Cognition across the Lifespan: Investigating Age, Sex, and Other Sociodemographic Influences
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Sociodemographic, Lifestyle, Psychological, and Sleep Questionnaire
2.2.2. Cognitive Battery
2.3. Procedure
2.4. Factor Analysis
2.5. Statistical Analyses
2.6. Secondary Analyses
3. Results
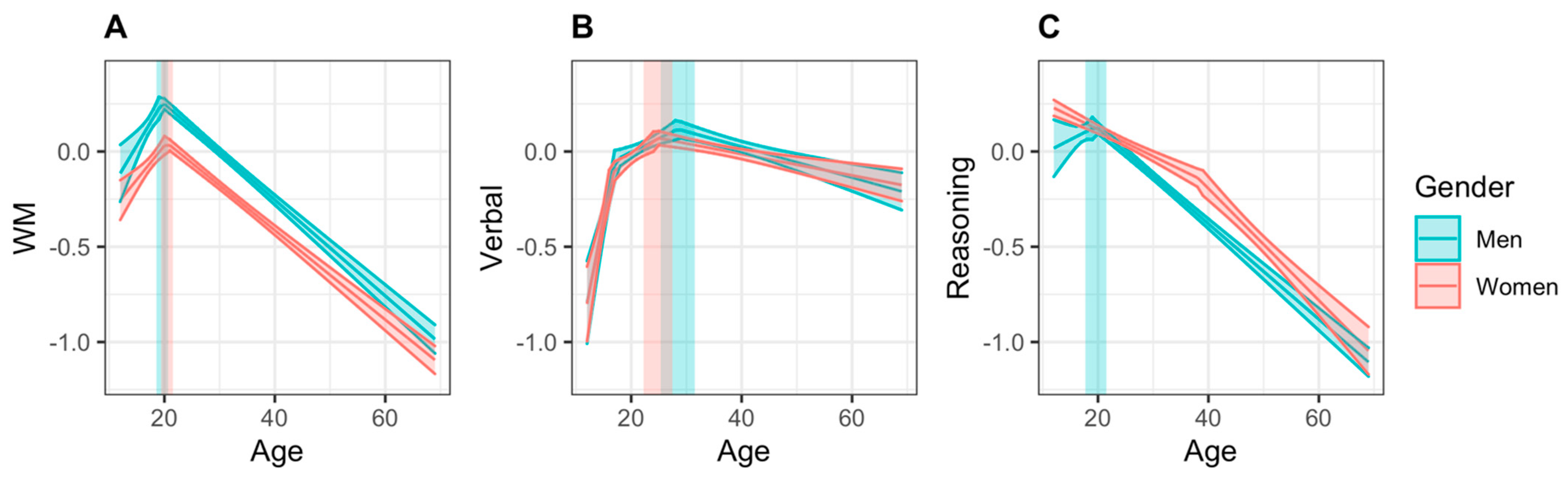
3.1. Cognitive Domain Scores
3.1.1. Working Memory
3.1.2. Verbal Abilities
3.1.3. Reasoning
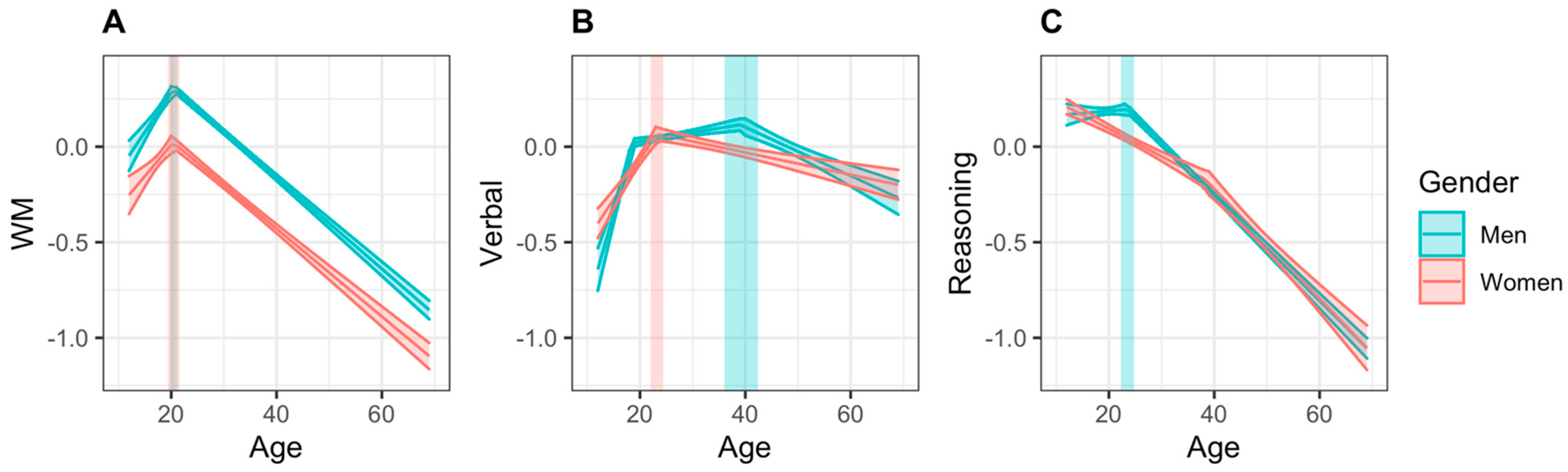
3.2. Unmatched Samples
3.2.1. Working Memory
3.2.2. Verbal Abilities
3.2.3. Reasoning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. World Population Prospects 2019; PUBLISHER United Nations Department of Economic and Social Affair: New York, NY, USA, 2019. [Google Scholar]
- Anderson, P.; Anderson, V.; Northam, E.; Taylor, H.G. Standardization of the Contingency Naming Test (CNT) for school-age children: A measure of reactive flexibility. Clin. Neuropsychol. Assess. 2000, 1, 247–273. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Standardization+of+the+Contingency+NamingTest+(CNT)+for+school+aged+children:+A+measure+of+reactive+flexibility#0 (accessed on 21 March 2021).
- Feng, J.; Spence, I.; Pratt, J. Playing an action video game in reduces gender differences spatial cognition. Psychol. Sci. 2007, 18, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Karapetsas, A.B.; Vlachos, F.M. Sex and handedness in development of visuomotor skills. Percept. Mot. Skills 1997, 85, 131–140. [Google Scholar] [CrossRef]
- Krikorian, R.; Bartok, J.A. Developmental data for the porteus maze test. Clin. Neuropsychol. 1998, 12, 305–310. [Google Scholar] [CrossRef]
- Anderson, P. Assessment and Development of Executive Function (EF) During Childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Anderson, P.; Northam, E.; Jacobs, R.; Catroppa, C. Development of executive functions through late childhood and adolescence in an australian sample. Dev. Neuropsychol. 2001, 20, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive function. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Rizeq, J.; Flora, D.B.; Toplak, M.E. Changing relations among cognitive abilities across development: Implications for measurement and research. Clin. Neuropsychol. 2017, 31, 1353–1374. [Google Scholar] [CrossRef]
- Lee, K.; Bull, R.; Ho, R. Developmental changes in executive functioning. Child Dev. 2013, 84, 1933–1953. [Google Scholar] [CrossRef]
- Hartshorne, J.K.; Germine, L.T. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol. Sci. 2015, 26, 433–443. [Google Scholar] [CrossRef]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Lee, J.; Shih, R.; Feeney, K.; Langa, K.M. Gender disparity in late-life cognitive functioning in India: Findings from the longitudinal aging study in India. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2014, 69, 603–611. [Google Scholar] [CrossRef]
- Comijs, H.C.; Dik, M.G.; Deeg, D.J.H.; Jonker, C. The course of cognitive decline in older persons: Results from the longitudinal aging study amsterdam. Dement Geriatr. Cogn. Disord. 2004, 17, 136–142. [Google Scholar] [CrossRef]
- Pronk, M.; Lissenberg-Witte, B.I.; van der Aa, H.P.; Comijs, H.C.; Smits, C.; Lemke, U.; Zekveld, A.A.; Kramer, S.E. Longitudinal Relationships Between Decline in Speech-in-Noise Recognition Ability and Cognitive Functioning: The Longitudinal Aging Study Amsterdam. J. Speech Lang Hear Res. 2019, 62, 1167–1187. [Google Scholar] [CrossRef]
- Sliwinski, M.; Buschke, H. Cross-sectional and longitudinal relationships among age, cognition, and processing speed. Psychol. Aging 1999, 14, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Krinzinger, H.; Wood, G.; Willmes, K. What accounts for individual and gender differences in the multi-digit number processing of primary school children? Z. Psychol. J. Psychol. 2012, 220, 78–89. [Google Scholar] [CrossRef]
- Sokolowski, H.M.; Hawes, Z.; Lyons, I.M. What explains sex differences in math anxiety? A closer look at the role of spatial processing. Cognition 2019, 182, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Huguet, P.; Régner, I. Counter-stereotypic beliefs in math do not protect school girls from stereotype threat. J. Exp. Soc. Psychol. 2009, 45, 1024–1027. [Google Scholar] [CrossRef]
- Scheuringer, A.; Wittig, R.; Pletzer, B. Sex differences in verbal fluency: The role of strategies and instructions. Cogn. Process. 2017, 18, 407–417. [Google Scholar] [CrossRef]
- Scheuringer, A.; Pletzer, B. Sex differences and menstrual cycle dependent changes in cognitive strategies during spatial navigation and verbal fluency. Front. Psychol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Burton, L.A.; Henninger, D.; Hafetz, J. Gender differences in relations of mental rotation, verbal fluency, and SAT scores to finger length ratios as hormonal indexes. Dev. Neuropsychol. 2005, 28, 493–505. [Google Scholar] [CrossRef]
- Griksiene, R.; Ruksenas, O. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology 2011, 36, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Pang, Z.; Oksuzyan, A.; Jiang, W.; Wang, S.; Li, S.; Kruse, T.; Christensen, K.; Tan, Q. Gender-specific patterns in age-related decline in general health among Danish and Chinese: A cross-national comparative study. Geriatr. Gerontol. Int. 2012, 12, 431–439. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Wang, Y.; Holland, T.; Agarwal, P.; Weintraub, S.; Morris, M.C. Age and cognitive decline in the UK Biobank. PLoS ONE 2019, 14, e213948. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Crawford, J.D.; Dutta, R.; Thalamuthu, A.; Kochan, N.A.; Andrews, G.; Lima-Costa, M.F.; Castro-Costa, E.; Brayne, C.; Matthews, F.E.; et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.R.; Mitchell, D.; Wersching, H.; Czira, M.E.; Berger, K.; Baune, B.T. Brain-derived neurotrophic factor (BDNF) gene: A gender-specific role in cognitive function during normal cognitive aging of the MEMO-Study? Age (Omaha) 2012, 34, 1011–1022. [Google Scholar] [CrossRef]
- Malpetti, M.; Ballarini, T.; Presotto, L.; Garibotto, V.; Tettamanti, M.; Perani, D. Gender differences in healthy aging and Alzheimer’s Dementia: A 18F-FDG-PET study of brain and cognitive reserve. Hum. Brain Mapp. 2017, 38, 4212–4227. [Google Scholar] [CrossRef]
- Miller, D.I.; Halpern, D.F. The new science of cognitive sex differences. Trends Cogn. Sci. 2014, 18, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Blums, A.; Belsky, J.; Grimm, K.; Chen, Z. Building links between early socioeconomic status, cognitive ability, and math and science achievement. J. Cogn. Dev. 2017, 18, 16–40. [Google Scholar] [CrossRef]
- Lubinski, D. Cognitive epidemiology: With emphasis on untangling cognitive ability and socioeconomic status. Intelligence 2009, 37, 625–633. [Google Scholar] [CrossRef]
- Zaremba, D.; Schulze Kalthoff, I.; Förster, K.; Redlich, R.; Grotegerd, D.; Leehr, E.J.; Meinert, S.; Dohm, K.; Bürger, C.; Enneking, V.; et al. The effects of processing speed on memory impairment in patients with major depressive disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2019, 92, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Crego, A.; Holguín, S.R.; Parada, M.; Mota, N.; Corral, M.; Cadaveira, F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol. Clin. Exp. Res. 2009, 33, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Highfield, R.R.; Parkin, B.L.; Owen, A.M. Fractionating human intelligence. Neuron 2012, 76, 1225–1237. [Google Scholar] [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Gender differences in depression. Int. Rev. Psychiatry 2010, 22, 429–436. [Google Scholar] [CrossRef]
- Compton, W.; Thomas, Y.F.; Stinson, F.S.; Grant, B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Arch. Gen. Psychiatry 2007, 64, 566–576. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2707116&tool=pmcentrez&rendertype=abstract (accessed on 21 March 2021). [CrossRef]
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013, results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Bratberg, G.H.; Wilsnack, S.C.; Wilsnack, R.; Håvås Haugland, S.; Krokstad, S.; Sund, E.R.; Bjørngaard, J.H. Gender differences and gender convergence in alcohol use over the past three decades (1984–2008), the HUNT Study, Norway. BMC Public Health 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Pliatsikas, C.; Veríssimo, J.; Babcock, L.; Pullman, M.Y.; Glei, D.A.; Weinstein, M.; Goldman, N.; Ullman, M.T. Working memory in older adults declines with age, but is modulated by sex and education. Q. J. Exp. Psychol. 2019, 72, 1308–1327. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.J.; Nichols, E.S.; Battista, M.E.; Stojanoski, B.; Owen, A.M. Dissociable effect of self-reported daily sleep duration on high-level cognitive abilities. Sleep 2018, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.S.; Wild, C.J.; Stojanoski, B.; Battista, M.E.; Owen, A.M. Bilingualism affords no general cognitive advantages: A population study of executive function in 11,000 people. Psychol. Sci. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sternin, A.; Burns, A.; Owen, A.M. Thirty-five years of computerized cognitive assessment of aging-Where are we now? Diagnostics 2019, 9, 114. [Google Scholar] [CrossRef]
- Stojanoski, B.; Lyons, K.M.; Pearce, A.A.A.; Owen, A.M. Targeted training: Converging evidence against the transferable benefits of online brain training on cognitive function. Neuropsychologia 2018, 117, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Wesnes, K.A.; Brooker, H.; Ballard, C.; Mccambridge, L.; Stenton, R.; Corbett, A. Utility, reliability, sensitivity and validity of an online test system designed to monitor changes in cognitive function in clinical trials. Int. J. Geriatr. Psychiatry 2017, 32, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Stojanoski, B.; Wild, C.J.; Battista, M.E.; Nichols, E.S.; Owen, A.M. Brain Training Habits Are Not Associated With Generalized Benefits to Cognition: An Online Study of Over 1000 “Brain Trainers”. J. Exp. Psychol. Gen. 2020. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Muggeo, V.M.R. Segmented: An R package to fit regression models with broken-line relationships. R News 2008, 8, 20–25. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for. J. Stat. Softw. 2011, 42, 1–28. Available online: http://www.jstatsoft.org/v42/i08 (accessed on 21 March 2021). [CrossRef]
- Canty, A.; Ripley, B. Boot: Bootstrap R(S-Plus) Functions. R Packag Version 13-20. 2019. Available online: https://cran.r-project.org/web/packages/boot/index.html (accessed on 21 February 2021).
- Wickham, H. Ggplot2, Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Muggeo, V.M.R. Estimating regression models with unknown break-points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Tiwari, R.C.; Cronin, K.A.; Davis, W.; Feuer, E.J.; Yu, B.; Chib, S. Bayesian model selection for join point regression with application to age-adjusted cancer rates. J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 919–939. [Google Scholar] [CrossRef]
- Davies, R.B. Hypothesis Testing When a Nuisance Parameter Is Present Only under the Alternatives; Oxford University Press: Oxford, UK, 2002; Volume 89, pp. 484–489. Available online: http://www.jstor.org/stable/2336019 (accessed on 21 March 2021).
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Kryspin-Exner, I.; Lamplmayr, E.; Felnhofer, A. Geropsychology: The gender gap in human aging-a mini-review. Gerontology 2011, 57, 539–548. [Google Scholar] [CrossRef]
- Miller, A.K.H.; Alston, R.L.; Corsellis, J.A.N. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: Measurements with an image analyser. Neuropathol. Appl. Neurobiol. 1980, 6, 119–132. [Google Scholar] [CrossRef]
- Sowell, E.R.; Peterson, B.S.; Kan, E.; Woods, R.P.; Yoshii, J.; Bansal, R.; Xu, D.; Zhu, H.; Thompson, P.M.; Toga, A.W. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex 2007, 17, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Anderson, P.; Northam, E.; Jacobs, R.; Catroppa, C. Developmental neuropsychology differential development of attention and executive functions in 3- to 12-year-old finnish children. Dev. Neuropsychol. 2001, 20, 407–428. [Google Scholar] [CrossRef]
- Moran, T.P. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol. Bull. 2016, 142. [Google Scholar] [CrossRef]
- Woods, A.J.; Porges, E.C.; Bryant, V.E.; Seider, T.; Gongvatana, A.; Kahler, C.W.; De La Monte, S.; Monti, P.M.; Cohen, R.A. Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcohol. Clin. Exp. Res. 2016, 40, 2435–2444. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]

| Measure | Mean (SD) or Percentage | χ2(df, N) or t(df) | p | Cohen’s d | BF10 | |
|---|---|---|---|---|---|---|
| Women | Men | |||||
| N | 9451 | 9451 | ||||
| Age (years) | 28.14 (10.95) | 28.28 (10.65) | −1.31(23,696) | 0.902 | 0.01 | 0.02 |
| Highest education completed | 10.18 (4, N = 18,902) | 0.281 | 0.05 | 9.06 × 10−5 | ||
| Some high school | 9.70% | 11.00% | ||||
| High School | 8.30% | 8.50% | ||||
| Some post-secondary | 28.00% | 27.50% | ||||
| Post-secondary degree | 27.80% | 27.10% | ||||
| Professional degree | 26.10% | 25.80% | ||||
| Level of employment | 6.57 (5, N = 18,902) | 0.902 | 0.04 | 4.76 × 10−7 | ||
| No answer | 3.70% | 4.10% | ||||
| Unemployed | 10.50% | 11.40% | ||||
| Full time student | 27.90% | 27.60% | ||||
| Employed and student | 14.90% | 14.60% | ||||
| Employed part time | 9.00% | 9.20% | ||||
| Employed full time | 34.00% | 33.10% | ||||
| Exercise | 4.07 (4, N = 18,902) | 0.902 | 0.03 | 3.77 × 10−6 | ||
| Never | 10.40% | 11.00% | ||||
| Infrequently | 36.40% | 36.90% | ||||
| Weekly | 19.80% | 19.80% | ||||
| Several times a week | 26.60% | 25.80% | ||||
| Every day | 6.90% | 6.50% | ||||
| Sleep (hours last night) | 7.02 (1.62) | 7.01 (1.63) | 0.40 (18,899) | 0.914 | −0.01 | 0.02 |
| Alcohol (units per week) | 1.72 (1.76) | 1.71 (1.76) | 0.25 (18,900) | 0.914 | <−0.01 | 4.14 × 10−23 |
| Caffeine (units per day) | 3.47 (4.80) | 3.52 (4.82) | −0.61 (18,900) | 0.902 | 0.01 | 0.02 |
| Cigarettes (per day) | 1.53 (4.63) | 1.68 (5.06) | −2.24 (18,749) | 0.281 | 0.03 | 0.20 |
| Depressive feelings | 2.19 (5, N = 18,902) | 0.914 | 0.02 | 1.35 × 10−8 | ||
| No answer | 1.10% | 1.30% | ||||
| Never | 10.90% | 11.10% | ||||
| Occasionally | 57.00% | 56.60% | ||||
| Quite often | 20.80% | 20.60% | ||||
| Nearly every day | 7.30% | 7.40% | ||||
| All the time | 3.00% | 3.00% | ||||
| Anxiety | 1.52 (5, N = 18,902) | 0.914 | 0.02 | 1.50 × 10−8 | ||
| No answer | 1.20% | 1.40% | ||||
| Never | 14.00% | 13.60% | ||||
| Occasionally | 50.20% | 50.30% | ||||
| Quite often | 20.00% | 20.20% | ||||
| Nearly every day | 10.00% | 9.90% | ||||
| All the time | 4.50% | 4.50% | ||||
| Tech savvy | 0.02 (1, N = 18,902) | 0.914 | <0.01 | 0.02 | ||
| Yes | 76.80% | 76.70% | ||||
| No | 23.20% | 23.30% | ||||
| Video games | 4.67 (3, N = 18,902) | 0.902 | 0.03 | 1.77 × 10−4 | ||
| Never | 33.80% | 32.50% | ||||
| Monthly | 26.50% | 26.40% | ||||
| Weekly | 23.50% | 24.30% | ||||
| Daily | 16.20% | 16.80% | ||||
| Political leaning | 1.29 (2, N = 18,902) | 0.902 | 0.02 | 6.63 × 10−4 | ||
| Liberal | 47.40% | 47.00% | ||||
| Middle | 44.60% | 44.60% | ||||
| Conservative | 7.90% | 8.40% | ||||
| Religiosity | 0.97 (4, N = 18,902) | 0.914 | 0.01 | 6.71 × 10−7 | ||
| Atheist | 33.50% | 33.10% | ||||
| Agnostic | 32.10% | 32.10% | ||||
| Religious lapsed | 18.70% | 18.70% | ||||
| Religious practicing | 11.90% | 12.00% | ||||
| Very religious | 3.90% | 4.10% | ||||
| Siblings | 2.30 (3, N = 18,902) | 0.902 | 0.02 | 4.64 × 10−5 | ||
| Only child | 12.40% | 12.40% | ||||
| Youngest | 30.30% | 30.50% | ||||
| Middle | 16.50% | 17.20% | ||||
| Oldest | 40.80% | 39.90% | ||||
| Score | Gender | Term | Coef | SE | t | p |
|---|---|---|---|---|---|---|
| WM | Women | Age | 0.04 | 0.01 | 4.10 | <0.001 |
| ∆Age | −0.06 | |||||
| Men | Age | 0.05 | 0.01 | 3.61 | <0.001 | |
| ∆Age | −0.07 | |||||
| Verbal | Women | Age | 0.15 | 0.01 | 7.58 | <0.001 |
| ∆Age1 | −0.13 | |||||
| ∆Age2 | −0.03 | |||||
| Men | Age | 0.15 | 0.03 | 5.36 | <0.001 | |
| ∆Age1 | −0.13 | |||||
| ∆Age2 | −0.02 | |||||
| Reasoning | Women | Age | −0.01 | 0.001 | −8.83 | <0.001 |
| ∆Age | −0.02 | |||||
| Men | Age | 0.01 | 0.01 | 1.10 | 0.272 | |
| ∆Age | −0.04 |
| Score | Measure | Women (95% CI) | Men (95% CI) | Difference (95% CI) | |||
|---|---|---|---|---|---|---|---|
| WM | Peak age | 20.42 | (19.36, 21.48) | 19.65 | (18.61, 20.69) | 0.76 | (−2.09, 4.32) |
| Peak score | 0.046 | (−0.009, 0.101) | 0.259 | (0.187, 0.330) | −0.213 | (−2.63, −0.159) | |
| Increase | 0.036 | (0.019, 0.053) | 0.049 | (0.022, 0.075) | −0.013 | (−0.132, 0.028) | |
| Decrease | −0.023 | (−0.025, −0.022) | −0.025 | (−0.027, −0.023) | 0.002 | (−0.001, 0.005) | |
| Verbal | Peak age | 24.89 | (22.26, 27.52) | 28.42 | (25.33, 31.52) | −3.53 | (−20.49, 6.10) |
| Peak score | 0.071 | (0.033, 0.108) | 0.104 | (0.050, 0.158) | −0.033 | (−0.091, 0.019) | |
| Increase | 0.035 | (0.016, 0.048) a | 0.022 | (0.006, 0.045) a | 0.013 | (−0.012, 0.036) | |
| Decrease | −0.006 | (−0.008, −0.003) | −0.008 | (−0.011, −0.005) | 0.002 | (−0.003, 0.014) | |
| Reasoning | Peak age | 12 | 19.62 | (17.70, 21.54) | −7.62 | (−12.82, −2.23) | |
| Peak score | 0.223 | (0.187, 0.271) | 0.131 | (0.060, 0.201) | 0.092 | (−0.047, 0.151) | |
| Increase | – | 0.015 | (−0.012, 0.041) | – | |||
| Decrease | −0.020 | (−0.021, −0.018) a | −0.025 | (−0.027, −0.023) | 0.005 | (0.003, 0.008) | |
| Score | Gender | Term | Coef | SE | t | p |
|---|---|---|---|---|---|---|
| WM | Women | Age | 0.03 | 0.01 | 3.83 | <0.001 |
| ∆Age | −0.05 | |||||
| Men | Age | 0.04 | 0.01 | 6.48 | <0.001 | |
| ∆Age | −0.07 | |||||
| Verbal | Women | Age | 0.04 | 0.01 | 8.16 | <0.001 |
| ∆Age | −0.05 | |||||
| Men | Age | 0.10 | 0.01 | 8.44 | <0.001 | |
| ∆Age1 | −0.09 | |||||
| ∆Age2 | −0.02 | |||||
| Reasoning | Women | Age | −0.01 | 0.001 | −9.62 | <0.001 |
| ∆Age | −0.01 | |||||
| Men | Age | 0.003 | 0.004 | 0.73 | 0.468 | |
| ∆Age | −0.03 |
| Score | Measure | Women (95% CI) | Men (95% CI) | Difference (95% CI) | |||
|---|---|---|---|---|---|---|---|
| WM | Peak age | 20.47 | (19.39, 21.55) | 20.48 | (19.85, 21.12) | −0.01 | (−4.70, 3.44) |
| Peak score | 0.021 | (−0.007, 0.049) | 0.304 | (0.286, 0.323) | −0.283 | (−0.331, −0.219) | |
| Increasing slope | 0.032 | (0.015, 0.048) | 0.042 | (0.029, 0.054) | 0.010 | (−0.071, 0.036) | |
| Decreasing slope | −0.023 | (−0.025, −0.021) | −0.024 | (−0.025, −0.023) | 0.001 | (−0.002, 0.005) | |
| Verbal | Peak age | 23.21 | (22.00, 24.42) | 39.20 | (35.99, 42.42) | −15.99 | (−26.36, −3.86) |
| Peak score | 0.067 | (0.033, 0.101) | 0.116 | (0.074, 0.157) | −0.049 | (−0.145, −0.002) | |
| Increasing slope | 0.042 | (0.032, 0.052) | 0.014 | (0.007, 0.027) a | 0.028 | (0.012, 0.176) | |
| Decreasing slope | −0.006 | (−0.008, −0.004) | −0.013 | (−0.017, −0.009) | 0.007 | (−0.001, 0.019) | |
| Reasoning | Peak age | 12 | 23.51 | (22.25, 24.78) | −11.51 | (−16.96, −4.22) | |
| Peak score | 0.208 | (0.168, 0.249) | 0.196 | (0.163, 0.228) | 0.012 | (−0.136, 0.046) | |
| Increasing slope | – | 0.003 | (−0.004, 0.010) | – | |||
| Decreasing slope | −0.019 | (−0.021, −0.018) a | −0.027 | (−0.029, −0.026) | 0.008 | (0.004, 0.012) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichols, E.S.; Wild, C.J.; Owen, A.M.; Soddu, A. Cognition across the Lifespan: Investigating Age, Sex, and Other Sociodemographic Influences. Behav. Sci. 2021, 11, 51. https://doi.org/10.3390/bs11040051
Nichols ES, Wild CJ, Owen AM, Soddu A. Cognition across the Lifespan: Investigating Age, Sex, and Other Sociodemographic Influences. Behavioral Sciences. 2021; 11(4):51. https://doi.org/10.3390/bs11040051
Chicago/Turabian StyleNichols, Emily S., Conor J. Wild, Adrian M. Owen, and Andrea Soddu. 2021. "Cognition across the Lifespan: Investigating Age, Sex, and Other Sociodemographic Influences" Behavioral Sciences 11, no. 4: 51. https://doi.org/10.3390/bs11040051
APA StyleNichols, E. S., Wild, C. J., Owen, A. M., & Soddu, A. (2021). Cognition across the Lifespan: Investigating Age, Sex, and Other Sociodemographic Influences. Behavioral Sciences, 11(4), 51. https://doi.org/10.3390/bs11040051






