Migraine and Mood in Children
Abstract
1. Introduction
1.1. Migraine
1.2. Mood
2. Methods Used to Study Mood in Children with Migraine
2.1. Migraines and Mood in Children
2.2. Migraine Phases and Mood in Children
2.3. Migraine and Psychiatric Comorbidity in Children
2.4. Proposed Mechanisms Underlying Co-Occurrence of Migraine and Psychobiological Response
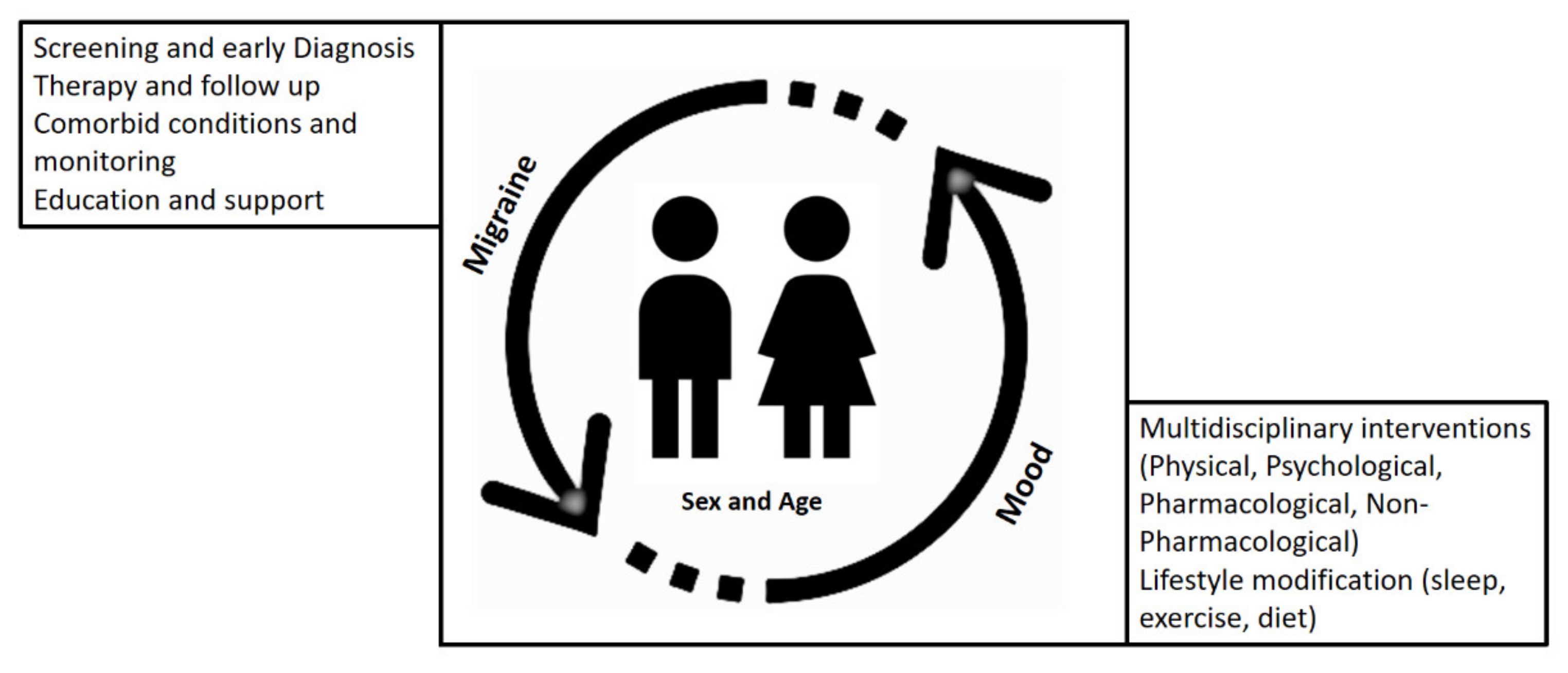
3. Considerations for Treatment Strategies in Children with Migraine
3.1. Pharmacological Treatments
3.2. Non-Pharmacological Treatments
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lewis, D.W.; Winner, P. The pharmacological treatment options for pediatric migraine: An evidence-based appraisal. NeuroRx 2006, 3, 181–191. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Gelfand, A.A. Episodic syndromes of childhood associated with migraine. Curr. Opin. Neurol. 2018, 31, 281–285. [Google Scholar] [CrossRef]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58 (Suppl. 1), 4–16. [Google Scholar] [CrossRef]
- Hamel, E. Serotonin and migraine: Biology and clinical implications. Cephalalgia 2007, 27, 1293–1300. [Google Scholar] [CrossRef]
- Deen, M.; Hansen, H.D.; Hougaard, A.; Norgaard, M.; Eiberg, H.; Lehel, S.; Ashina, M.; Knudsen, G.M. High brain serotonin levels in migraine between attacks: A 5-HT4 receptor binding PET study. Neuroimage Clin. 2018, 18, 97–102. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Smith, R.A.; Griffiths, L.R. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J. Headache Pain 2017, 18, 20. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Youssef, P.E.; Mack, K.J. Episodic and chronic migraine in children. Dev. Med. Child. Neurol. 2020, 62, 34–41. [Google Scholar] [CrossRef]
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P.; et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef]
- Wober-Bingol, C. Epidemiology of Migraine and Headache in Children and Adolescents. Curr. Pain Headache R 2013, 17, 341, 1–11. [Google Scholar] [CrossRef]
- Abu-Arafeh, I.; Razak, S.; Sivaraman, B.; Graham, C. Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Dev. Med. Child. Neurol. 2010, 52, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Morichi, S.; Suzuki, S.; Go, S.; Takeshita, M.; Kanou, K.; Ishida, Y.; Oana, S.; Kawashima, H. A Review on the Triggers of Pediatric Migraine with the Aim of Improving Headache Education. J. Clin. Med. 2020, 9, 3717. [Google Scholar] [CrossRef] [PubMed]
- Kacperski, J.; Kabbouche, M.A.; O’Brien, H.L.; Weberding, J.L. The optimal management of headaches in children and adolescents. Ther. Adv. Neurol. Disord. 2016, 9, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.W.; Hu, X.; Campbell, J.C.; Buse, D.C.; Lipton, R.B. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia 2010, 30, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.D.; Powers, S.W.; Vockell, A.L.; LeCates, S.; Kabbouche, M.A.; Maynard, M.K. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology 2001, 57, 2034–2039. [Google Scholar] [CrossRef]
- Bille, B. A 40-year follow-up of school children with migraine. Cephalalgia 1997, 17, 488–491, discussion 487. [Google Scholar] [CrossRef]
- Koller, L.S.; Diesner, S.C.; Voitl, P. Quality of life in children and adolescents with migraine: An Austrian monocentric, cross-sectional questionnaire study. BMC Pediatr. 2019, 19, 164. [Google Scholar] [CrossRef]
- Renaud, S.M.; Zacchia, C. Toward a definition of affective instability. Harv Rev. Psychiatry 2012, 20, 298–308. [Google Scholar] [CrossRef]
- Bowen, R.C.; Wang, Y.; Balbuena, L.; Houmphan, A.; Baetz, M. The relationship between mood instability and depression: Implications for studying and treating depression. Med. Hypotheses 2013, 81, 459–462. [Google Scholar] [CrossRef]
- Marwaha, S.; He, Z.; Broome, M.; Singh, S.P.; Scott, J.; Eyden, J.; Wolke, D. How is affective instability defined and measured? A systematic review. Psychol. Med. 2014, 44, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Greenberg, B.R.; Serper, M.R. The affective lability scales: Development, reliability, and validity. J. Clin. Psychol. 1989, 45, 786–793. [Google Scholar] [CrossRef]
- Aas, M.; Pedersen, G.; Henry, C.; Bjella, T.; Bellivier, F.; Leboyer, M.; Kahn, J.P.; Cohen, R.F.; Gard, S.; Aminoff, S.R.; et al. Psychometric properties of the Affective Lability Scale (54 and 18-item version) in patients with bipolar disorder, first-degree relatives, and healthy controls. J. Affect. Disord. 2015, 172, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.J.; Copeland, W.; Angold, A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? J. Child. Psychol. Psychiatry 2011, 52, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Fulford, D.; Peckham, A.D.; Johnson, K.; Johnson, S.L. Emotion perception and quality of life in bipolar I disorder. J. Affect. Disord. 2014, 152-154, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.; Balbuena, L.; Peters, E.M.; Leuschen-Mewis, C.; Baetz, M. The Relationship between Mood Instability and Suicidal Thoughts. Arch. Suicide Res. 2015, 19, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.W.; Gilman, D.K.; Hershey, A.D. Headache and psychological functioning in children and adolescents. Headache 2006, 46, 1404–1415. [Google Scholar] [CrossRef]
- Braccili, T.; Montebello, D.; Verdecchia, P.; Crenca, R.; Redondi, A.; Turri, E.; Turaccio, R.; Lendvai, D. Evaluation of anxiety and depression in childhood migraine. Eur. Rev. Med. Pharmacol. Sci. 1999, 3, 37–39. [Google Scholar] [PubMed]
- Crombez, G.; Bijttebier, P.; Eccleston, C.; Mascagni, T.; Mertens, G.; Goubert, L.; Verstraeten, K. The child version of the pain catastrophizing scale (PCS-C): A preliminary validation. Pain 2003, 104, 639–646. [Google Scholar] [CrossRef]
- Genizi, J.; Halevy, A.; Schertz, M.; Osman, K.; Assaf, N.; Segal, I.; Srugo, I.; Kessel, A.; Engel-Yeger, B. Sensory processing patterns affect headache severity among adolescents with migraine. J. Headache Pain 2020, 21, 48. [Google Scholar] [CrossRef]
- Margari, F.; Lucarelli, E.; Craig, F.; Petruzzelli, M.G.; Lecce, P.A.; Margari, L. Psychopathology in children and adolescents with primary headaches: Categorical and dimensional approaches. Cephalalgia 2013, 33, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Messer, S.C.; Beidel, D.C. Psychosocial correlates of childhood anxiety disorders. J. Am. Acad. Child. Adolesc. Psychiatry 1994, 33, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Genizi, J.; Khourieh Matar, A.; Schertz, M.; Zelnik, N.; Srugo, I. Pediatric mixed headache -The relationship between migraine, tension-type headache and learning disabilities—In a clinic-based sample. J. Headache Pain 2016, 17, 42. [Google Scholar] [CrossRef][Green Version]
- El-Heneedy, Y.A.E.; Bahnasy, W.S.; ELAhwal, S.A.; Amer, R.A.R.; Abohammar, S.D.A.; Salem, H.A.M. Psychiatric and sleep abnormalities in school-age children with migraine. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55. [Google Scholar] [CrossRef]
- Pakalnis, A.; Splaingard, M.; Splaingard, D.; Kring, D.; Colvin, A. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache 2009, 49, 1486–1492. [Google Scholar] [CrossRef]
- Zarea, K.; Rahmani, M.; Hassani, F.; Hakim, A. Epidemiology and associated factors of migraine headache among iranian medical students: A descriptive-analytical study. Clin. Epidemiol. Glob. Health 2017, 6. [Google Scholar] [CrossRef]
- Dyb, G.; Stensland, S.; Zwart, J.A. Psychiatric comorbidity in childhood and adolescence headache. Curr. Pain Headache Rep. 2015, 19, 5. [Google Scholar] [CrossRef]
- Arruda, M.A.; Bigal, M.E. Behavioral and emotional symptoms and primary headaches in children: A population-based study. Cephalalgia 2012, 32, 1093–1100. [Google Scholar] [CrossRef]
- Bellini, B.; Panunzi, S.; Bruni, O.; Guidetti, V. Headache and sleep in children. Curr. Pain Headache Rep. 2013, 17, 335. [Google Scholar] [CrossRef]
- Karlson, C.W.; Litzenburg, C.C.; Sampilo, M.L.; Rapoff, M.A.; Connelly, M.; Bickel, J.L.; Hershey, A.D.; Powers, S.W. Relationship between daily mood and migraine in children. Headache 2013, 53, 1624–1634. [Google Scholar] [CrossRef]
- McGrath, P.A.; Seifert, C.E.; Speechley, K.N.; Booth, J.C.; Stitt, L.; Gibson, M.C. A new analogue scale for assessing children’s pain: An initial validation study. Pain 1996, 64, 435–443. [Google Scholar] [CrossRef]
- Connelly, M.; Bickel, J. An electronic daily diary process study of stress and health behavior triggers of primary headaches in children. J. Pediatr. Psychol. 2011, 36, 852–862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huss, D.; Derefinko, K.; Milich, R.; Farzam, F.; Baumann, R. Examining the stress response and recovery among children with migraine. J. Pediatr. Psychol. 2009, 34, 707–715. [Google Scholar] [CrossRef]
- Karsan, N.; Prabhakar, P.; Goadsby, P.J. Characterising the premonitory stage of migraine in children: A clinic-based study of 100 patients in a specialist headache service. J. Headache Pain 2016, 17, 94. [Google Scholar] [CrossRef]
- Cuvellier, J.C. Pediatric vs. Adult Prodrome and Postdrome: A Window on Migraine Pathophysiology? Front. Neurol. 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.M.; Buse, D.C.; Sollars, C.M.; Haut, S.; Lipton, R.B. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache 2014, 54, 1670–1679. [Google Scholar] [CrossRef]
- Bose, P.; Karsan, N.; Goadsby, P.J. The Migraine Postdrome. Continuum Minneap. Minn. 2018, 24, 1023–1031. [Google Scholar] [CrossRef]
- Jacobs, H.; Pakalnis, A. Premonitory Symptoms in Episodic and Chronic Migraine From a Pediatric Headache Clinic. Pediatr. Neurol. 2019, 97, 26–29. [Google Scholar] [CrossRef]
- Fonseca, E.; Torres-Ferrus, M.; Gallardo, V.J.; Macaya, A.; Pozo-Rosich, P. Impact of Puberty in Pediatric Migraine: A Pilot Prospective Study. J. Clin. Neurol. 2020, 16, 416–422. [Google Scholar] [CrossRef]
- Orr, S.L.; Potter, B.K.; Ma, J.; Colman, I. Migraine and Mental Health in a Population-Based Sample of Adolescents. Can. J. Neurol. Sci. 2017, 44, 44–50. [Google Scholar] [CrossRef]
- Gelfand, A.A. Psychiatric comorbidity and paediatric migraine: Examining the evidence. Curr. Opin. Neurol. 2015, 28, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Qubty, W.; Gelfand, A.A. Psychological and Behavioral Issues in the Management of Migraine in Children and Adolescents. Curr. Pain Headache Rep. 2016, 20, 69. [Google Scholar] [CrossRef]
- Bruijn, J.; Locher, H.; Passchier, J.; Dijkstra, N.; Arts, W.F. Psychopathology in children and adolescents with migraine in clinical studies: A systematic review. Pediatrics 2010, 126, 323–332. [Google Scholar] [CrossRef]
- Blaauw, B.A.; Dyb, G.; Hagen, K.; Holmen, T.L.; Linde, M.; Wentzel-Larsen, T.; Zwart, J.A. The relationship of anxiety, depression and behavioral problems with recurrent headache in late adolescence—A Young-HUNT follow-up study. J. Headache Pain 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Pine, D.S.; Cohen, P.; Brook, J. The association between major depression and headache: Results of a longitudinal epidemiologic study in youth. J. Child. Adolesc. Psychopharmacol. 1996, 6, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Arita, J.H.; Lin, J.; Pinho, R.S.; Minett, T.S.; de Souza Vitalle, M.S.; Fisberg, M.; Peres, M.F.; Vilanova, L.C.; Masruha, M.R. Adolescents with chronic migraine commonly exhibit depressive symptoms. Acta Neurol. Belg. 2013, 113, 61–65. [Google Scholar] [CrossRef]
- Rammohan, K.; Mundayadan, S.M.; Das, S.; Shaji, C.V. Migraine and Mood Disorders: Prevalence, Clinical Correlations and Disability. J. Neurosci. Rural Pract. 2019, 10, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Kopala-Sibley, D.C.; Noel, M. The Co-occurrence of Pediatric Chronic Pain and Depression: A Narrative Review and Conceptualization of Mutual Maintenance. Clin. J. Pain 2019, 35, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Molnar, Z. Thomas Willis (1621–1675), the founder of clinical neuroscience. Nat. Rev. Neurosci. 2004, 5, 329–335. [Google Scholar] [CrossRef]
- Guidetti, V.; Faedda, N.; Siniatchkin, M. Migraine in childhood: Biobehavioural or psychosomatic disorder? J. Headache Pain 2016, 17, 82. [Google Scholar] [CrossRef]
- Maleki, N.; Becerra, L.; Borsook, D. Migraine: Maladaptive brain responses to stress. Headache 2012, 52 (Suppl. 2), 102–106. [Google Scholar] [CrossRef]
- Borsook, D.; Maleki, N.; Becerra, L.; McEwen, B. Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 2012, 73, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Loder, E. What is the evolutionary advantage of migraine? Cephalalgia 2002, 22, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.J.; McGrath, P.J.; Ferguson, H.B.; Humphreys, P.; D’Astous, J.; Latter, J.; Goodman, J.T.; Firestone, P. Personality and behavioural characteristics in pediatric migraine. Headache 1987, 27, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ostkirchen, G.G.; Andler, F.; Hammer, F.; Pohler, K.D.; Snyder-Schendel, E.; Werner, N.K.; Markett, S.; Horacek, U.; Jockel, K.H.; Diener, H.C. Prevalences of primary headache symptoms at school-entry: A population-based epidemiological survey of preschool children in Germany. J. Headache Pain 2006, 7, 331–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radat, F. Stress and migraine. Rev. Neurol. Paris 2013, 169, 406–412. [Google Scholar] [CrossRef]
- Breslau, N.; Lipton, R.B.; Stewart, W.F.; Schultz, L.R.; Welch, K.M. Comorbidity of migraine and depression: Investigating potential etiology and prognosis. Neurology 2003, 60, 1308–1312. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Juang, K.D.; Lu, S.R. Migraine and suicidal ideation in adolescents aged 13 to 15 years. Neurology 2009, 72, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Schrumm, M.; Brennenstuhl, S. Migraine and Despair: Factors Associated with Depression and Suicidal Ideation among Canadian Migraineurs in a Population-Based Study. Depress. Res. Treat. 2013, 2013, 401487. [Google Scholar] [CrossRef]
- Balottin, U.; Fusar Poli, P.; Termine, C.; Molteni, S.; Galli, F. Psychopathological symptoms in child and adolescent migraine and tension-type headache: A meta-analysis. Cephalalgia 2013, 33, 112–122. [Google Scholar] [CrossRef]
- Kemper, K.J.; Heyer, G.; Pakalnis, A.; Binkley, P.F. What Factors Contribute to Headache-Related Disability in Teens? Pediatr. Neurol. 2016, 56, 48–54. [Google Scholar] [CrossRef]
- Oztop, D.B.; Tasdelen, B.I.; PoyrazogLu, H.G.; Ozsoy, S.; Yilmaz, R.; Sahin, N.; Per, H.; Bozkurt, S. Assessment of Psychopathology and Quality of Life in Children and Adolescents With Migraine. J. Child. Neurol. 2016, 31, 837–842. [Google Scholar] [CrossRef]
- Vannatta, K.; Getzoff, E.A.; Gilman, D.K.; Noll, R.B.; Gerhardt, C.A.; Powers, S.W.; Hershey, A.D. Friendships and social interactions of school-aged children with migraine. Cephalalgia 2008, 28, 734–743. [Google Scholar] [CrossRef]
- Langdon, R.; DiSabella, M.; Strelzik, J.; Fletcher, A. Pediatric Migraine and Academics. Curr. Pain Headache Rep. 2020, 24, 40. [Google Scholar] [CrossRef]
- Rains, J.C.; Poceta, J.S. Sleep-related headaches. Neurol. Clin. 2012, 30, 1285–1298. [Google Scholar] [CrossRef]
- Grengs, L.R.; Mack, K.J. New Daily Persistent Headache Is Most Likely to Begin at the Start of School. J. Child. Neurol. 2016, 31, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Wang, S.J.; Liu, C.Y.; Juang, Y.Y.; Yang, C.H.; Hung, C.I. The impact of anxiety and migraine on quality of sleep in patients with major depressive disorder. Compr. Psychiatry 2009, 50, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cook, C. Acute Treatment of Pediatric Migraine: A Review of the Updated Guidelines. Adv. Emerg. Nurs. J. 2020, 42, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.L.; Kabbouche, M.A.; O’Brien, H.L.; Kacperski, J.; Powers, S.W.; Hershey, A.D. Paediatric migraine: Evidence-based management and future directions. Nat. Rev. Neurol. 2018, 14, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.A.; Peterlin, B.L.; Rapoport, A.M.; Linder, S.L.; Kabbouche, M.A.; Sheftell, F.D. Favorable outcome of early treatment of new onset child and adolescent migraine-implications for disease modification. J. Headache Pain 2009, 10, 227–233. [Google Scholar] [CrossRef]
- Kabbouche, M.A.; Powers, S.W.; Vockell, A.L.; LeCates, S.L.; Ellinor, P.L.; Segers, A.; Manning, P.; Burdine, D.; Hershey, A.D. Outcome of a multidisciplinary approach to pediatric migraine at 1, 2, and 5 years. Headache 2005, 45, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Potrebic, S.; Billinghurst, L.; Gloss, D.; Hershey, A.D.; Licking, N.; Sowell, M.; Victorio, M.C.; et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Faedda, N.; Cerutti, R.; Verdecchia, P.; Migliorini, D.; Arruda, M.; Guidetti, V. Behavioral management of headache in children and adolescents. J. Headache Pain 2016, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Faria, V.; Linnman, C.; Lebel, A.; Borsook, D. Harnessing the placebo effect in pediatric migraine clinic. J. Pediatr. 2014, 165, 659–665. [Google Scholar] [CrossRef]
- Singer, A.B.; Buse, D.C.; Seng, E.K. Behavioral treatments for migraine management: Useful at each step of migraine care. Curr. Neurol. Neurosci. Rep. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Wood, M.E.; Black, R.A.; Surette, D.A.; Zacharoff, K.L.; Chiauzzi, E.J. A randomized trial of a web-based intervention to improve migraine self-management and coping. Headache 2012, 52, 244–261. [Google Scholar] [CrossRef]
- Sorbi, M.J.; Kleiboer, A.M.; van Silfhout, H.G.; Vink, G.; Passchier, J. Medium-term effectiveness of online behavioral training in migraine self-management: A randomized trial controlled over 10 months. Cephalalgia 2015, 35, 608–618. [Google Scholar] [CrossRef]
- Soee, A.B.; Skov, L.; Skovgaard, L.T.; Thomsen, L.L. Headache in children: Effectiveness of multidisciplinary treatment in a tertiary paediatric headache clinic. Cephalalgia 2013, 33, 1218–1228. [Google Scholar] [CrossRef]
- Esparham, A.; Herbert, A.; Pierzchalski, E.; Tran, C.; Dilts, J.; Boorigie, M.; Wingert, T.; Connelly, M.; Bickel, J. Pediatric Headache Clinic Model: Implementation of Integrative Therapies in Practice. Child. Basel 2018, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Eidlitz-Markus, T.; Haimi-Cohen, Y.; Steier, D.; Zeharia, A. Effectiveness of nonpharmacologic treatment for migraine in young children. Headache 2010, 50, 219–223. [Google Scholar] [CrossRef]
- Albers, L.; Heinen, F.; Landgraf, M.; Straube, A.; Blum, B.; Filippopulos, F.; Lehmann, S.; Mansmann, U.; Berger, U.; Akboga, Y.; et al. Headache cessation by an educational intervention in grammar schools: A cluster randomized trial. Eur. J. Neurol. 2015, 22, 270–276.e22. [Google Scholar] [CrossRef] [PubMed]
- Zebenholzer, K.; Frantal, S.; Pablik, E.; Lieba-Samal, D.; Salhofer-Polanyi, S.; Wober-Bingol, C.; Wober, C. Reliability of assessing lifestyle and trigger factors in patients with migraine—Findings from the PAMINA study. Eur. J. Neurol. 2016, 23, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; Fily, A.; Cuvellier, J.C.; Vallee, L. The prevalence of triggers in paediatric migraine: A questionnaire study in 102 children and adolescents. J. Headache Pain 2012, 13, 61–65. [Google Scholar] [CrossRef]
- Fraga, M.D.; Pinho, R.S.; Andreoni, S.; Vitalle, M.S.; Fisberg, M.; Peres, M.F.; Vilanova, L.C.; Masruha, M.R. Trigger factors mainly from the environmental type are reported by adolescents with migraine. Arq. Neuropsiquiatr. 2013, 71, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Sieberg, C.B.; Huguet, A.; von Baeyer, C.L.; Seshia, S. Psychological interventions for headache in children and adolescents. Can. J. Neurol. Sci. 2012, 39, 26–34. [Google Scholar] [CrossRef]
- Bellini, B.; Arruda, M.; Cescut, A.; Saulle, C.; Persico, A.; Carotenuto, M.; Gatta, M.; Nacinovich, R.; Piazza, F.P.; Termine, C.; et al. Headache and comorbidity in children and adolescents. J. Headache Pain 2013, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Penzien, D.B.; Taylor, F.R. Headache toolbox. Behavioral and other nonpharmacologic treatments for headache. Headache 2014, 54, 955–956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kabbouche, M.A.; Gilman, D.K. Management of migraine in adolescents. Neuropsychiatr. Dis. Treat. 2008, 4, 535–548. [Google Scholar] [CrossRef]
- Kroner-Herwig, B. Headache in children and adolescents. Epidemiology, biopsychosocial correlates, and psychological treatment approaches. Bundesgesundheitsblatt Gesundh. Gesundh. 2014, 57, 928–934. [Google Scholar] [CrossRef]
- Powers, S.W.; Kashikar-Zuck, S.M.; Allen, J.R.; LeCates, S.L.; Slater, S.K.; Zafar, M.; Kabbouche, M.A.; O’Brien, H.L.; Shenk, C.E.; Rausch, J.R.; et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: A randomized clinical trial. JAMA 2013, 310, 2622–2630. [Google Scholar] [CrossRef]
- Oskoui, M.; Pringsheim, T.; Billinghurst, L.; Potrebic, S.; Gersz, E.M.; Gloss, D.; Holler-Managan, Y.; Leininger, E.; Licking, N.; Mack, K.; et al. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 500–509. [Google Scholar] [CrossRef]
- Szperka, C.L.; VanderPluym, J.H.; Oakley, C.B. Pharmacologic Acute and Preventive Treatment for Migraine in Children and Adolescents. JAMA Neurol. 2020, 77, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Raucci, U.; Della Vecchia, N.; Ossella, C.; Paolino, M.C.; Villa, M.P.; Reale, A.; Parisi, P. Management of Childhood Headache in the Emergency Department. Review of the Literature. Front. Neurol. 2019, 10, 886. [Google Scholar] [CrossRef]
- Kacperski, J.; Green, A.; Qaiser, S. Management of Chronic Migraine in Children and Adolescents: A Brief Discussion on Preventive Therapies. Paediatr. Drugs 2020, 22, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. A Bidirectional View of Migraine and Diet Relationship. Neuropsychiatr. Dis. Treat. 2021, 17, 435–451. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Millichap, J.G.; Yee, M.M. The diet factor in pediatric and adolescent migraine. Pediatr. Neurol. 2003, 28, 9–15. [Google Scholar] [CrossRef]
- Hershey, A.D.; Powers, S.W.; Vockell, A.L.; Lecates, S.L.; Ellinor, P.L.; Segers, A.; Burdine, D.; Manning, P.; Kabbouche, M.A. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 2007, 47, 73–80. [Google Scholar] [CrossRef]
- Slater, S.K.; Nelson, T.D.; Kabbouche, M.A.; LeCates, S.L.; Horn, P.; Segers, A.; Manning, P.; Powers, S.W.; Hershey, A.D. A randomized, double-blinded, placebo-controlled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 2011, 31, 897–905. [Google Scholar] [CrossRef]
- Rainero, I.; Vacca, A.; Roveta, F.; Govone, F.; Gai, A.; Rubino, E. Targeting MTHFR for the treatment of migraines. Expert Opin. Ther. Targets 2019, 23, 29–37. [Google Scholar] [CrossRef]
- Gordon, C.M.; DePeter, K.C.; Feldman, H.A.; Grace, E.; Emans, S.J. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 531–537. [Google Scholar] [CrossRef]
- Yamanaka, G.; Kanou, K.; Takamatsu, T.; Takeshita, M.; Morichi, S.; Suzuki, S.; Ishida, Y.; Watanabe, Y.; Go, S.; Oana, S.; et al. Complementary and Integrative Medicines as Prophylactic Agents for Pediatric Migraine: A Narrative Literature Review. J. Clin. Med. 2021, 10, 138. [Google Scholar] [CrossRef]
- Bruni, O.; Galli, F.; Guidetti, V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999, 19 (Suppl. 25), 57–59. [Google Scholar] [CrossRef]
- Amin, F.M.; Aristeidou, S.; Baraldi, C.; Czapinska-Ciepiela, E.K.; Ariadni, D.D.; Di Lenola, D.; Fenech, C.; Kampouris, K.; Karagiorgis, G.; Braschinsky, M.; et al. The association between migraine and physical exercise. J. Headache Pain 2018, 19, 83. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J.; Tripathi, G.M.; Bhoi, S.K. Is beta endorphin related to migraine headache and its relief? Cephalalgia 2013, 33, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Triantafillou, S.; Saeb, S.; Lattie, E.G.; Mohr, D.C.; Kording, K.P. Relationship Between Sleep Quality and Mood: Ecological Momentary Assessment Study. JMIR Ment. Health 2019, 6, e12613. [Google Scholar] [CrossRef]
- Hearing, C.M.; Chang, W.C.; Szuhany, K.L.; Deckersbach, T.; Nierenberg, A.A.; Sylvia, L.G. Physical Exercise for Treatment of Mood Disorders: A Critical Review. Curr. Behav. Neurosci. Rep. 2016, 3, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Stubberud, A.; Varkey, E.; McCrory, D.C.; Pedersen, S.A.; Linde, M. Biofeedback as Prophylaxis for Pediatric Migraine: A Meta-analysis. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.K.; Brockman, L.N.; Breuner, C.C. Biofeedback therapy for pediatric headache: Factors associated with response. Headache 2012, 52, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Siniatchkin, M.; Hierundar, A.; Kropp, P.; Kuhnert, R.; Gerber, W.D.; Stephani, U. Self-regulation of slow cortical potentials in children with migraine: An exploratory study. Appl. Psychophysiol. Biofeedback 2000, 25, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Kroon Van Diest, A.M.; Powers, S.W. Cognitive Behavioral Therapy for Pediatric Headache and Migraine: Why to Prescribe and What New Research Is Critical for Advancing Integrated Biobehavioral Care. Headache 2019, 59, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mahato, A.K. Cognitive Behavior Therapy for Children and Adolescents: Challenges and Gaps in Practice. Indian J. Psychol. Med. 2019, 41, 279–283. [Google Scholar] [CrossRef]
- Shah, U.H.; Kalra, V. Pediatric migraine. Int. J. Pediatr. 2009, 2009, 424192. [Google Scholar] [CrossRef] [PubMed]
- Oar, E.L.; Johnco, C.; Ollendick, T.H. Cognitive Behavioral Therapy for Anxiety and Depression in Children and Adolescents. Psychiatr. Clin. N. Am. 2017, 40, 661–674. [Google Scholar] [CrossRef]
- Fristad, M.A.; MacPherson, H.A. Evidence-based psychosocial treatments for child and adolescent bipolar spectrum disorders. J. Clin. Child. Adolesc. Psychol. 2014, 43, 339–355. [Google Scholar] [CrossRef][Green Version]
- Kroner, J.W.; Hershey, A.D.; Kashikar-Zuck, S.M.; LeCates, S.L.; Allen, J.R.; Slater, S.K.; Zafar, M.; Kabbouche, M.A.; O’Brien, H.L.; Shenk, C.E.; et al. Cognitive Behavioral Therapy plus Amitriptyline for Children and Adolescents with Chronic Migraine Reduces Headache Days to ≤4 Per Month. Headache 2016, 56, 711–716. [Google Scholar] [CrossRef]
- Powers, S.W.; Gilman, D.K.; Hershey, A.D. Suggestions for a biopsychosocial approach to treating children and adolescents who present with headache. Headache 2006, 46 (Suppl. 3), S149–S150. [Google Scholar] [CrossRef]
- Hershey, A.D.; Powers, S.W.; Coffey, C.S.; Eklund, D.D.; Chamberlin, L.A.; Korbee, L.L.; Group, C.S. Childhood and Adolescent Migraine Prevention (CHAMP) study: A double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache 2013, 53, 799–816. [Google Scholar] [CrossRef]
- Powers, S.W.; Coffey, C.S.; Chamberlin, L.A.; Ecklund, D.J.; Klingner, E.A.; Yankey, J.W.; Korbee, L.L.; Porter, L.L.; Hershey, A.D.; Investigators, C. Trial of Amitriptyline, Topiramate, and Placebo for Pediatric Migraine. N. Engl. J. Med. 2017, 376, 115–124. [Google Scholar] [CrossRef]
- Popova, V.; Berk, T. Pediatric Migraine-An Updated Review. US Neurol. 2019, 15, 68–73. [Google Scholar] [CrossRef]
| Prodrome | Aura | Headache | Postdrome |
|---|---|---|---|
| Gastrointestinal disturbances (e.g., constipation or diarrhea) Food craving or thirst Neck stiffness Frequent yawning, Fatigue Mood changes (depression, irritability, anger, and anxiety) | Visual symptoms (e.g., flashing lights, blank spots, and blurry vision) Olfactory or auditory hallucinations Hypersensitivity or reduced sensation Tingling or numbness (e.g., in face or extremities) Difficulty speaking Confusion, dizziness, or vertigo | Head pain that can become bilateral Sensitivity to light and sound Nausea and vomiting Depression or anxiety Dizziness | Feeling tired Confused Mood changes (feeling melancholy or depression) Poor concentration Poor memory |
| Child’s personality traits | Education |
| Individual child (sex and age) | Monitoring |
| Children family | Treatment strategies |
| School and community | Intervention feature |
| Children communication feature | Stressors |
| Care system | Adherence |
| Comorbid conditions | Compliance |
| Lifestyle (sleep, diet, and exercise) | Placebo effect |
| Puberty | Nocebo effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazerani, P. Migraine and Mood in Children. Behav. Sci. 2021, 11, 52. https://doi.org/10.3390/bs11040052
Gazerani P. Migraine and Mood in Children. Behavioral Sciences. 2021; 11(4):52. https://doi.org/10.3390/bs11040052
Chicago/Turabian StyleGazerani, Parisa. 2021. "Migraine and Mood in Children" Behavioral Sciences 11, no. 4: 52. https://doi.org/10.3390/bs11040052
APA StyleGazerani, P. (2021). Migraine and Mood in Children. Behavioral Sciences, 11(4), 52. https://doi.org/10.3390/bs11040052






