Chloroquine/Hydroxychloroquine Use and Suicide Risk: Hypotheses for Confluent Etiopathogenetic Mechanisms?
Abstract
:1. Introduction
2. Case Report
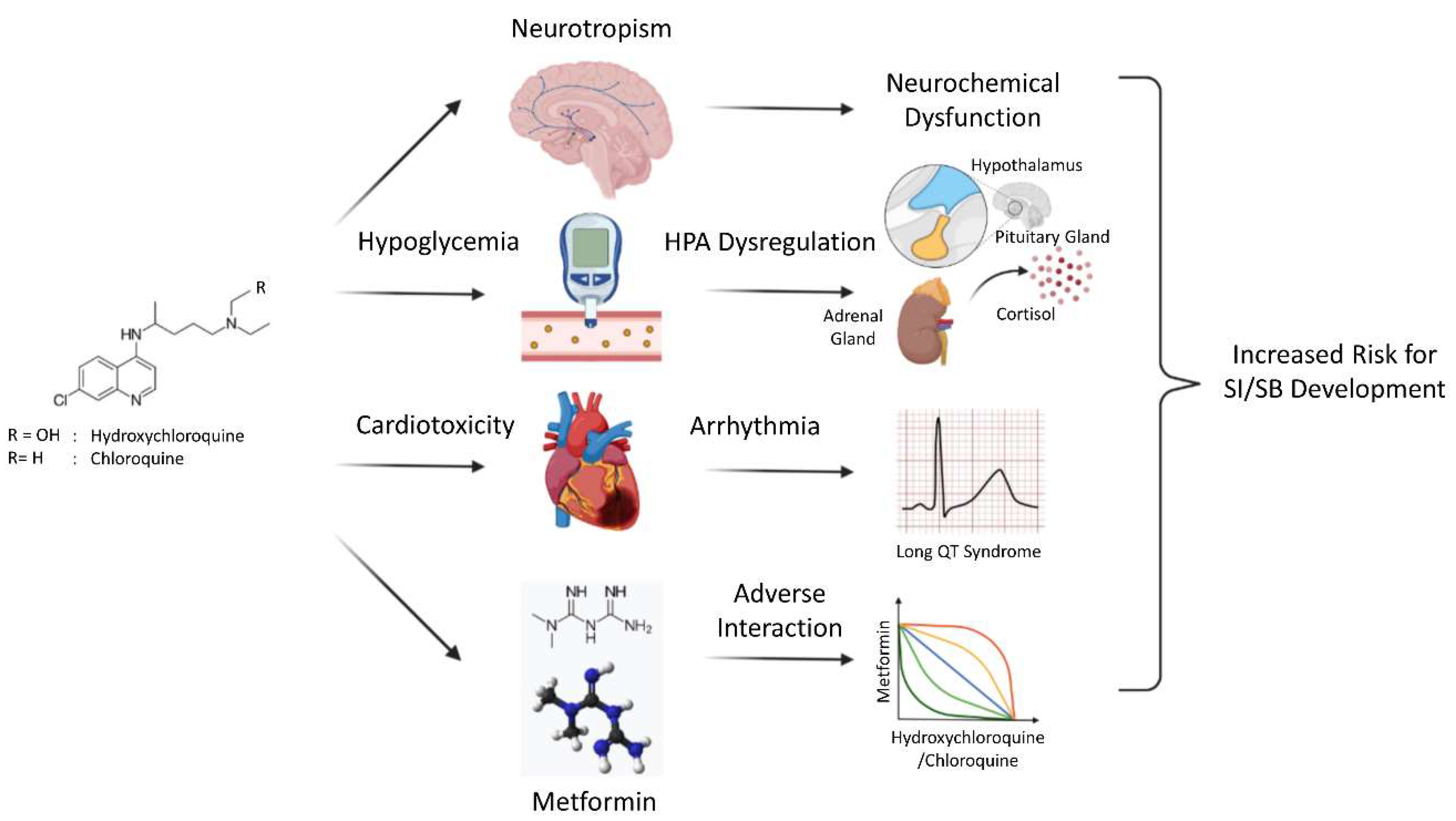
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Ganne, P.; Srinivasan, R. Chloroquine Retinopathy. JAMA Ophthalmol. 2015, 133, 603–604. [Google Scholar] [CrossRef]
- Posada, C.; García-Cruz, A.; García-Doval, I.; Millán, B.S.; Teijeira, S. Chloroquine-induced myopathy. Lupus 2011, 20, 773–774. [Google Scholar] [CrossRef]
- White, N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 2007, 7, 549–558. [Google Scholar] [CrossRef]
- Mustakallio, K.; Putkonen, T.; Pihkanen, T. Chloroquine psychosis? Lancet 1962, 280, 1387–1388. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Request for Emergency Use Authorization for Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied from the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease. Available online: https://www.fda.gov/media/136534/download (accessed on 22 November 2020).
- European Medicines Agency. COVID-19: Chloroquine and Hydroxychloroquine Only to Be Used in Clinical Trials or Emergency Use Programs. Available online: https://www.ema.europa.eu/en/documents/press-release/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes_en.pdf (accessed on 22 November 2020).
- Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: A systematic review. Clin. Microbiol. Infect. 2020, 26, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, N.J.; Yen, C.F.; Shim, D.J.; Maher, T.R.; McCoy, C.M.; Zimetbaum, P.J.; Gold, H.S. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1036–1041. [Google Scholar] [CrossRef]
- US Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and (accessed on 15 June 2020).
- Amerio, A.; Aguglia, A.; Odone, A.; Gianfredi, V.; Serafini, G.; Signorelli, C.; Amore, M. Covid-19 pandemic impact on mental health of vulnerable populations. Acta Biomed. 2020, 91, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.; Amerio, A.; Radomska, M.; Ambrosetti, J.; Di Marco, S.; Prelati, M.; Aguglia, A.; Serafini, G.; Amore, M.; Bondolfi, G.; et al. Suicidality Assessment of the Elderly with Physical Illness in the Emergency Department. Front Psychiatry 2020, 11, 558974. [Google Scholar] [CrossRef]
- Aguglia, A.; Amerio, A.; Costanza, A.; Parodi, N.; Copello, F.; Serafini, G.; Amore, M. Hopelessness and Post-Traumatic Stress Symptoms among Healthcare Workers during the COVID-19 Pandemic: Any Role for Mediating Variables? Int. J. Environ. Res. Public Heal. 2021, 18, 6579. [Google Scholar] [CrossRef]
- Odone, A.; Salvati, S.; Bucci, D.; Gaetti, G.; Amerio, A.; Signorelli, C. The runaway science: A bibliometric analysis of the COVID-19 scientific literature. Acta Biomed. 2020, 91, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Amerio, A.; Odone, A.; Aguglia, A.; Gianfredi, V.; Bellini, L.; Bucci, D.; Gaetti, G.; Capraro, M.; Salvati, S.; Serafini, G.; et al. La casa de papel: A pandemic in a pandemic. J. Affect. Disord. 2020, 277, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.; Baertschi, M.; Weber, K.; Canuto, A. Maladies neurologiques et suicide: De la neurobiologie au manque d’espoir. [Neurological diseases and suicide: From neurobiology to hopelessness]. Rev. Med. Suisse 2015, 11, 402–405. [Google Scholar] [PubMed]
- Costanza, A.; Amerio, A.; Aguglia, A.; Escelsior, A.; Serafini, G.; Berardelli, I.; Pompili, M.; Amore, M. When Sick Brain and Hopelessness Meet: Some Aspects of Suicidality in the Neurological Patient. CNS Neurol. Disord. Drug Targ. 2020, 19, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.; Di Marco, S.; Burroni, M.; Corasaniti, F.; Santinon, P.; Prelati, M.; Chytas, V.; Cedraschi, C.; Ambrosetti, J. Meaning in life and demoralization: A mental-health reading perspective of suicidality in the time of COVID-19. Acta Biomed 2020, 91, e2020163. [Google Scholar] [CrossRef]
- Costanza, A.; Baertschi, M.; Richard-Lepouriel, H.; Weber, K.; Berardelli, I.; Pompili, M.; Canuto, A. Demoralization and Its Relationship with Depression and Hopelessness in Suicidal Patients Attending an Emergency Department. Int. J. Environ. Res. Public Health 2020, 17, 2232. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.; Revet, A.; Yrondi, A.; Rousseau, V.; Degboe, Y.; Montastruc, F. Psychiatric Disorders and Hydroxychloroquine for Coronavirus Disease 2019 (COVID-19): A VigiBase Study. Drug Saf. 2020, 43, 1315–1322. [Google Scholar] [CrossRef]
- Hamm, B.S.; Rosenthal, L.J. Psychiatric Aspects of Chloroquine and Hydroxychloroquine Treatment in the Wake of Coronavirus Disease-2019: Psychopharmacological Interactions and Neuropsychiatric Sequelae. J. Psychosom. Res. 2020, 61, 597–606. [Google Scholar] [CrossRef]
- Montastruc, J.-L.; Toutain, P.-L. A New Drug–Drug Interaction Between Hydroxychloroquine and Metformin? A Signal Detection Study. Drug Saf. 2020, 43, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neuropsychiatric adverse events of chloroquine: A real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci. Trends 2020, 14, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, J.C.E.; Weaver, J.; Kotska, K.; Duarte-Salles, T.; Abrahao, M.T.F.; Alghoul, H.; Alser, O.; Alshammari, T.M.; Areia, C.; Biedermann, P.; et al. OHDSI-COVID-19 consortium. Risk of depression, suicide and psychosis with hydroxychloroquine treatment for rheumatoid arthritis: A multinational network cohort study. Rheumatology 2021, 60, 3222–3234. [Google Scholar] [CrossRef]
- Mascolo, A.; Berrino, P.M.; Gareri, P.; Castagna, A.; Capuano, A.; Manzo, C.; Berrino, L. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: A review article. Inflammopharmacology 2018, 26, 1141–1149. [Google Scholar] [CrossRef]
- De Oliveira, N.P.R.; de Mello Schier, A.R.; Pessoa, T.M.; Pereira, V.; Machado, S.; Arias-Carrión, O.; Nardi, A.; Cardoso, A. Depression as a comorbidity in Behcet’s syndrome. CNS Neurol. Disord. Drug Targ. 2014, 13, 1041–1048. [Google Scholar] [CrossRef]
- Meier, C.R.; Wilcock, K.; Jick, S.S. The risk of severe depression, psychosis or panic attacks with prophylactic antimalarials. Drug Saf. 2004, 27, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Good, M.I.; Shader, R.I. Behavioral toxicity and equivocal suicide associated with chloroquine and its derivatives. Am. J. Psychiatry 1977, 134, 798–801. [Google Scholar] [CrossRef]
- Costanza, A.; Xekardaki, A.; Kovari, E.; Gold, G.; Bouras, C.; Giannakopoulos, P. Microvascular burden and Alzheimer-type lesions across the age spectrum. J. Alzheimer Dis. 2012, 32, 643–652. [Google Scholar] [CrossRef]
- Telgt, D.S.; van der Ven, A.J.; Schimmer, B.; Droogleever-Fortuyn, H.; Sauerwein, R.W. Serious Psychiatric Symptoms after Chloroquine Treatment following Experimental Malaria Infection. Ann. Pharmacother. 2005, 39, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; Ilett, K.F.; Karunajeewa, H.A.; Batty, K.T.; Davis, T.M. Role of P-glycoprotein in absorption of novel antimalarial drugs. Antimicrob. Agents Chemother. 2006, 50, 3504–3506. [Google Scholar] [CrossRef] [Green Version]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A Specific Inflammatory Profile Underlying Suicide Risk? Systematic Review of the Main Literature Findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef] [Green Version]
- Aguglia, A.; Solano, P.; Giacomini, G.; Caprino, M.; Conigliaro, C.; Romano, M.; Aguglia, E.; Serafini, G.; Amore, M. The Association Between Dyslipidemia and Lethality of Suicide Attempts: A Case-Control Study. Front. Psychiatry 2019, 10, 70. [Google Scholar] [CrossRef]
- Aguglia, A.; Solano, P.; Parisi, V.M.; Asaro, P.; Caprino, M.; Trabucco, A.; Amerio, A.; Amore, M.; Serafini, G. Predictors of relapse in high lethality suicide attempters: A six-month prospective study. J. Affect. Disord. 2020, 271, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Amerio, A.; Asaro, P.; Caprino, M.; Conigliaro, C.; Giacomini, G.; Parisi, V.M.; Trabucco, A.; Amore, M.; Serafini, G. High-lethality of suicide attempts associated with platelet to lymphocyte ratio and mean platelet volume in psychiatric inpatient setting. World J. Biol. Psychiatry 2021, 22, 119–127. [Google Scholar] [CrossRef]
- Wong, S.K. Repurposing New Use for Old Drug Chloroquine against Metabolic Syndrome: A Review on Animal and Human Evidence. Int. J. Med. Sci. 2021, 18, 2673–2688. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Linden-Torres, E. Effect of Hydroxychloroquine on Lipid Levels: A Systematic Review and Metaanalysis. Curr. Pharm. Des. 2021, 27, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, T.J.; Zim, B.; Ma, W.; Shaffer, K.M.; Stenger, D.A.; Zamani, K.; Gross, G.W.; Pancrazio, J.J. Acute neuropharmacologic action of chloroquine on cortical neurons in vitro. Brain Res. 2003, 959, 280–286. [Google Scholar] [CrossRef]
- Bhatia, M.S.; Singhal, P.K.; Dhar, N.K. Psychiatric complications of chloroquine. Ann. Nat. Acad. Med. Sci. 1988, 24, 223–228. [Google Scholar]
- David, K.L.; Berger, P.A. Pharmacological investigations of the cholinergic imbalance hypothesis of movement disorder and psychosis. Biol. Psychiatry 1978, 13, 23–26. [Google Scholar]
- Abbas, H.M.; Al-Jumaili, A.; Nassir, K.F.; Al-Obaidy, M.W.; Al Jubouri, A.M.; Dakhil, B.D.; Abdulelah, M.M.; Al Khames, Q.A. Assessment of COVID-19 Treatment containing both Hydroxychloroquine and Azithromycin: A natural clinical trial. Int. J. Clin. Pract. 2021, 75, e13856. [Google Scholar] [CrossRef]
- Drummond, J.B.; Soares, B.S.; Pedrosa, W.; Ribeiro-Oliveira, A., Jr. Revisiting peak serum cortisol response to insulin-induced hypoglycemia in children. J. Endocrinol. Investig. 2021, 44, 1291–1299. [Google Scholar] [CrossRef]
- Taheri, S.; Karaca, Z.; Rassoulzadegan, M.; Mehmetbeyoglu, E.; Zararsiz, G.; Sener, E.F.; Bayram, K.K.; Tufan, E.; Sahin, M.C.; Marasli, M.K.; et al. The Characterization of Sex Differences in Hypoglycemia-Induced Activation of HPA Axis on the Transcriptomic Level. Cell. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Hernández-Díaz, Y.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Genis-Mendoza, A.D.; Nicolini, H. The role of peripheral cortisol levels in suicide behavior: A systematic review and meta-analysis of 30 studies. Psychiatry Res. 2020, 293, 113448. [Google Scholar] [CrossRef]
- Shalev, A.; Porta, G.; Biernesser, C.; Zelazny, J.; Walker-Payne, M.; Melhem, N.; Brent, D. Cortisol response to stress as a predictor for suicidal ideation in youth. J. Affect. Disord. 2019, 257, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, I.; Serafini, G.; Cortese, N.; Fiaschè, F.; O’Connor, R.C.; Pompili, M. The Involvement of Hypothalamus-Pituitary-Adrenal (HPA) Axis in Suicide Risk. Brain Sci. 2020, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Zeng-Treitler, Q.; Cheng, Y.; Kerr, G.S.; Nashel, D.J.; Liappis, A.P.; Weintrob, A.C.; Karasik, P.E.; Arundel, C.; Boehm, D.; et al. Cardiovascular Safety of Hydroxychloroquine in US Veterans with Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, I.; Kaboudian, A.; Iravanian, S.; Siles-Paredes, J.G.; Gumbart, J.C.; Ashikaga, H.; Bhatia, N.; Gilmour, R.F.; Cherry, E.M.; Fenton, F.H. Quantifying arrhythmic long QT effects of hydroxychloroquine and azithromycin with whole-heart optical mapping and simulations. Heart Rhythm. O2 2021, 2, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.D.; Stenager, E.; Mogensen, C.B.; Erlangsen, A. The association between heart diseases and suicide: A nationwide cohort study. J. Intern. Med. 2020, 287, 558–568. [Google Scholar] [CrossRef]
- Lee, D.; Baek, J.H.; Cho, Y.J.; Hong, K.S. Association of Resting HeartRate and HeartRate Variability With Proximal SuicidalRisk in Patients With Diverse Psychiatric Diagnoses. Front. Psychiatry 2021, 12, 652340. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Yabuuchi, S.; Pai, S.G.; Maitra, A.; Hidalgo, M.; Dang, C.V. Fatal toxicity of chloroquine or hydroxychloroquine with metformin in mice. Biorxiv 2020. [Google Scholar] [CrossRef]
- Zortea, T.C.; Brenna, C.T.A.; Joyce, M.; McClelland, H.; Tippet, M.; Tran, M.M.; Arensman, E.; Corcoran, P.; Hatcher, S.; Heisel, M.J.; et al. The impact of infectious disease-related public health emergencies on suicide, suicidal behavior, and suicidal thoughts. Crisis 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Schimmenti, A.; Billieux, J.; Starcevic, V. The four horsemen of fear: An integrated model of understanding fear experiences during the COVID-19 pandemic. Clinical Neuropsychiatry 2020, 17, 41–45. [Google Scholar]
- Costanza, A.; Mazzola, V.; Radomska, M.; Amerio, A.; Aguglia, A.; Prada, P.; Bondolfi, G.; Sarasin, F.; Ambrosetti, J. Who Consult an Adult Psychiatric Emergency Department? Pertinence of Admissions and Opportunities for Telepsychiatry. Medicina 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mengual, N.; Aragonés-Barbera, I.; Moret-Tatay, C.; Moliner-Albero, A.R. The Relationship of Fear of Death Between Neuroticism and Anxiety During the Covid-19 Pandemic. Front. Psychiatry 2021, 12, 648498. [Google Scholar] [CrossRef] [PubMed]
| Authors | Psychiatric AEs | SI/SB | Use |
|---|---|---|---|
| Garcia et al., 2020 [21] 1754 reports of HCQ treatment in COVID-19(VigiBase ICSRs *) | 56 cases (50% considered serious: 12 cases of psychosis and 7 cases of SB, see beside); others: insomnia, anxiety, and confusion. Increased risk of psychiatric AEs with HCQ vs. other antiviral drugs (ROR 6.27, CI 2.74–14.35, 95%) | 4 deaths by suicide (men, within 4 days after HCQ treatment) 3 cases of suicidal self-harm | C |
| Hamm et al., 2020 [22] Literature review of HCQ treatment in COVID-19 (PubMed 1950–2020) | Psychosis (most studied); delirium, adjustment disorder (most common); anxiety (weak association) | Weak association with increased suicide risk Suicide risk should be screened prior to CQ/HCQ prescription | C |
| Montastruc et al., 2020 [23] Disproportionality analysis of 10,771 reports of CQ/HCQ treatment with metformin and other antidiabetic medications(VigiBase ICSRs *) | - | HCQ-metformin interaction associated with fatal outcomes, particularly death by suicide (in comparison with HCQ alone, HCQ + metformin: ROR 57.7, CI 23.9–139.3, 95%; in comparison with metformin alone, HCQ + metformin: ROR 6.0, CI 2.6–13.8, 95%) | C |
| Sato et al., 2020 [24] Disproportionality analysis of 2,389,474 cases with CQ/HCQ treatment (FAERS database) | - 520 cases Significant higher reports of delirium, loss of consciousness, amnesia, hallucinations, and depression (ROR > 1, 95%) No statistically significant higher reporting of other neuropsychiatric AE, including psychosis and agitation | No association with increased suicide risk | C |
| Lane et al., 2021 [25] Cohort studies of 918,144 cases with HCQ vs. 290,383 cases with other medications (records from 10 sources in Germany, UK, and USA) | No association with depression and psychosis with both short and long-term use | No association with increased SI/SB with both short and long-term use | R |
| Mascolo et al., 2018 [26] Literature review of HCQ treatment in elderly patients | Psychiatric AEs depend on different risk factors in patients with autoimmunity | Patients and their relatives should be informed of the risk of developing SB and other neuropsychiatric AEs and eventually of the possibility of switching medications However, these possible AEs should not discourage the use of HCQ in a rheumatological setting | R |
| De Oliveira Ribeiro et al., 2014 [27] Naturalistic study of rheumatoid arthritis patients treated with HCQ and other medications | Increased risk of anxiety and depression with HCQ or biological drugs vs. leflunomide/methotrexate (p < 0.001) | Increased SI risk with HCQ or biological drugs vs. leflunomide /methotrexate (p < 0.001) | R |
| Meier et al., 2004 [28] Observational study of 35,370 patients with CQ/HCQ and other medications (medical records) | Low risk of psychosis and panic attacks with all antimalarial medications No association of mefloquine with depression when compared with other antimalarial medications | 2 deaths by suicide (men, treated with mefloquine) | M |
| Good et al., 1977 [29] Literature review of patients with CQ/HCQ treatment | Psychosis, “personality change”, depression and delirium | SI could be consequence of CQ treatment | M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, A.; Placenti, V.; Amerio, A.; Aguglia, A.; Serafini, G.; Amore, M.; Macchiarulo, E.; Branca, F.; Merli, R.; Bondolfi, G.; et al. Chloroquine/Hydroxychloroquine Use and Suicide Risk: Hypotheses for Confluent Etiopathogenetic Mechanisms? Behav. Sci. 2021, 11, 154. https://doi.org/10.3390/bs11110154
Costanza A, Placenti V, Amerio A, Aguglia A, Serafini G, Amore M, Macchiarulo E, Branca F, Merli R, Bondolfi G, et al. Chloroquine/Hydroxychloroquine Use and Suicide Risk: Hypotheses for Confluent Etiopathogenetic Mechanisms? Behavioral Sciences. 2021; 11(11):154. https://doi.org/10.3390/bs11110154
Chicago/Turabian StyleCostanza, Alessandra, Valeria Placenti, Andrea Amerio, Andrea Aguglia, Gianluca Serafini, Mario Amore, Elena Macchiarulo, Francesco Branca, Roberto Merli, Guido Bondolfi, and et al. 2021. "Chloroquine/Hydroxychloroquine Use and Suicide Risk: Hypotheses for Confluent Etiopathogenetic Mechanisms?" Behavioral Sciences 11, no. 11: 154. https://doi.org/10.3390/bs11110154
APA StyleCostanza, A., Placenti, V., Amerio, A., Aguglia, A., Serafini, G., Amore, M., Macchiarulo, E., Branca, F., Merli, R., Bondolfi, G., & Nguyen, K. D. (2021). Chloroquine/Hydroxychloroquine Use and Suicide Risk: Hypotheses for Confluent Etiopathogenetic Mechanisms? Behavioral Sciences, 11(11), 154. https://doi.org/10.3390/bs11110154










