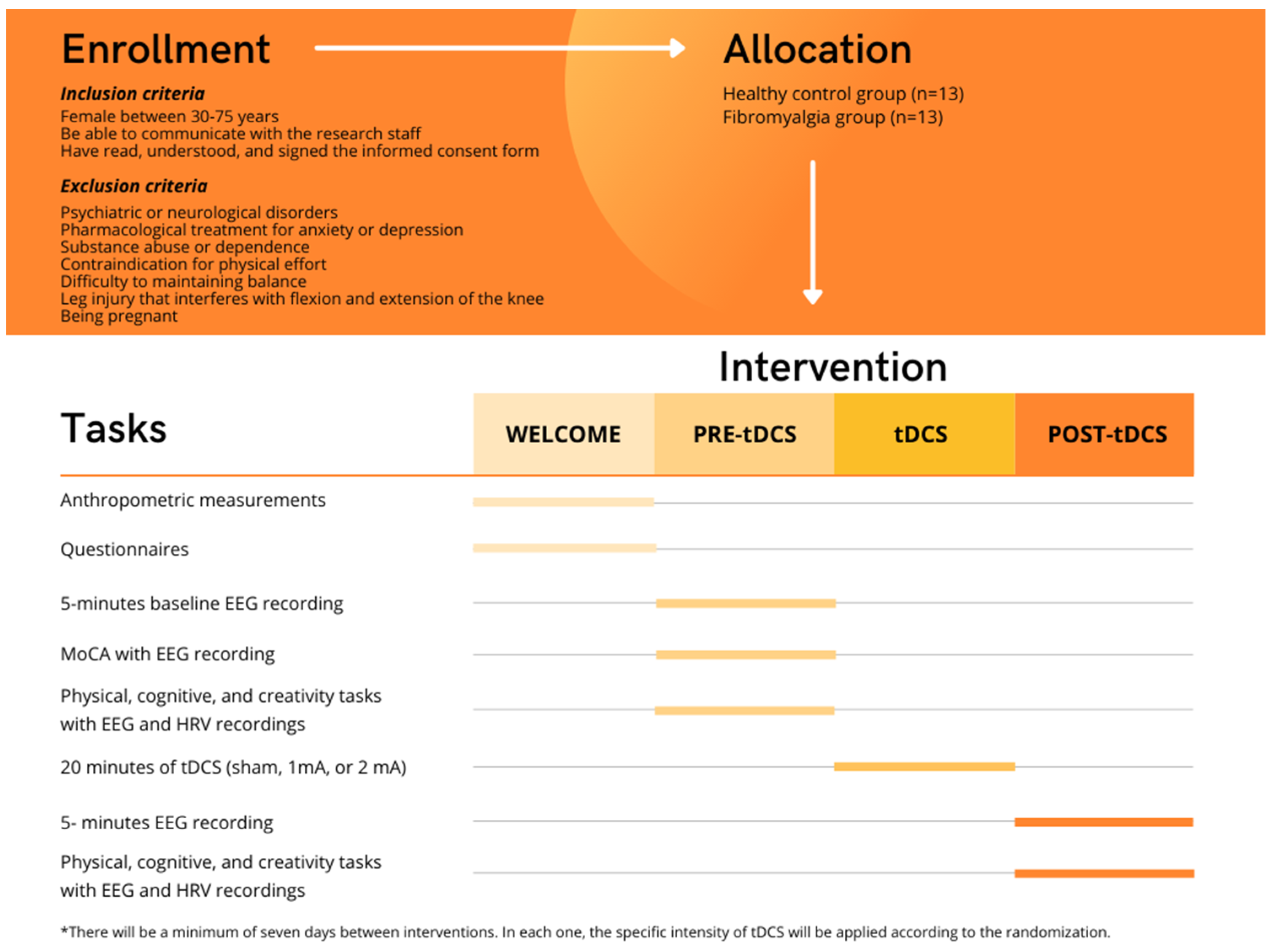
2.1. Study Design
2.2. Participants
2.2.1. Eligibility Criteria
2.2.2. Recruitment
2.2.3. Randomization
2.3. Ethical Approval
2.4. Intervention
2.5. Procedures and Outcome Measures
2.5.1. Procedure
2.5.2. Primary Outcomes
Neurophysiological Variables
- Electroencephalography (EEG) and Heart Rate Variability (HRV)
Physical Fitness
- Balance
- Strength
- Dual-Task Condition
- Creativity
2.5.3. Secondary Outcomes
Sociodemographic Information
Physical Activity Level
Impact of the Disease
Fear of Falling
Health-Related Quality of Life
Sleep Quality
Cognitive Impairment
Sensations Related to tDCS
Anthropometric Measurement
2.6. Data Analysis
3. Discussion
3.1. Future Perspectives in Clinical and Research Issues
3.2. Limitations
4. Ethics and Dissemination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Gaudreault, N.; Boulay, P. Cardiorespiratory fitness among adults with fibromyalgia. Breathe 2018, 14, e25–e33. [Google Scholar] [CrossRef] [PubMed]
- Maquet, D.; Croisier, J.L.; Renard, C.; Crielaard, J.M. Muscle performance in patients with fibromyalgia. Jt. Bone Spine 2002, 69, 293–299. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Gallego-Diaz, J.M.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; Olivares, P.R.; Gusi, N. Fear of falling in women with fibromyalgia and its relation with number of falls and balance performance. BioMed Res. Int. 2015, 2015, 589014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, J. Fibromyalgia: Clinical features, diagnosis and management. Nurs. Stand. 2016, 31, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Trost, Z.; Buelow, M.T.; Clay, O.; Younger, J.; Moore, D.; Crowe, M. Meta-analysis of cognitive performance in fibromyalgia. J. Clin. Exp. Neuropsychol. 2018, 40, 698–714. [Google Scholar] [CrossRef]
- Glass, J.M. Review of cognitive dysfunction in fibromyalgia: A convergence on working memory and attentional control impairments. Rheum. Dis. Clin. N. Am. 2009, 35, 299–311. [Google Scholar] [CrossRef]
- Fallon, N.; Chiu, Y.; Nurmikko, T.; Stancak, A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur. J. Pain 2018, 22, 49–57. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Fuentes-García, J.P.; Cano-Plasencia, R.; Gusi, N. Impact of fibromyalgia on alpha-2 EEG power spectrum in the resting condition: A descriptive correlational study. BioMed Res. Int. 2019, 2019, 7851047. [Google Scholar] [CrossRef]
- Meeus, M.; Goubert, D.; De Backer, F.; Struyf, F.; Hermans, L.; Coppieters, I.; De Wandele, I.; Da Silva, H.; Calders, P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Semin. Arthritis Rheum. 2013, 43, 279–287. [Google Scholar] [CrossRef]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993, 20, 475–479. [Google Scholar]
- Huijnen, I.P.; Verbunt, J.A.; Meeus, M.; Smeets, R.J. Energy expenditure during functional daily life performances in patients with fibromyalgia. Pain Pract. Off. J. World Inst. Pain 2015, 15, 748–756. [Google Scholar] [CrossRef]
- Yuan, J.; Blumen, H.M.; Verghese, J.; Holtzer, R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: A resting-state fMRI study. Hum. Brain Mapp. 2015, 36, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Pedullà, L.; Tacchino, A.; Podda, J.; Bragadin, M.M.; Bonzano, L.; Battaglia, M.A.; Bove, M.; Brichetto, G.; Ponzio, M. The patients’ perspective on the perceived difficulties of dual-tasking: Development and validation of the Dual-task Impact on Daily-living Activities Questionnaire (DIDA-Q). Mult. Scler. Relat. Disord. 2020, 46, 102601. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef]
- Watanabe, K.; Funahashi, S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat. Neurosci. 2014, 17, 601–611. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Domínguez-Muñoz, F.J.; Fuentes-García, J.P.; Gusi, N. Impact of adding a cognitive task while performing physical fitness tests in women with fibromyalgia: A cross-sectional descriptive study. Medicine 2018, 97, e13791. [Google Scholar] [CrossRef]
- Sempere-Rubio, N.; López-Pascual, J.; Aguilar-Rodríguez, M.; Cortés-Amador, S.; Espí-López, G.; Villarrasa-Sapiña, I.; Serra-Añó, P. Characterization of postural control impairment in women with fibromyalgia. PLoS ONE 2018, 13, e0196575. [Google Scholar] [CrossRef]
- Villafaina, S.; Gusi, N.; Rodriguez-Generelo, S.; Martin-Gallego, J.D.; Fuentes-García, J.P.; Collado-Mateo, D. Influence of a cell-phone conversation on balance performance in women with fibromyalgia: A cross-sectional descriptive study. BioMed Res. Int. 2019, 2019, 5132802. [Google Scholar] [CrossRef]
- Villafaina, S.; Sitges, C.; Collado-Mateo, D.; Fuentes-García, J.P.; Gusi, N. Influence of depressive feelings in the brain processing of women with fibromyalgia: An EEG study. Medicine 2019, 98, e15564. [Google Scholar] [CrossRef] [PubMed]
- Kübler, S.; Soutschek, A.; Schubert, T. The causal role of the lateral prefrontal cortex for task-order coordination in dual-task situations: A study with transcranial magnetic stimulation. J. Cogn. Neurosci. 2019, 31, 1840–1856. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Stelzel, C.; Brandt, S.A.; Schubert, T. Neural mechanisms of concurrent stimulus processing in dual tasks. NeuroImage 2009, 48, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Szameitat, A.J.; Lepsien, J.; von Cramon, D.Y.; Sterr, A.; Schubert, T. Task-order coordination in dual-task performance and the lateral prefrontal cortex: An event-related fMRI study. Psychol. Res. 2006, 70, 541–552. [Google Scholar] [CrossRef]
- Wrightson, J.G.; Twomey, R.; Ross, E.Z.; Smeeton, N.J. The effect of transcranial direct current stimulation on task processing and prioritisation during dual-task gait. Exp. Brain Res. 2015, 233, 1575–1583. [Google Scholar] [CrossRef]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Potini, B.R.; Azam, A.T. Diagnostic challenges and management of fibromyalgia. Cureus 2021, 13, e18692. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.A.; Taylor, J.L.; Chew, T.; Gálvez, V.; Alonzo, A.; Bai, S.; Dokos, S.; Loo, C.K. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: Evidence from single and repeated sessions. Brain Stimul. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Wrightson, J.G.; Twomey, R.; Yeung, S.T.Y.; Millet, G.Y. No effect of tDCS of the primary motor cortex on isometric exercise performance or perceived fatigue. Eur. J. Neurosci. 2020, 52, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, D.J.; Daly, R.M.; Young, K.; Lum, J.; Tooley, G.; Jaberzadeh, S.; Zoghi, M.; Pearce, A.J. Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast. 2013, 2013, 603502. [Google Scholar] [CrossRef] [PubMed]
- Papazova, I.; Strube, W.; Wienert, A.; Henning, B.; Schwippel, T.; Fallgatter, A.J.; Padberg, F.; Falkai, P.; Plewnia, C.; Hasan, A. Effects of 1 mA and 2 mA transcranial direct current stimulation on working memory performance in healthy participants. Conscious. Cogn. 2020, 83, 102959. [Google Scholar] [CrossRef]
- Tadini, L.; El-Nazer, R.; Brunoni, A.R.; Williams, J.; Carvas, M.; Boggio, P.; Priori, A.; Pascual-Leone, A.; Fregni, F. Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. J. ECT 2011, 27, 134–140. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Vanneste, S. Optimization of transcranial direct current stimulation of dorsolateral prefrontal cortex for tinnitus: A non-linear dose-response effect. Sci. Rep. 2018, 8, 8311. [Google Scholar] [CrossRef]
- Ehrhardt, S.E.; Filmer, H.L.; Wards, Y.; Mattingley, J.B.; Dux, P.E. The influence of tDCS intensity on decision-making training and transfer outcomes. J. Neurophysiol. 2021, 125, 385–397. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Vignaud, P.; Mondino, M.; Poulet, E.; Palm, U.; Brunelin, J. Duration but not intensity influences transcranial direct current stimulation (tDCS) after-effects on cortical excitability. Neurophysiol. Clin. 2018, 48, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Hassanzahraee, M.; Nitsche, M.A.; Zoghi, M.; Jaberzadeh, S. Determination of anodal tDCS intensity threshold for reversal of corticospinal excitability: An investigation for induction of counter-regulatory mechanisms. Sci. Rep. 2020, 10, 16108. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Babayev, J.; Mahmoud, R.; Rafiq, B.; Chusid, E.; Aguglia, E.; Coira, D.; Battaglia, F. Modulation of prefrontal cortex with anodal tDCS prevents post-exercise facilitation interference during dual task. Somatosens. Mot. Res. 2017, 34, 80–84. [Google Scholar] [CrossRef]
- Lee, J.; Dong, S.; Jeong, J.; Yoon, B. Effects of transcranial direct current stimulation over the dorsolateral prefrontal cortex (PFC) on cognitive-motor dual control skills. Percept. Mot. Skill 2020, 127, 803–822. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, Y.; Wang, Y.; Jor’dan, A.; Pascual-Leone, A.; Zhang, J.; Fang, J.; Manor, B. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur. J. Neurosci. 2014, 39, 1343–1348. [Google Scholar] [CrossRef]
- Abedanzadeh, R.; Alboghebish, S.; Barati, P. The effect of transcranial direct current stimulation of dorsolateral prefrontal cortex on performing a sequential dual task: A randomized experimental study. Psicol. Reflex. Crit. 2021, 34, 30. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.Y.; Travison, T.G.; Hausdorff, J.M.; Pascual-Leone, A.; Lipsitz, L. Transcranial direct current stimulation may improve cognitive-motor function in functionally limited older adults. Neurorehabilit. Neural Repair 2018, 32, 788–798. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Jor’dan, A.; Zhang, J.; Fang, J.; Pascual-Leone, A. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J. Cogn. Neurosci. 2016, 28, 275–281. [Google Scholar] [CrossRef]
- Zhu, C.E.; Yu, B.; Zhang, W.; Chen, W.H.; Qi, Q.; Miao, Y. Effiectiveness and safety of transcranial direct current stimulation in fibromyalgia: A systematic review and meta-analysis. J. Rehabil. Med. 2017, 49, 2–9. [Google Scholar] [CrossRef]
- Brietzke, A.P.; Zortea, M.; Carvalho, F.; Sanches, P.R.S.; Silva, D.P.J.; Torres, I.; Fregni, F.; Caumo, W. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: A proof of concept sham-randomized clinical study. J. Pain 2020, 21, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Roizenblatt, S.; Botte, S.; Zaghi, S.; Riberto, M.; Tufik, S.; Boggio, P.S.; Fregni, F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2009, 2, 353–361. [Google Scholar] [PubMed]
- Roizenblatt, S.; Fregni, F.; Gimenez, R.; Wetzel, T.; Rigonatti, S.P.; Tufik, S.; Boggio, P.S.; Valle, A.C. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: A randomized, sham-controlled study. Pain Pract. Off. J. World Inst. Pain 2007, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Zortea, M.; Alves, R.L.; Naziazeno, C.; Saldanha, J.S.; Carvalho, S.; Leite, A.; Torres, I.; Souza, A.; Calvetti, P.; et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: A randomized clinical trial. Sci. Rep. 2018, 8, 12477. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Maeda, K.; Yamaguchi, T.; Tatemoto, T.; Kondo, K.; Otaka, Y.; Tanaka, S. Transcranial direct current stimulation does not affect lower extremity muscle strength training in healthy individuals: A triple-blind, sham-controlled study. Front. Neurosci. 2017, 11, 179. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, J.; Chen, H.; Manor, B.; Lin, J.; Zhang, J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp. Brain Res. 2015, 233, 2401–2409. [Google Scholar] [CrossRef]
- Pineau, N.; Robin, A.; Bulteau, S.; Thomas-Ollivier, V.; Sauvaget, A.; Deschamps, T. Does the transcranial direct current stimulation improve dual-task postural control in young healthy adults? Cogn. Process. 2020, 22, 291–298. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Antal, A.; Lang, N.; Tergau, F.; Paulus, W. Modulation of cortical excitability by weak direct current stimulation—Technical, safety and functional aspects. Suppl. Clin. Neurophysiol. 2003, 56, 255–276. [Google Scholar] [CrossRef]
- Paulus, W. Transcranial direct current stimulation (tDCS). Suppl. Clin. Neurophysiol. 2003, 56, 249–254. [Google Scholar] [CrossRef]
- Ruffini, G.; Dunne, S.; Farres, E.; Cester, I.; Watts, P.C.P.; Silva, S.R.P.; Grau, C.; Fuentemilla, L.; Marco-Pallares, J.; Vandecasteele, B.; et al. ENOBIO dry electrophysiology electrode; first human trial plus wireless electrode system. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6690–6694. [Google Scholar]
- Collado-Mateo, D.; Adsuar, J.C.; Olivares, P.R.; Cano-Plasencia, R.; Gusi, N. Using a dry electrode EEG device during balance tasks in healthy young-adult males: Test-retest reliability analysis. Somatosens. Mot. Res. 2015, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cheron, G.; Petit, G.; Cheron, J.; Leroy, A.; Cebolla, A.; Cevallos, C.; Petieau, M.; Hoellinger, T.; Zarka, D.; Clarinval, A.-M. Brain oscillations in sport: Toward EEG biomarkers of performance. Front. Psychol. 2016, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.R.; Kothe, C.A.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Jung, T.-P.; Cauwenberghs, G. Real-time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Trans. Biomed. Eng. 2015, 62, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 2000, 111, 1745–1758. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Trevisan, D.C.; Driusso, P.; Avila, M.A.; Gramani-Say, K.; Moreira, F.M.A.; Parizotto, N.A. Static postural sway of women with and without fibromyalgia syndrome: A cross-sectional study. Clin. Biomech. 2017, 44, 83–89. [Google Scholar] [CrossRef]
- Chen, B.; Liu, P.; Xiao, F.; Liu, Z.; Wang, Y. Review of the upright balance assessment based on the force plate. Int. J. Environ. Res. Public Health 2021, 18, 2696. [Google Scholar] [CrossRef]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions—Acquisition interval—Sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Baltzopoulos, V.; Brodie, D.A. Isokinetic dynamometry. Applications and limitations. Sport. Med. 1989, 8, 101–116. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.C.; Maffulli, N.; Chan, K.M.; Chan, J.L. Relationship between isokinetic concentric and eccentric contraction modes in the knee flexor and extensor muscle groups. J. Orthop. Sport. Phys. Ther. 1997, 26, 143–149. [Google Scholar] [CrossRef]
- Coban, O.; Yildirim, N.U.; Yasa, M.E.; Akinoglu, B.; Kocahan, T. Determining the number of repetitions to establish isokinetic knee evaluation protocols specific to angular velocities of 60°/second and 180°/second. J. Bodyw. Mov. Ther. 2021, 25, 255–260. [Google Scholar] [CrossRef]
- Reichard, L.B.; Croisier, J.L.; Malnati, M.; Katz-Leurer, M.; Dvir, Z. Testing knee extension and flexion strength at different ranges of motion: An isokinetic and electromyographic study. Eur. J. Appl. Physiol. 2005, 95, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Leon-Llamas, J.L.; Villafaina, S.; Murillo-Garcia, A.; Collado-Mateo, D.; Javier Domínguez-Muñoz, F.; Sánchez-Gómez, J.; Gusi, N. Strength assessment under dual task conditions in women with fibromyalgia: A test-retest reliability study. Int. J. Environ. Res. Public Health 2019, 16, 4971. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.; Graif, B.; Neubauer, A.C. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. NeuroImage 2009, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International physical activity questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Bennett, R.M.; Friend, R.; Jones, K.D.; Ward, R.; Han, B.K.; Ross, R.L. The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Res. Ther. 2009, 11, R120. [Google Scholar] [CrossRef]
- Salgueiro, M.; García-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual. Life Outcomes 2013, 11, 132. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Rodríguez-Almagro, D.; Peinado-Rubia, A.B.; Zagalaz-Anula, N.; Molina, F.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J.; Osuna-Pérez, M.C. Joint assessment of equilibrium and neuromotor function: A validation study in patients with fibromyalgia. Diagnostics 2020, 10, 1057. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Hita-Contreras, F.; Mendoza, N.; Martínez-Amat, A. Cross-cultural adaptation and validation of the Falls Efficacy Scale International in Spanish postmenopausal women. Menopause 2012, 19, 904–908. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Garcia, A.; Leon-Llamas, J.L.; Villafaina, S.; Rohlfs-Dominguez, P.; Gusi, N. MoCA vs. MMSE of fibromyalgia patients: The possible role of dual-task tests in detecting cognitive impairment. J. Clin. Med. 2021, 10, 125. [Google Scholar] [CrossRef]
- Carson, N.; Leach, L.; Murphy, K.J. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 2018, 33, 379–388. [Google Scholar] [CrossRef]
- Hobson, J. The Montreal Cognitive Assessment (MoCA). Occup. Med. 2015, 65, 764–765. [Google Scholar] [CrossRef]
- Neri, F.; Mencarelli, L.; Menardi, A.; Giovannelli, F.; Rossi, S.; Sprugnoli, G.; Rossi, A.; Pascual-Leone, A.; Salvador, R.; Ruffini, G.; et al. A novel tDCS sham approach based on model-driven controlled shunting. Brain Stimul. 2020, 13, 507–516. [Google Scholar] [CrossRef]
- Fertonani, A.; Rosini, S.; Cotelli, M.; Rossini, P.M.; Miniussi, C. Naming facilitation induced by transcranial direct current stimulation. Behav. Brain Res. 2010, 208, 311–318. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Rasyada, A.R.; Zulaika, H.; Akutagawa, M.; Emoto, T.; Kinouchi, Y.; Azhim, A. The impact of visceral fat and blood flow velocity in hypertensive subjects running head: The impact of visceral fat and blood flow velocity. Health Sci. J. 2016, 10, 1. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mishra, R.K.; Thrasher, A.T. Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: A double blind, sham-controlled study. Gait Posture 2021, 84, 11–16. [Google Scholar] [CrossRef]
- Villafaina, S.; Fuentes-García, J.P.; Cano-Plasencia, R.; Gusi, N. Neurophysiological differences between women with fibromyalgia and healthy controls during dual task: A pilot study. Front. Psychol. 2020, 11, 558849. [Google Scholar] [CrossRef]
- Chew, T.; Ho, K.A.; Loo, C.K. Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Tremblay, S.; Larochelle-Brunet, F.; Lafleur, L.P.; El Mouderrib, S.; Lepage, J.F.; Théoret, H. Systematic assessment of duration and intensity of anodal transcranial direct current stimulation on primary motor cortex excitability. Eur. J. Neurosci. 2016, 44, 2184–2190. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.A.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Bhattacharjee, S.; Arumugam, R.; Bharath, R.D.; Udupa, K.; Oishi, K.; Desmond, J.E.; Chen, S.H.A.; Guan, C. Focality-oriented selection of current dose for transcranial direct current stimulation. J. Personal. Med. 2021, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Splittgerber, M.; Salvador, R.; Brauer, H.; Breitling-Ziegler, C.; Prehn-Kristensen, A.; Krauel, K.; Nowak, R.; Ruffini, G.; Moliadze, V.; Siniatchkin, M. Individual baseline performance and electrode montage impact on the effects of anodal tDCS over the left dorsolateral prefrontal cortex. Front. Hum. Neurosci. 2020, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Kamholz, J. Response variability in transcranial direct current stimulation: Why sex matters. Front. Psychiatry 2020, 11, 585. [Google Scholar] [CrossRef]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; McIntire, J.P. The effects of anodal transcranial direct current stimulation on sleep time and efficiency. Front. Hum. Neurosci. 2020, 14, 357. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Boggio, P.S.; Fregni, F.; Pascual-Leone, A. Treatment of depression with transcranial direct current stimulation (tDCS): A review. Exp. Neurol. 2009, 219, 14–19. [Google Scholar] [CrossRef]
- Lloyd, D.M.; Wittkopf, P.G.; Arendsen, L.J.; Jones, A.K.P. Is transcranial direct current stimulation (tDCS) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. J. Pain 2020, 21, 1085–1100. [Google Scholar] [CrossRef]
- Montenegro, R.A.; Farinatti, P.d.T.V.; Fontes, E.B.; Soares, P.P.; Cunha, F.A.; Gurgel, J.L.; Porto, F.; Cyrino, E.S.; Okano, A.H. Transcranial direct current stimulation influences the cardiac autonomic nervous control. Neurosci. Lett. 2011, 497, 32–36. [Google Scholar] [CrossRef]
- Okano, A.H.; Fontes, E.B.; Montenegro, R.A.; Farinatti, P.d.T.V.; Cyrino, E.S.; Li, L.M.; Bikson, M.; Noakes, T.D. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sport. Med. 2015, 49, 1213–1218. [Google Scholar] [CrossRef]
- Nikolin, S.; Boonstra, T.W.; Loo, C.K.; Martin, D. Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PLoS ONE 2017, 12, e0181833. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Butcher, S.; Ko, J.; Zello, G.A.; Chilibeck, P.D. Wearing of cloth or disposable surgical face masks has no effect on vigorous exercise performance in healthy individuals. Int. J. Environ. Res. Public Health 2020, 17, 8110. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.R.; Dominelli, P.B.; Davis, C.K.; Guenette, J.A.; Luks, A.M.; Molgat-Seon, Y.; Sá, R.C.; Sheel, A.W.; Swenson, E.R.; Stickland, M.K. Face masks and the cardiorespiratory response to physical activity in health and disease. Ann. Am. Thorac. Soc. 2021, 18, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.; Korytny, A.; Isenberg, Y.; Marcusohn, E.; Zukermann, R.; Bishop, B.; Minha, S.; Raz, A.; Miller, A. Return to training in the COVID-19 era: The physiological effects of face masks during exercise. Scand. J. Med. Sci. Sport. 2021, 31, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lässing, J.; Falz, R.; Pökel, C.; Fikenzer, S.; Laufs, U.; Schulze, A.; Hölldobler, N.; Rüdrich, P.; Busse, M. Effects of surgical face masks on cardiopulmonary parameters during steady state exercise. Sci. Rep. 2020, 10, 22363. [Google Scholar] [CrossRef]
- Fikenzer, S.; Uhe, T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 109, 1522–1530. [Google Scholar] [CrossRef]
- Alkan, B.; Ozalevli, S.; Akkoyun Sert, O. Maximal exercise outcomes with a face mask: The effects of gender and age differences on cardiorespiratory responses. Ir. J. Med Sci. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Alvaro, M.C.; Villafaina, S.; Leon-Llamas, J.L.; Murillo-Garcia, A.; Melo-Alonso, M.; Sánchez-Gómez, J.; Molero, P.; Cano-Plasencia, R.; Gusi, N. Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol. Behav. Sci. 2022, 12, 37. https://doi.org/10.3390/bs12020037
Gomez-Alvaro MC, Villafaina S, Leon-Llamas JL, Murillo-Garcia A, Melo-Alonso M, Sánchez-Gómez J, Molero P, Cano-Plasencia R, Gusi N. Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol. Behavioral Sciences. 2022; 12(2):37. https://doi.org/10.3390/bs12020037
Chicago/Turabian StyleGomez-Alvaro, Mari Carmen, Santos Villafaina, Juan Luis Leon-Llamas, Alvaro Murillo-Garcia, María Melo-Alonso, Jesús Sánchez-Gómez, Pablo Molero, Ricardo Cano-Plasencia, and Narcis Gusi. 2022. "Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol" Behavioral Sciences 12, no. 2: 37. https://doi.org/10.3390/bs12020037
APA StyleGomez-Alvaro, M. C., Villafaina, S., Leon-Llamas, J. L., Murillo-Garcia, A., Melo-Alonso, M., Sánchez-Gómez, J., Molero, P., Cano-Plasencia, R., & Gusi, N. (2022). Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol. Behavioral Sciences, 12(2), 37. https://doi.org/10.3390/bs12020037








