Long-Term Management of Generalised Anxiety Disorder with Low-Dose Continuous Infusions of Flumazenil: A Case Series
Abstract
1. Introduction
2. Method
2.1. Design
2.2. Clinical Setting
2.3. Participants
2.4. Inclusion and Exclusion Criteria
2.5. Intervention
2.6. Outcome Measures
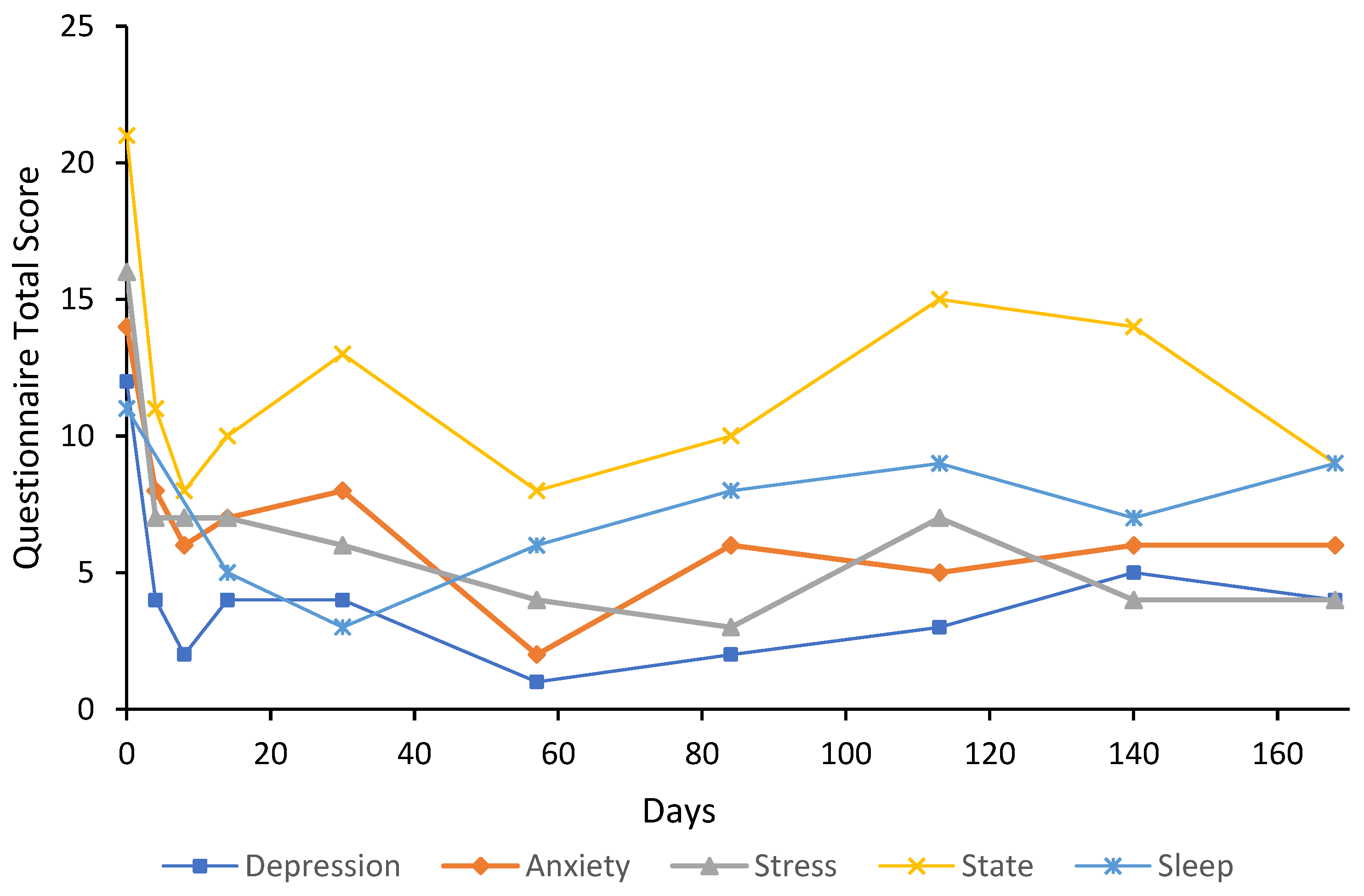
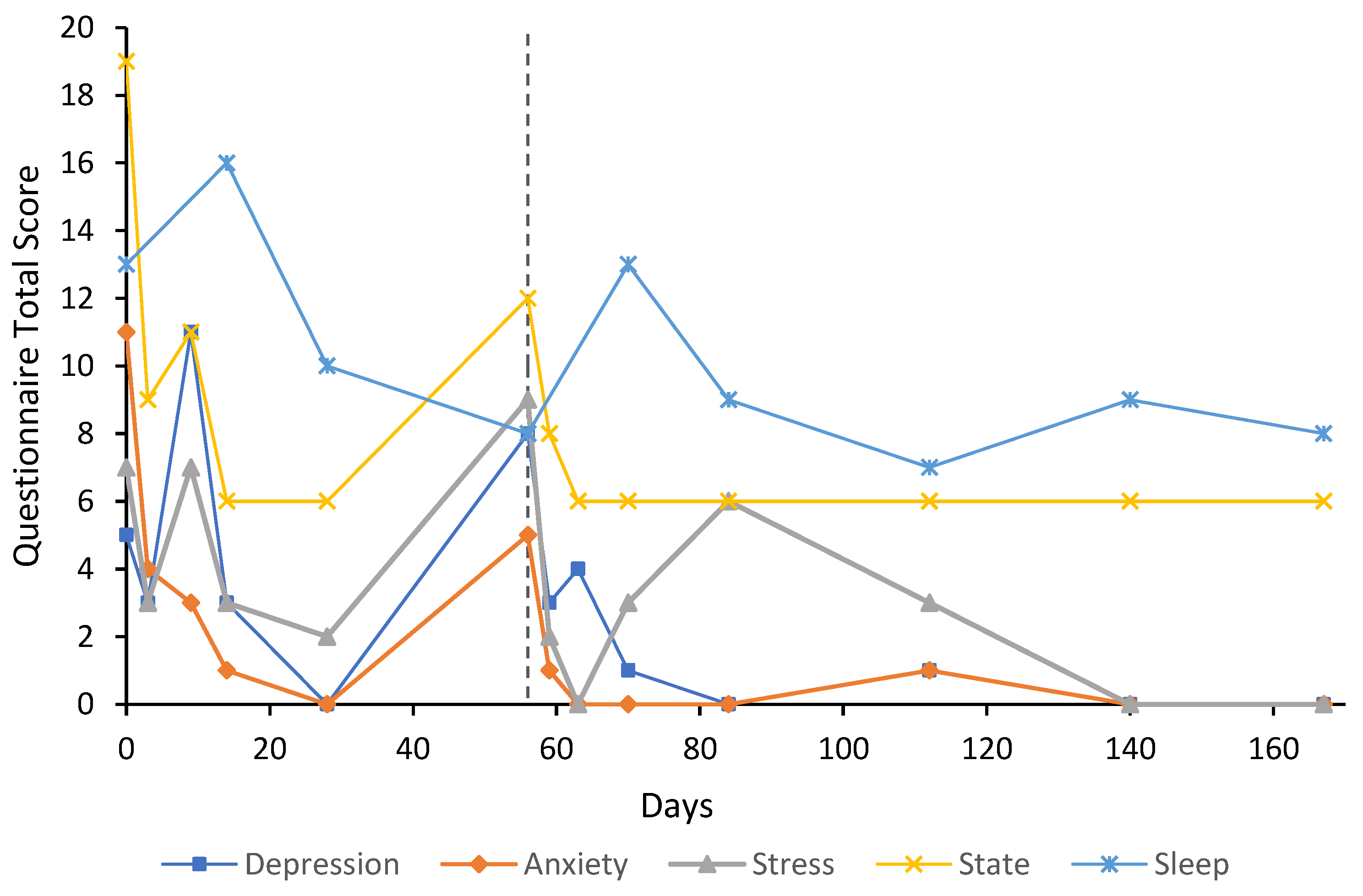
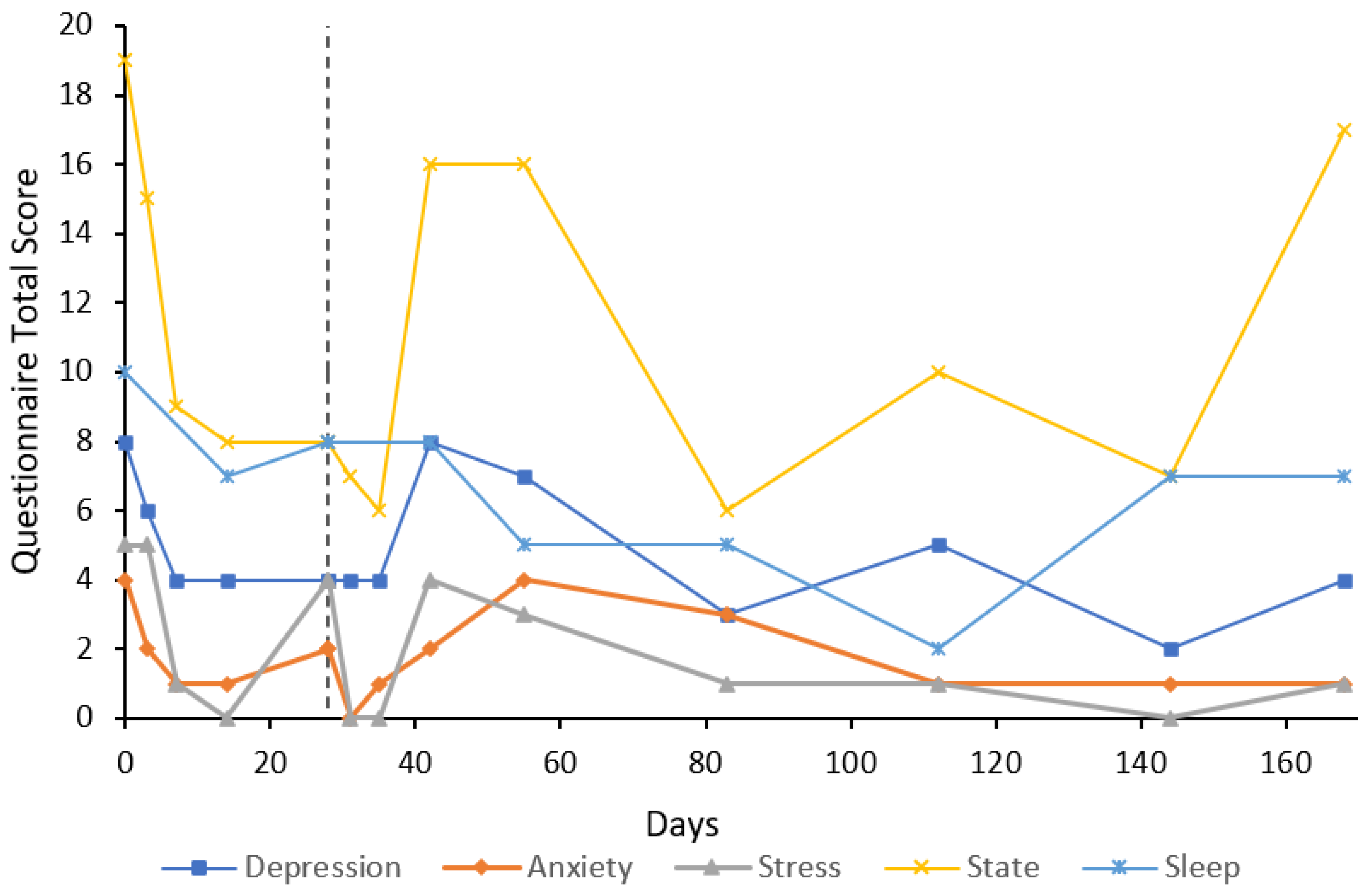
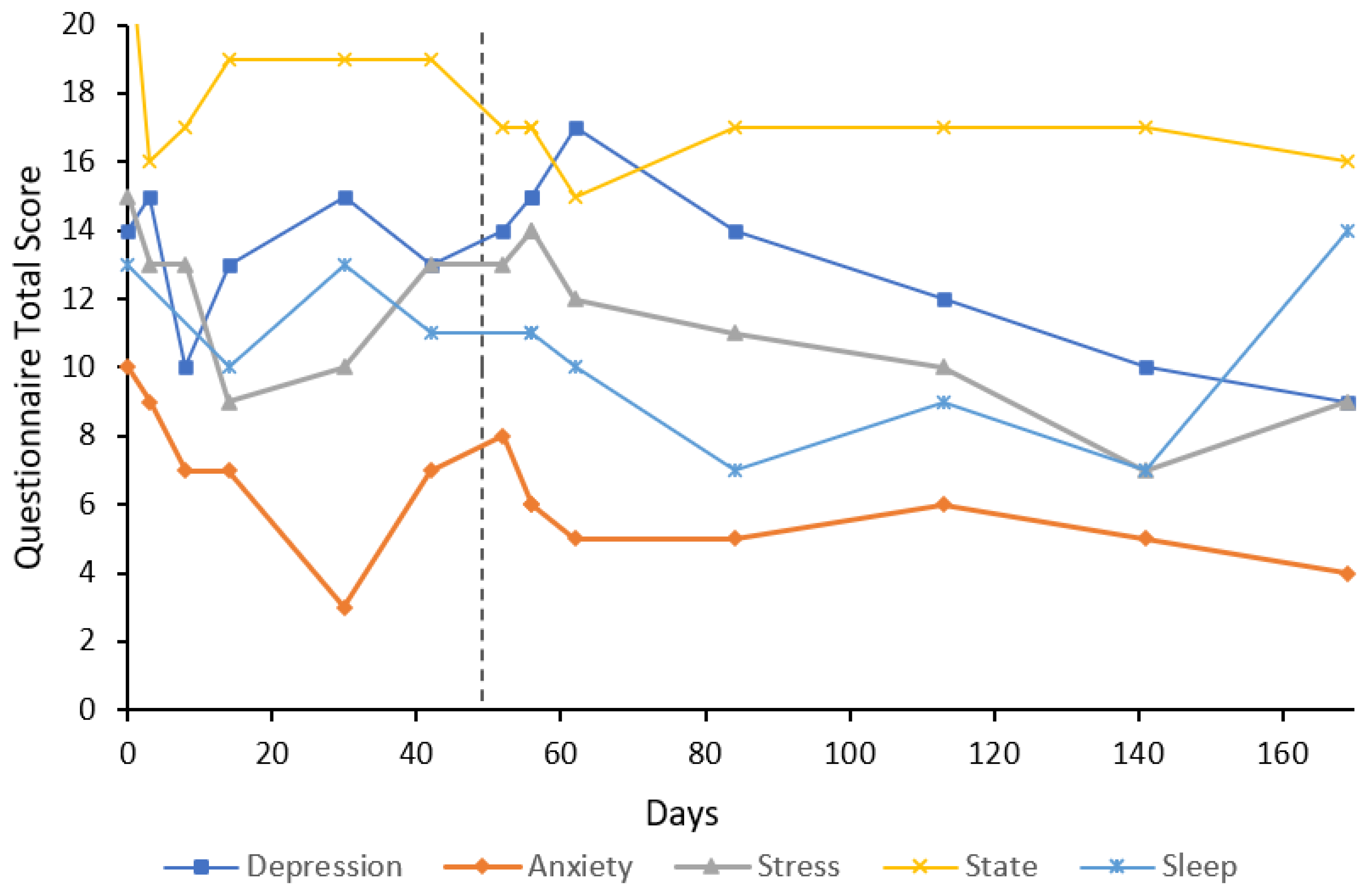
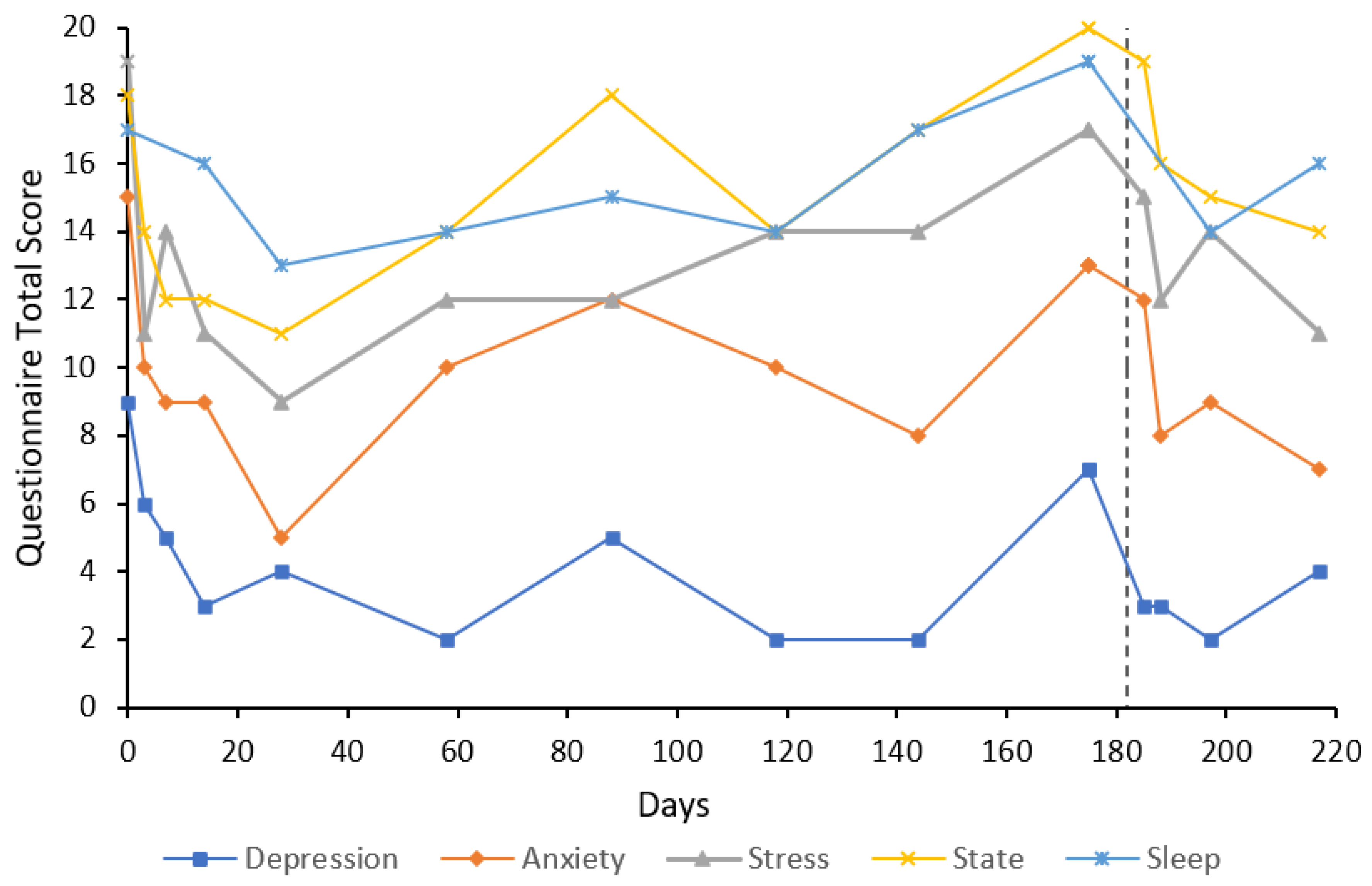
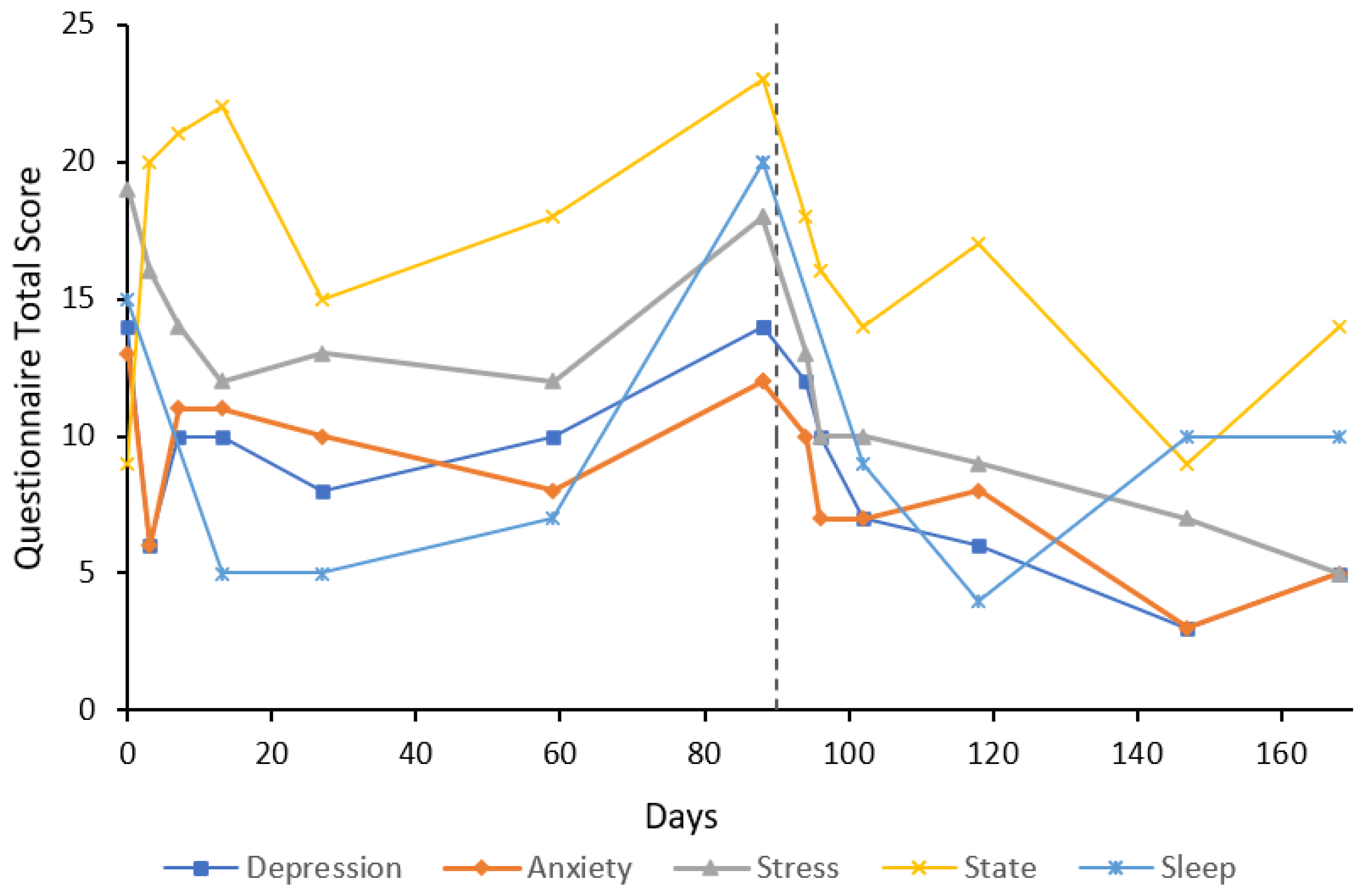
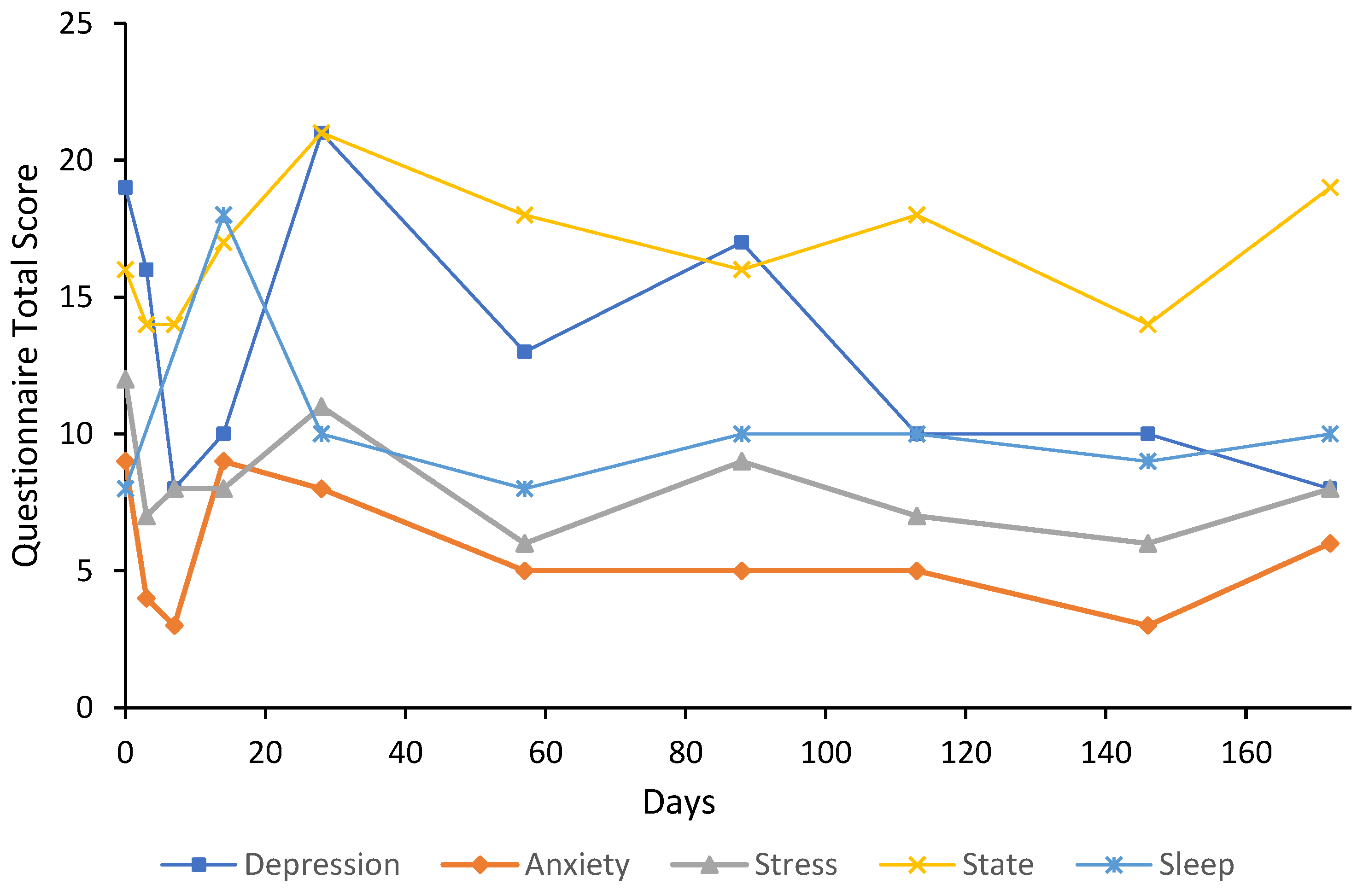
3. Case Reports
3.1. Participant Characteristics
3.2. Cases
4. Discussion
5. Strengths and Limitations
6. Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Fisher, P.L. Psychopathology of generalized anxiety disorder. Psychiatry 2007, 6, 171–175. [Google Scholar] [CrossRef]
- Yonkers, K.A.; Bruce, S.E.; Dyck, I.R.; Keller, M.B. Chronicity, relapse, and illness—Course of panic disorder, social phobia, and generalized anxiety disorder: Findings in men and women from 8 years of follow-up. Depress. Anxiety 2003, 17, 173–179. [Google Scholar] [CrossRef]
- Weisberg, R.B. Overview of generalized anxiety disorder: Epidemiology, presentation, and course. J. Clin. Psychiatry 2009, 70, 4–9. [Google Scholar] [CrossRef]
- Millan, M.J. Agomelatine for the treatment of generalized anxiety disorder: Focus on its distinctive mechanism of action. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221105128. [Google Scholar] [CrossRef]
- De Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the first melatonergic antidepressant: Discovery, characterization and development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef]
- Levitan, M.N.; Papelbaum, M.; Nardi, A.E. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr. Dis. Treat. 2015, 11, 1149–1155. [Google Scholar] [CrossRef]
- Wang, S.M.; Woo, Y.S.; Kim, N.Y.; Na, H.R.; Lim, H.K.; Bahk, W.M. Agomelatine for the treatment of generalized anxiety disorder: A meta-analysis. Clin. Psychopharmacol. Neurosci. 2020, 18, 423. [Google Scholar] [CrossRef]
- Stein, D.J.; Ahokas, A.; Márquez, M.S.; Höschl, C.; Oh, K.S.; Jarema, M.; Avedisova, A.S.; Albarran, C.; Olivier, V. Agomelatine in generalized anxiety disorder: An active comparator and placebo-controlled study. J. Clin. Psychiatry 2014, 75, 663. [Google Scholar] [CrossRef]
- Bystritsky, A. Treatment-resistant anxiety disorders. Mol. Psychiatry 2006, 11, 805–814. [Google Scholar] [CrossRef]
- Gallo, A.T.; Hulse, G.K. A theory of the anxiolytic action of flumazenil in anxiety disorders. J. Psychopharmacol. 2022, 36, 439–448. [Google Scholar] [CrossRef]
- Kuver, A.; Smith, S.S. Flumazenil decreases surface expression of α4β2δ GABAA receptors by increasing the rate of receptor internalization. Brain Res. Bull. 2016, 120, 131–143. [Google Scholar] [CrossRef]
- Klotz, U.; Kanto, J. Pharmacokinetics and Clinical Use of Flumazenil (Ro 15-1788). Clin. Pharmacokinet. 1988, 14, 1–12. [Google Scholar] [CrossRef]
- Hulse, G.; O’Neil, G.; Morris, N.; Bennett, K.; Norman, A.; Hood, S. Withdrawal and psychological sequelae, and patient satisfaction associated with subcutaneous flumazenil infusion for the management of benzodiazepine withdrawal: A case series. J. Psychopharmacol. 2013, 27, 222–227. [Google Scholar] [CrossRef]
- MacDonald, T.; Gallo, A.T.; Basso-Hulse, G.M.; Bennett, K.S.; Hulse, G.K. A double-blind randomised crossover trial of low-dose flumazenil for benzodiazepine withdrawal: A proof of concept. Drug Alcohol Depend. 2022, 236, 109501. [Google Scholar] [CrossRef]
- Government of Western Australia Department of Health. List of Licensed Private Day Hospitals—Class B Sedation. 2022. Available online: https://ww2.health.wa.gov.au/Articles/J_M/List-of-licensed-private-day-hospitals-class-B-sedation#:~:text=Definition%20of%20a%20day%20hospital,the%20Health%20Services%20Act%202016%22 (accessed on 1 September 2022).
- Gerra, G.; Zaimovic, A.; Giusti, F.; Moi, G.; Brewer, C. Intravenous flumazenil versus oxazepam tapering in the treatment of benzodiazepine withdrawal: A randomized, placebo-controlled study. Addict. Biol. 2002, 7, 385–395. [Google Scholar] [CrossRef]
- Tamburin, S.; Faccini, M.; Casari, R.; Federico, A.; Morbioli, L.; Franchini, E.; Bongiovanni, L.G.; Lugoboni, F. Low risk of seizures with slow flumazenil infusion and routine anticonvulsant prophylaxis for high-dose benzodiazepine dependence. J. Psychopharmacol. 2017, 31, 1369–1373. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales; Psychology Foundation of Australia: Sydney, Australia, 1996. [Google Scholar]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Spielberger, C.; Gorsuch, R.; Lushene, R. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Dowling, N.L.; Bonwick, R.; Dharwadkar, N.P.; Ng, C.H. Repetitive transcranial magnetic stimulation for major depression: A naturalistic observational study in an Australian private hospital. Psychiatry Res. 2020, 291, 113275. [Google Scholar] [CrossRef]
- Norton, P.J. Depression Anxiety and Stress Scales (DASS-21): Psychometric analysis across four racial groups. Anxiety Stress Coping 2007, 20, 253–265. [Google Scholar] [CrossRef]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176. [Google Scholar] [CrossRef]
- Brown, T.A.; Chorpita, B.F.; Korotitsch, W.; Barlow, D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997, 35, 79–89. [Google Scholar] [CrossRef]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Jenkins Sleep Scale. In Stop, That and One Hundred Other Sleep Scales; Shahid, A., Wilkinson, K., Marcu, S., Eds.; Springer: New York, NY, USA, 2012; pp. 203–204. [Google Scholar]
- Jenkins, C.D.; Stanton, B.A.; Niemcryk, S.J.; Rose, R.M. A scale for the estimation of sleep problems in clinical research. J. Clin. Epidemiol. 1988, 41, 313–321. [Google Scholar] [CrossRef]
- Barbaccia, M.L.; Roscetti, G.; Trabucchi, M.; Purdy, R.H.; Mostallino, M.C.; Concas, A.; Biggio, G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997, 120, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Matsumoto, K.; Uzunova, V.; Sugaya, I.; Takahata, H.; Nomura, H.; Watanabe, H.; Costa, E.; Guidotti, A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. USA 2001, 98, 2849–2854. [Google Scholar] [CrossRef]
- Evans, J.; Sun, Y.; McGregor, A.; Connor, B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology 2012, 63, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Pinna, G.; Puia, G.; Guidotti, A.; Costa, E. Social isolation stress-induced aggression in mice: A model to study the pharmacology of neurosteroidogenesis. Stress 2005, 8, 85–93. [Google Scholar] [CrossRef]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J. Neurochem. 2000, 75, 732–740. [Google Scholar] [CrossRef]
- Gulinello, M.; Gong, Q.H.; Li, X.; Smith, S. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001, 910, 55–66. [Google Scholar] [CrossRef]
- Gulinello, M.; Gong, Q.H.; Smith, S.S. Progesterone withdrawal increases the α4 subunit of the GABAA receptor in male rats in association with anxiety and altered pharmacology—A comparison with female rats. Neuropharmacology 2002, 43, 701–714. [Google Scholar] [CrossRef]
- Kuver, A.; Shen, H.; Smith, S.S. Regulation of the surface expression of α4β2δ GABAA receptors by high efficacy states. Brain Res. 2012, 1463, 1–20. [Google Scholar] [CrossRef]
- Gong, Q.H.; Smith, S.S. Characterization of neurosteroid effects on hyperpolarizing current at α4β2δ GABAA receptors. Psychopharmacology 2014, 231, 3525–3535. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gong, Q.H.; Aoki, C.; Yuan, M.; Ruderman, Y.; Dattilo, M.; Williams, K.; Smith, S.S. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 2007, 10, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Mohammad, A.; Ramroop, J.; Smith, S.S. A stress steroid triggers anxiety via increased expression of α4βδ GABAA receptors in methamphetamine dependence. Neuroscience 2013, 254, 452–475. [Google Scholar] [CrossRef] [PubMed]
- Poisbeau, P.; Gazzo, G.; Calvel, L. Anxiolytics targeting GABAA receptors: Insights on etifoxine. World J. Biol. Psychiatry 2018, 19, S36–S45. [Google Scholar] [CrossRef] [PubMed]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar]
- Jongen-Rêlo, A.L.; Amaral, D.G. Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: A combined retrograde tracing and in situ hybridization study. Eur. J. Neurosci. 1998, 10, 2924–2933. [Google Scholar] [CrossRef]
- Kelty, E.; Martyn, V.; O’Neil, G.; Hulse, G. Use of subcutaneous flumazenil preparations for the treatment of idiopathic hypersomnia: A case report. J. Psychopharmacol. 2014, 28, 703–706. [Google Scholar] [CrossRef]
- Gallo, A.; MacDonald, T.; Bennett, K.; Basso-Hulse, G.; Hulse, G. Is the Precipitation of Anxiety Symptoms Associated with Bolus Doses of Flumazenil a Barrier to Its Use at Low Continuous Doses in Benzodiazepine Withdrawal? J. Clin. Med. 2022, 11, 5948. [Google Scholar] [CrossRef]

| ID | Age at First Infusion (Years) | Total Cumulative Flumazenil Dose (mg) * | Duration of GAD (Years) | Comorbid Psychiatric Conditions | Current GAD Drug Therapy (mg) | Previously Taken Medication for GAD ^ | Sodium Valproate Given | Re-Treatment Day |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 32 | 10 | ADHD | Lisdexamfetamine Agomelatine 25 | Y | N | - |
| 2 | 35 | 48 | 15 | Alcohol abuse | Escitalopram 20 | Y | Y | 56 |
| 3 | 64 | 48 | 15 | PTSD | - | Y | N | 28 |
| 4 | 51 | 64 | 20 | Social anxiety | - | Y | N | 49 |
| 5 | 20 | 64 | 8 | Social anxiety | - | Y | N | 182 |
| 6 | 54 | 64 | 5 | - | Bupropion SR 150 Fluoxetine 20 | Y | N | 90 |
| 7 | 31 | 32 | 20 | Depression | - | N | N | - |
| 8 | 50 | 48 | 10 | AUD | - | N | Y | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, A.T.; Addis, S.; Martyn, V.; Ramanathan, H.; Wilkerson, G.K.; Hood, S.D.; Stampfer, H.; Hulse, G.K. Long-Term Management of Generalised Anxiety Disorder with Low-Dose Continuous Infusions of Flumazenil: A Case Series. Behav. Sci. 2022, 12, 430. https://doi.org/10.3390/bs12110430
Gallo AT, Addis S, Martyn V, Ramanathan H, Wilkerson GK, Hood SD, Stampfer H, Hulse GK. Long-Term Management of Generalised Anxiety Disorder with Low-Dose Continuous Infusions of Flumazenil: A Case Series. Behavioral Sciences. 2022; 12(11):430. https://doi.org/10.3390/bs12110430
Chicago/Turabian StyleGallo, Alexander T., Stephen Addis, Vlad Martyn, Hishani Ramanathan, Grace K. Wilkerson, Sean D. Hood, Hans Stampfer, and Gary K. Hulse. 2022. "Long-Term Management of Generalised Anxiety Disorder with Low-Dose Continuous Infusions of Flumazenil: A Case Series" Behavioral Sciences 12, no. 11: 430. https://doi.org/10.3390/bs12110430
APA StyleGallo, A. T., Addis, S., Martyn, V., Ramanathan, H., Wilkerson, G. K., Hood, S. D., Stampfer, H., & Hulse, G. K. (2022). Long-Term Management of Generalised Anxiety Disorder with Low-Dose Continuous Infusions of Flumazenil: A Case Series. Behavioral Sciences, 12(11), 430. https://doi.org/10.3390/bs12110430






