Behavioral Sciences in the Optimization of Pharmacological and Non-Pharmacological Therapy for Type 2 Diabetes
Abstract
1. Introduction
| Country | No. of Studies | Prevalence | Country | No. of Studies | Prevalence |
|---|---|---|---|---|---|
| Belgium | 1 | 16.6% | South Africa | 2 | 5.8% |
| Canada | 1 | 14.8% | France | 1 | 5.6% |
| USA | 3 | 13.0% | Greece | 1 | 4.8% |
| Trinidad | 1 | 12.2% | Jordan | 2 | 4.2% |
| India | 2 | 11.6% | China | 10 | 4.1% |
| Norway | 1 | 10.4% | Uganda | 1 | 4.0% |
| Cameroon | 3 | 9.9% | Ireland | 1 | 3.9% |
| Italy | 1 | 9.7% | Turkey | 1 | 3.1% |
| Thailand | 2 | 8.8% | Spain | 5 | 3.0% |
| Denmark | 1 | 7.8% | Germany | 2 | 2.8% |
| Pakistan | 4 | 7.4% | Saudi Arabia | 1 | 2.3% |
| Tanzania | 2 | 7.3% | Japan | 1 | 2.0% |
| Pacific island countries | 1 | 6.8% | Netherlands | 2 | 1.8% |
| United Kingdom | 4 | 6.3% | Korea | 2 | 1.7% |
| Egypt | 2 | 6% | Poland | 1 | 1.7% |
| Bahrain | 1 | 5.9% | Australia | 2 | 1.5% |
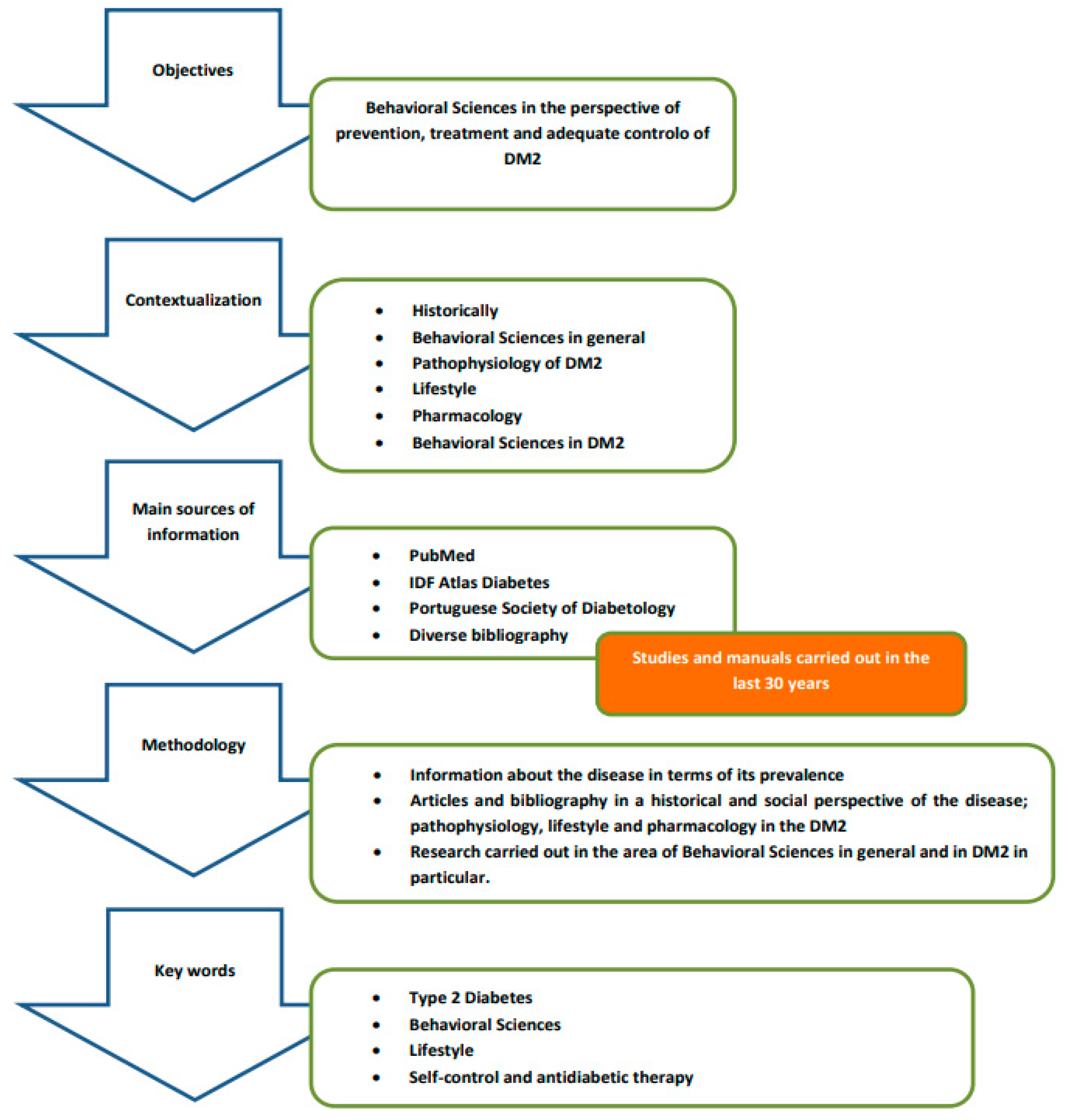
2. Materials and Methods
- -
- The guiding principle used in this review, which, in the end, defines the central issue of this work, is the importance of behavioral sciences in the perspective of prevention, treatment and adequate control of DM2, so the adoption of assertive behaviors assumes an extreme preponderance in achieving positive results, with health professionals having a decisive role in defining effective strategies to ensure that patients can assume a commitment, at a behavioral level, leading to the success of pharmacological and non-pharmacological treatments instituted.
- -
- This narrative review intends to contextualize the disease from a historical point of view; summarize the essence of behavioral sciences and what is its framework; discuss what kind of approach should be taken both in terms of lifestyle and pharmacologically, given the pathophysiology of DM2, reviewing the therapeutic options currently available and its main characteristics that can influence the behavior of patients; and address the potentially modifiable aspects that are decisive in influencing the behavior of patients with DM2.
- -
- The main sources of information were databases such as PubMed, IDF Diabetes Atlas, the Portuguese Society of Diabetology, as well as manuals in the field of Pharmacology/Pharmacotherapy, focusing generically on search terms such as behavioral sciences, history, pathophysiology, lifestyle and complications/comorbidities in diabetes, and specifically guidelines for the treatment of DM2 and behavioral sciences in diabetes.
- -
- The key statements are supported by references to studies carried out in the past 30 years in the field of behavioral sciences as well as on the history, incidence, pathophysiology and drug therapy of DM2. We used a three-stage approach to review the literature:
- The first stage consisted of researching statistical information about the disease in terms of its prevalence.
- The second stage refers to the research of articles and bibliography in a historical and social perspective of the disease; pathophysiology, lifestyle and pharmacology in the DM2.
- The third stage reports on research carried out in the area of behavioral sciences in general and in DM2 in particular.
- -
- The initial keywords were organized into the following conceptual categories: type 2 diabetes, behavioral sciences, lifestyle, self-control and antidiabetic therapy. Search terms were developed and customized for each database.
- -
- The data and information gathered are presented establishing a logical sequence that aims to demonstrate how it is possible to identify and act to ensure adequate control of DM2 from the behavioral perspective of patients with the disease.
3. Main Findings
3.1. Historical and Social Context of the Disease and Behaviors
3.2. The Essence of Behavioral Sciences
- -
- The number of variables involved is small and the dynamics can be significantly evaluated in just a few points of time;
- -
- The change process is the same for all individuals, e.g., following the same sequence;
- -
- The dynamics between variables is linear, additive and does not change over time; and
- -
- Included variables are not diluted in context or omitted.
3.3. Pathophysiology of Type 2 Diabetes
3.4. Healthy Lifestyle
- Lifestyle changes with the adoption of adequate eating habits and physical exercise throughout the course of the disease;
- Individualization of therapy and patient-centeredness; and
- Therapeutic Education (TE) or DSMES (Diabetes Self-Management Education and Support), which is essential in the care provided to people with type 2 diabetes.
3.5. Pharmacological Treatment
3.6. Potentially Modifiable Factors in Which Efforts Should Be Made to Influence the Behavior of Patients with DM2
3.6.1. The Effectiveness of the Treatment According to the Patient’s Perception
3.6.2. The Incidence of Hypoglycemia
3.6.3. Complexity and Convenience of Treatment
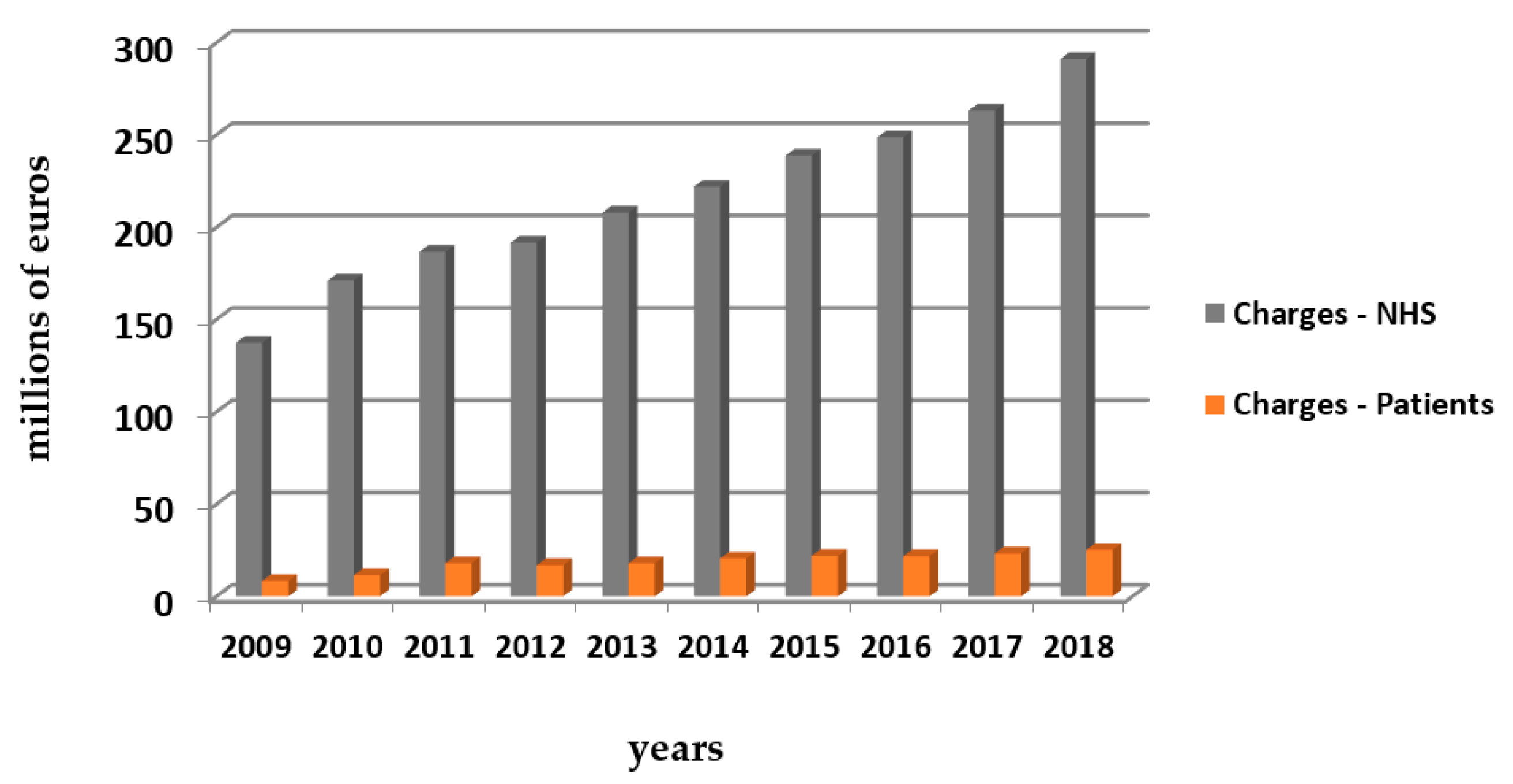
3.6.4. Costs of Treatment
3.6.5. Beliefs Regarding Medication
3.6.6. Trust in Health Professionals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sokol, M.C.; McGuigan, K.A.; Verbrugge, R.R.; Epstein, R.S. Impact of Medication Adherence on Hospitalization Risk and Healthcare Cost. Med. Care 2005, 43, 521–530. [Google Scholar] [CrossRef]
- Whitley, B.E.; Kite, M.E. Principles of Research in Behavioral Science, 3rd ed.; Routledge: Oxfordshire, UK, 2013. [Google Scholar] [CrossRef]
- Grarup, N.; Sandholt, C.H.; Hansen, T.; Pedersen, O. Genetic susceptibility to type 2 diabetes and obesity: From genome-wide association studies to rare variants and beyond. Diabetologia 2014, 57, 1528–1541. [Google Scholar] [CrossRef]
- Katzung, B.G.; Masters, S.B.; Trevor, A.J. Basic and Clinical Pharmacology, 12th ed.; Mcgraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Bacha, F.; Lee, S.; Gungor, N.; Arslanian, S.A. From Pre-Diabetes to Type 2 Diabetes in Obese Youth: Pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010, 33, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Morello, C.M.; Chynoweth, M.; Kim, H.; Singh, R.F.; Hirsch, J.D. Strategies to Improve Medication Adherence Reported by Diabetes Patients and Caregivers: Results of a Taking Control of Your Diabetes Survey. Ann. Pharmacother. 2011, 45, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Abate, N.; Chandalia, M. The impact of ethnicity on type 2 diabetes. J. Diabetes Its Complicat. 2003, 17, 39–58. [Google Scholar] [CrossRef]
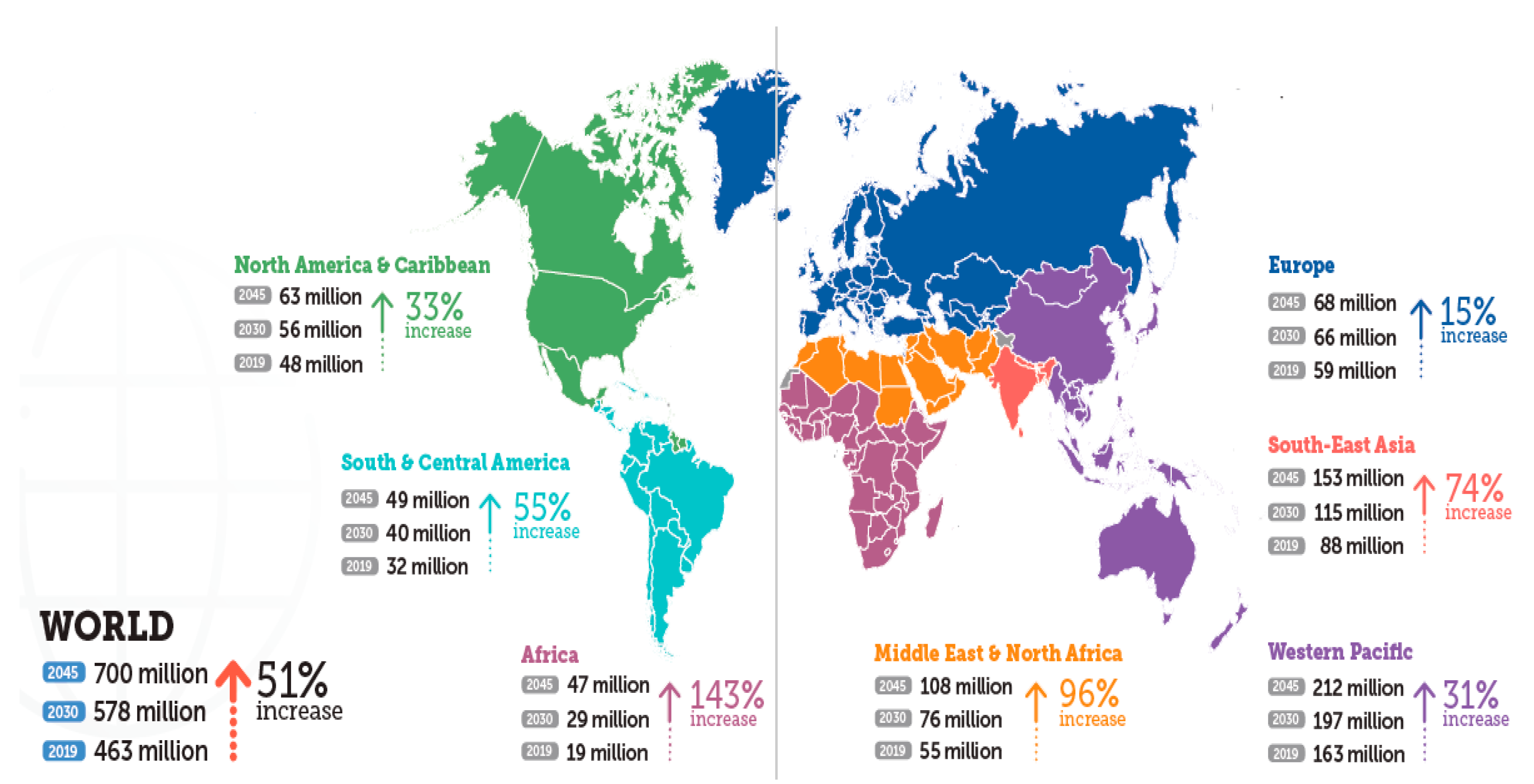
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
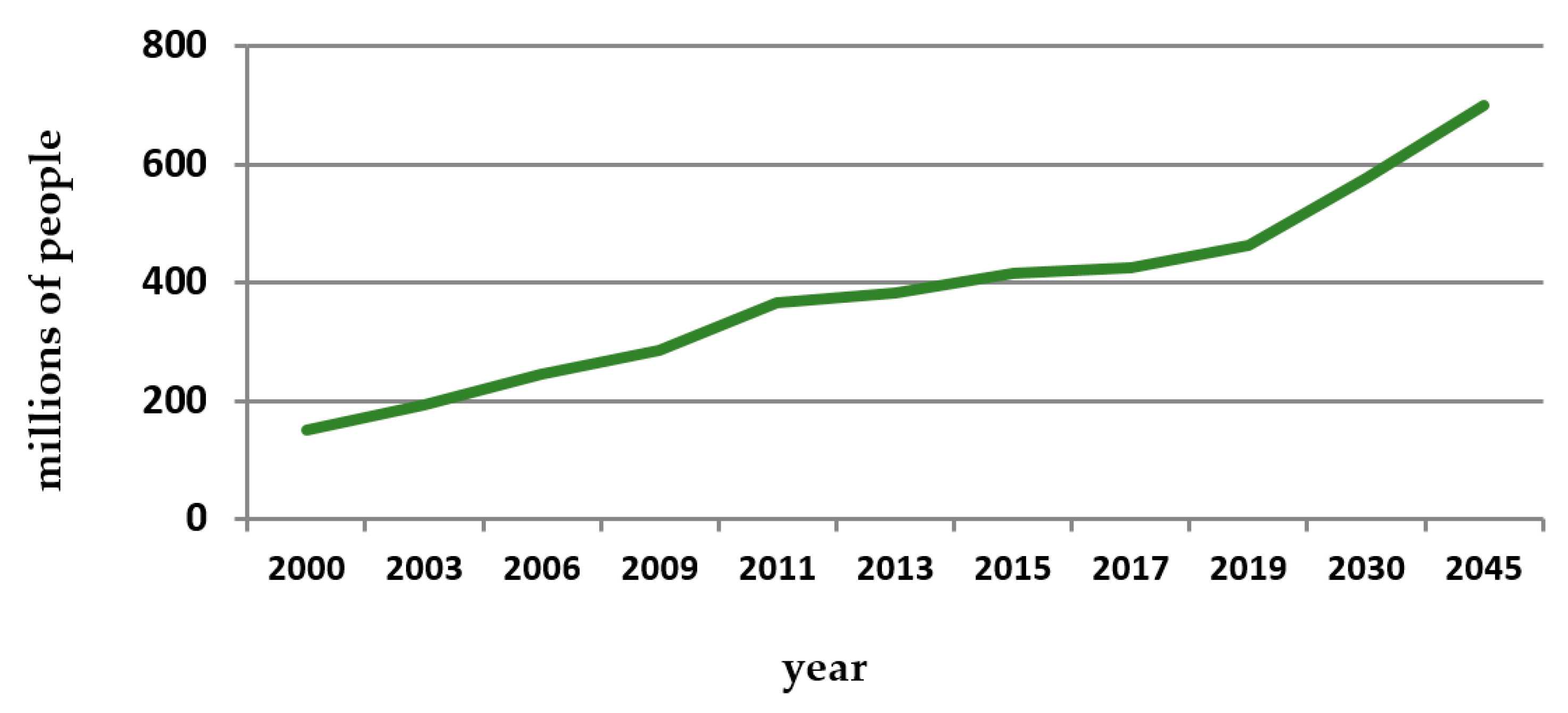
- Kaiser, A.B.; Zhang, N.; van der Pluijm, W. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes 2018, 67, 202. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Gæde, P.; Vedel, P.; Larsen, N.; Jensen, G.V.H.; Parving, H.-H.; Pedersen, O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2016, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
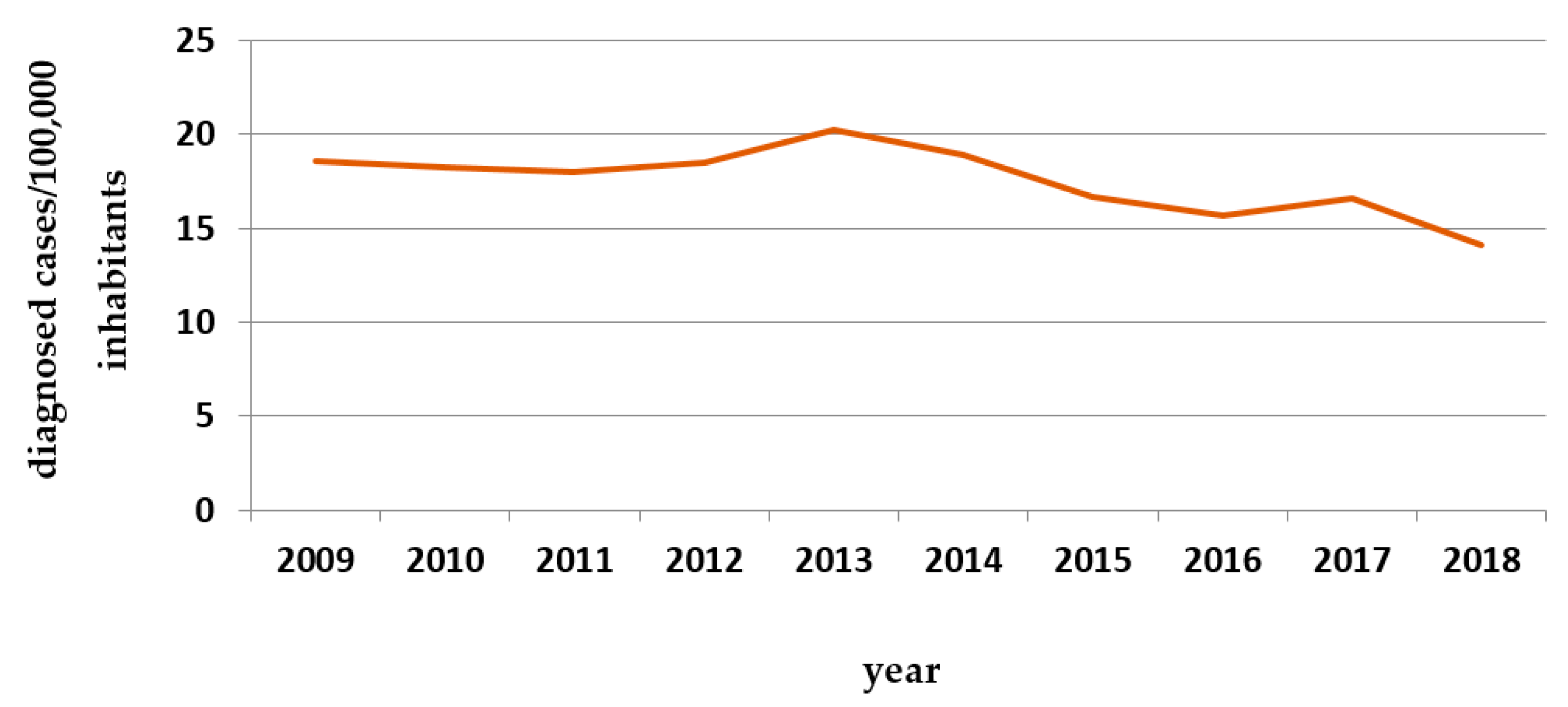
- Raposo, J. Diabetes: Factos e Números 2016, 2017 e 2018. Rev. Port. Diabetes 2020, 15, 19–27. [Google Scholar]
- Fortington, L.; Geertzen, J.; van Netten, J.J.; Postema, K.; Rommers, G.; Dijkstra, P. Short and Long Term Mortality Rates after a Lower Limb Amputation. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 124–131. [Google Scholar] [CrossRef] [PubMed]
- AB Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Meo, S.A.; Sheikh, S.A.; Sattar, K.; Akram, A.; Hassan, A.; Meo, A.S.; Usmani, A.M.; Qalbani, E.; Ullah, A. Prevalence of Type 2 Diabetes Mellitus Among Men in the Middle East: A Retrospective Study. Am. J. Mens Health 2019, 13, 1557988319848577. [Google Scholar] [CrossRef] [PubMed]
- Noshad, S.; Afarideh, M.; Heidari, B.; Mechanick, J.I.; Esteghamati, A. Diabetes Care in Iran: Where We Stand and Where We Are Headed. Ann. Glob. Health 2015, 81, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Moini, J. (Ed.) Epidemiology of Diabetes; Chapter 3: Pathophysiology of Diabetes; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wing, R.R.; Goldstein, M.G.; Acton, K.J.; Birch, L.L.; Jakicic, J.M.; Sallis, J.F.; Smith-West, D.; Jeffery, R.W.; Surwit, R.S. Behavioral Science Research in Diabetes: Lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care 2001, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cragg, R. A Brief History of Diabetes. Available online: https://www.eyescreening.org.uk/userFiles/File/Conference%202017/2017%20BARS%20presentations/07%20Brief%20History%20of%20Diabetes.pdf; (accessed on 21 October 2021).
- Laios, K.; Karamanou, M.; Saridaki, Z.; Androutsos, G. Aretaeus of Cappadocia and the first description of diabetes. Hormones 2012, 11, 109–113. [Google Scholar] [CrossRef]
- Mandal, A. História do diabetes. News-Medical. 2019. Available online: https://www.news-medical.net/health/History-of-Diabetes.aspx (accessed on 27 June 2021).
- Chast, F.; Slama, G. Apollinaire Bouchardat et le diabète. Hist. Sci. Med. 2007, 41, 287–301. (In French) [Google Scholar] [PubMed]
- Mazur, A. Why were “starvation diets” promoted for diabetes in the pre-insulin period? Nutr. J. 2011, 10, 23. [Google Scholar] [CrossRef]
- Lakhtakia, R. The History of Diabetes Mellitus. Sultan Qaboos Univ. Med. J. 2013, 13, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Higuera, V. Medical News Today. Diabetes: Past Treatments, New Discoveries. 2020. Available online: https://www.medicalnewstoday.com/articles/317484 (accessed on 1 September 2019).
- Coleman, J.S. Foundations of Social Theory; Belknap Press of Harvard University: Cambridge, MA, USA, 1994. [Google Scholar]
- Michie, S.; West, R.; Campbell, R.; Brown, J.; Gainforth, H. ABC of Behaviour Change Theories; Silverback: Sutton, UK, 2014. [Google Scholar]
- Carey, R.N.; Connell, L.E.; Johnston, M.; Rothman, A.J.; de Bruin, M.; Kelly, M.P.; Michie, S. Behavior Change Techniques and Their Mechanisms of Action: A Synthesis of Links Described in Published Intervention Literature. Ann. Behav. Med. 2018, 53, 693–707. [Google Scholar] [CrossRef]
- Short, S.E.; Mollborn, S. Social determinants and health behaviors: Conceptual frames and empirical advances. Curr. Opin. Psychol. 2015, 5, 78–84. [Google Scholar] [CrossRef]
- Matthews, L.; Simpson, S.A. Evaluation of Behavior Change Interventions. In The Handbook of Behavior Change; Hamilton, K., Cameron, L.D., Hagger, M.S., Hankonen, N., Lintunen, T., Eds.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Green, D.; Ha, S.E.; Bullock, J.G. Enough Already about “Black Box” Experiments: Studying Mediation Is More Difficult than Most Scholars Suppose. Ann. Am. Acad. Political Soc. Sci. 2010, 628, 200–208. [Google Scholar] [CrossRef]
- Eiler, B.A.; Kallen, R.W.; Richardson, M.J. Interaction-dominant dynamics, timescale enslavement, and the emergence of social behavior. In Computational Social Psychology; Vallacher, R.R., Read, S.J., Nowak, A., Eds.; Taylor & Francis: Oxfordshire, UK, 2017; pp. 105–126. [Google Scholar] [CrossRef]
- Roe, R. What is wrong with mediators and moderators. Eur. Health Psychol. 2012, 14, 4–10. [Google Scholar]
- Hofmann, S.G.; Curtiss, J.E.; Hayes, S. Beyond linear mediation: Toward a dynamic network approach to study treatment processes. Clin. Psychol. Rev. 2020, 76, 101824. [Google Scholar] [CrossRef] [PubMed]
- Rickles, D. Causality in complex interventions. Med. Health Care Philos. 2008, 12, 77–90. [Google Scholar] [CrossRef]
- Meehl, P.E. Why summaries of research on psychological theories are often uninterpretable. Psychol. Rep. 1990, 66, 195–244. [Google Scholar] [CrossRef]
- Heino, M.; Knittle, K.; Noone, C.; Hasselman, F.; Hankonen, N. Studying Behaviour Change Mechanisms under Complexity. Behav. Sci. 2021, 11, 77. [Google Scholar] [CrossRef]
- Gomersall, T. Complex adaptive systems: A new approach for understanding health practices. Health Psychol. Rev. 2018, 12, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Plsek, P.E.; Greenhalgh, T. Complexity science: The challenge of complexity in health care. BMJ 2001, 323, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, R.; Cohen, M. Harnessing Complexity: Organizational Implications of a Scientific Frontier; Free Press: New York, NY, USA, 2001. [Google Scholar]
- Stacey, R. Strategic Management and Organisational Dynamics: The Challenge of Complexity to Ways of Thinking about Organisations; Financial Times/Prentice Hall: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pype, P.; Mertens, F.; Helewaut, F.; Krystallidou, D. Healthcare teams as complex adaptive systems: Understanding team behaviour through team members’ perception of interpersonal interaction. BMC Health Serv. Res. 2018, 18, 570. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Freeman, A.M.; Pennings, N. Insulin Resistance; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Saisho, Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 109–124. [Google Scholar] [CrossRef]
- Christensen, A.; Gannon, M. The Beta Cell in Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Wells, B.; DiPiro, J.; Schwinghammer, T.; Dipiro, C. Manual de Farmacoterapia; McGraw Hill Eduaction: New York, NY, USA, 2016. [Google Scholar]
- Leiter, L.A.; Lundman, P.; da Silva, P.M.; Drexel, H.; Jünger, C.; Gitt, A.K. on behalf of the DYSIS investigators Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: Results of the Dyslipidaemia International Study. Diabet. Med. 2011, 28, 1343–1351. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88–98. [Google Scholar] [CrossRef]
- Caramona, M.; Vitória, I.; Teixeira, M.; Alcobia, A.; Almeida, P.; Horta, R.; Reis, L.; Grupo de Farmacoterapia da Ordem dos Farmacêuticos. Normas de Orientação Terapêutica; Cadaval Gráfica: Terrugem, Portugal, 2012. [Google Scholar]
- Eckstein, M.L.; Williams, D.M.; O’Neil, L.; Hayes, J.; Stephens, J.W.; Bracken, R. Physical exercise and non-insulin glucose-lowering therapies in the management of Type 2 diabetes mellitus: A clinical review. Diabet. Med. 2018, 36, 349–358. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.; Walker, E.A.; Nathan, D.M. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Charlesworth, S.; Ivey, A.; Nettlefold, L.; Bredin, S.S. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 39. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M.; et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Earley, A.; Raman, G.; Avendano, E.A.; Pittas, A.G.; Remington, P.L. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann. Intern. Med. 2015, 163, 437–451. [Google Scholar] [CrossRef]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadizadeh, A.; Khorshidsavar, H.; Seif, M.; Sharifi, M.H. Adherence to Medication, Diet and Physical Activity and the Associated Factors Amongst Patients with Type 2 Diabetes. Diabetes Ther. 2020, 11, 479–494. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Rowan-Martin, M.T.; Levin, R.; Fonseca, V.A.; Schmittdiel, J.A.; Herman, W.H.; Aubert, R.E. Determinants of Adherence to Diabetes Medications: Findings from a Large Pharmacy Claims Database. Diabetes Care 2015, 38, 604–609. [Google Scholar] [CrossRef]
- Capoccia, K.L.; Odegard, P.S.; Letassy, N. Medication Adherence with Diabetes Medication. Diabetes Educ. 2015, 42, 34–71. [Google Scholar] [CrossRef]
- Kisa, A.; Sabaté, E.; Nuño-Solinís, R. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- SPD National Recommendations for the Treatment of Hyperglycemia in Type 2 Diabetes—Update Based in the ADA/EASD JointPositionStatement. Available online: http://www.revportdiabetes.com/wp-content/uploads/2019/01/RPD-DEzembro-2018-Recomenda%C3%A7%C3%B5es-p%C3%A1gs-154-180.pdf (accessed on 21 October 2021).
- Mayberry, L.S.; Bergner, E.M.; Chakkalakal, R.J.; Elasy, T.A.; Osborn, C.Y. Self-Care Disparities Among Adults with Type 2 Diabetes in the USA. Curr. Diabetes Rep. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Rolnick, S.J.; Pawloski, P.; Hedblom, B.D.; Asche, S.E.; Bruzek, R.J. Patient Characteristics Associated with Medication Adherence. Clin. Med. Res. 2013, 11, 54–65. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Seligman, H.K.; Choudhry, N.K. Treat or Eat: Food Insecurity, Cost-related Medication Underuse, and Unmet Needs. Am. J. Med. 2014, 127, 303–310.e3. [Google Scholar] [CrossRef] [PubMed]
- Demoz, G.T.; Wahdey, S.; Bahrey, D.; Kahsay, H.; Woldu, G.; Niriayo, Y.L.; Collier, A. Predictors of poor adherence to antidiabetic therapy in patients with type 2 diabetes: A cross-sectional study insight from Ethiopia. Diabetol. Metab. Syndr. 2020, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guerci, B.; Chanan, N.; Kaur, S.; Jasso-Mosqueda, J.G.; Lew, E. Lack of Treatment Persistence and Treatment Nonadherence as Barriers to Glycaemic Control in Patients with Type 2 Diabetes. Diabetes Ther. 2019, 10, 437–449. [Google Scholar] [CrossRef] [PubMed]
- McSharry, J.; Byrne, M.; Casey, B.; Dinneen, S.F.; Fredrix, M.; Hynes, L.; Lake, A.J.; Morrissey, E. Behaviour change in diabetes: Behavioural science advancements to support the use of theory. Diabet. Med. 2019, 37, 455–463. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Shinnick, M.A. What do we know about adherence and self-care? J. Cardiovasc. Nurs. 2008, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.D.; Fisher, W.A.; Amico, K.R.; Harman, J.J. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006, 25, 462–473. [Google Scholar] [CrossRef]
- Klonoff, D.C. Behavioral Theory: The Missing Ingredient for Digital Health Tools to Change Behavior and Increase Adherence. J. Diabetes Sci. Technol. 2018, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Henry, R.R. Poor medication adherence in type 2 diabetes: Recognizing the scope of the problem and its key contributors. Patient Prefer. Adherence 2016, 10, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Lauffenburger, J.C.; Lewey, J.; Jan, S.; Lee, J.; Ghazinouri, R.; Choudhry, N.K. Association of Potentially Modifiable Diabetes Care Factors with Glycemic Control in Patients with Insulin-Treated Type 2 Diabetes. JAMA Netw. Open 2020, 3, e1919645. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, M.A.; Tlou, B. Modifiable risk factors associated with non-communicable diseases among adult outpatients in Manzini, Swaziland: A cross-sectional study. BMC Public Health 2020, 20, 665. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.; Weinman, J.; Barber, N.; Elliott, R.; Morgan, M. Concordance, Adherence and Compliance in Medicine Taking; A Report for the National Coordinating Centre for NHS Service Delivery and Organisation R&D; National Coordinating Centre for NHS Service Delivery and Organisation R&D: London, UK, 2005. [Google Scholar]
- Gadkari, A.S.; McHorney, C.A. Medication nonfulfillment rates and reasons: Narrative systematic review. Curr. Med. Res. Opin. 2010, 26, 683–705. [Google Scholar] [CrossRef] [PubMed]
- Burrell, A.; Wong, P.; Ollendorf, D.; Fuldeore, M.; Roy, A.; Fairchild, C.; Cramer, J. PHP46 defining compliance/adherence and persistence: ISPOR special interest working group. Value Health 2005, 8, A194–A195. [Google Scholar] [CrossRef]
- Brawley, L.R.; Culos-Reed, S. Studying Adherence to Therapeutic Regimens: Overview, Theories, Recommendations. Control. Clin. Trials 2000, 21, S156–S163. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Cockram, C.S.; Flyvbjerg, A.; Goldstein, B.J. (Eds.) Textbook of Diabetes, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Duarte, R. Inibidores da DPP-4 (Gliptinas)—10 anos depois (2007–2017). Rev. Port. Diabetes 2017, 12, 62–67. [Google Scholar]
- Pape, E.; Nascimento, E.; Jordão, A. Fármacos na Diabetes; LIDEL: Lisboa, Portugal, 2019. [Google Scholar]
- Committee Physical Activity Guidelines Advisory. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; Department of Health and Human Services: Washington, DC, USA, 2018.
- Cahn, A.; Miccoli, R.; Dardano, A.; del Prato, S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 638–652. [Google Scholar] [CrossRef]
- Mahmoodi, H.; Nahand, F.J.; Shaghaghi, A.; Shooshtari, S.; Jafarabadi, M.A.; Allahverdipour, H. Gender Based Cognitive Determinants of Medication Adherence in Older Adults with Chronic Conditions. Patient Prefer. Adherence 2019, 13, 1733–1744. [Google Scholar] [CrossRef]
- Brown, M.T.; Bussell, J.; Dutta, S.; Davis, K.; Strong, S.; Mathew, S. Medication Adherence: Truth and Consequences. Am. J. Med. Sci. 2016, 351, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.N.; Wei, W.; Garg, S. Clinical Impact of Initiating Insulin Glargine Therapy with Disposable Pen Ve;rsus Vial in Patients with Type 2 Diabetes Mellitus in a Managed Care Setting. Endocr. Pract. 2011, 17, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Anderbro, T.; Amsberg, S.; Adamson, U.; Bolinder, J.; Lins, P.-E.; Wredling, R.; Moberg, E.; Lisspers, J.; Johansson, U.-B. Fear of hypoglycaemia in adults with Type 1 diabetes. Diabet. Med. 2010, 27, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Walz, L.; Pettersson, B.; Rosenqvist, U.; Deleskog, A.; Journath, G.; Wändell, P. Impact of symptomatic hypoglycemia on medication adherence, patient satisfaction with treatment, and glycemic control in patients with type 2 diabetes. Patient Prefer. Adherence 2014, 8, 593–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Wittmann, I.; Kempler, P. Persistence to Treatment with Novel Antidiabetic Drugs (Dipeptidyl Peptidase-4 Inhibitors, Sodium-Glucose Co-Transporter-2 Inhibitors, and Glucagon-Like Peptide-1 Receptor Agonists) in People with Type 2 Diabetes: A Nationwide Cohort Study. Diabetes Ther. 2018, 9, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Annunziata, K.; Bailey, R.; E Morisky, D.; Rupnow, M. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer. Adherence 2014, 8, 683–692. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, L.-E.; Álvarez, M.; Dilla, T.; Gil-Guillén, V.F.; Orozco-Beltran, D. Adherence to Therapies in Patients with Type 2 Diabetes. Diabetes Ther. 2013, 4, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, E. Management of Type 2 Diabetes Mellitus in Older Patients: Current and Emerging Treatment Options. Diabetes Ther. 2013, 4, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Melikian, C.; White, T.; Vanderplas, A.; Dezii, C.M.; Chang, E. Adherence to oral antidiabetic therapy in a managed care organization: A comparison of monotherapy, combination therapy, and fixed-dose combination therapy. Clin. Ther. 2002, 24, 460–467. [Google Scholar] [CrossRef]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zagrean, A.-M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar]
- Holt, R.I.G.; de Groot, M.; Golden, S.H. Diabetes and Depression. Curr. Diabetes Rep. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hasanovic, E.; Trifunovic, N.; Dzambo, I.; Erkocevic, H.; Cemerlic, A.; Jatic, Z.; Kulenovic, A.D. The Association among Glycemic Control and Depression Symptoms in Patients with Type 2 Diabetes. Mater. Soc. Med. 2020, 32, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Iglay, K.; Davies, M.J.; Zhang, Q.; Radican, L. Glycemic effectiveness and medication adherence with fixed-dose combination or coadministered dual therapy of antihyperglycemic regimens: A meta-analysis. Curr. Med. Res. Opin. 2012, 28, 969–977. [Google Scholar] [CrossRef]
- Khunti, N.; Khunti, N.; Khunti, K. Adherence to type 2 diabetes management. Br. J. Diabetes Vasc. Dis. 2019, 19, 99–104. [Google Scholar] [CrossRef]
- Sefah, I.A.; Okotah, A.; Afriyie, D.K.; Amponsah, S.K. Adherence to Oral Hypoglycemic Drugs among Type 2 Diabetic Patients in a Resource-Poor Setting. Int. J. Appl. Basic Med. Res. 2020, 10, 102–109. [Google Scholar]
- Mikulic, M. Statistics & Facts, Global Pharmaceutical, Industry; Statista: Hamburg, Germany, 2020. [Google Scholar]
- Osterberg, L.; Blaschke, T. Adherence to Medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, e016982. [Google Scholar] [CrossRef] [PubMed]
- Turcu-Stiolica, A.; Subtirelu, M.-S.; Taerel, A.-E.; Boboia, A.; Berbecaru-Iovan, A. Analysis of Financial Losses due to Poor Adherence of Patients with Chronic Diseases and Their Impact on Health Economics. In Financial Management from an Emerging Market Perspective; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Aikens, J.E.; Piette, J.D. Diabetic Patients’ Medication Underuse, Illness Outcomes, and Beliefs About Antihyperglycemic and Antihypertensive Treatments. Diabetes Care 2008, 32, 19–24. [Google Scholar] [CrossRef][Green Version]
- Shahin, W.; A Kennedy, G.; Stupans, I. The impact of personal and cultural beliefs on medication adherence of patients with chronic illnesses: A systematic review. Patient Prefer. Adherence 2019, 13, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Straßner, C.; Mahler, C.; Strauss, B.; Wehrmann, U.; Krug, K.; Szecsenyi, J.; Haefeli, W.E.; Seidling, H.M. Medication beliefs and use of medication lists—Is there a connection? Results from a before-and-after study in Germany. BMC Geriatr. 2020, 20, 116. [Google Scholar] [CrossRef]
- Nasir, N.M.; Ariffin, F.; Yasin, S.M. Physician-patient interaction satisfaction and its influence on medication adherence and type-2 diabetic control in a primary care setting. Med. J. Malays. 2018, 73, 163–169. [Google Scholar]
- Kerse, N.; Buetow, S.; Iii, A.G.M.; Young, G.; Coster, G.; Arroll, B. Physician-Patient Relationship and Medication Compliance: A Primary Care Investigation. Ann. Fam. Med. 2004, 2, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Golin, C.E.; Jones, C.D.; Ashok, M.; Blalock, S.J.; Wines, R.C.; Coker-Schwimmer, E.J.; Rosen, D.L.; Sista, P.; Lohr, K.N. Interventions to Improve Adherence to Self-administered Medications for Chronic Diseases in the United States. Ann. Intern. Med. 2012, 157, 785–795. [Google Scholar] [CrossRef]
- Soto, C.; Strain, W.D. Tackling clinical inertia: Use of coproduction to improve patient engagement. J. Diabetes 2018, 10, 942–947. [Google Scholar] [CrossRef]
- Werble, C. Health Policy Brief: Pharmacy Benefit Managers. Health Aff. J. 2017. [Google Scholar] [CrossRef]
- Carroll, A.E. The Unsung Role of the Pharmacist in Patient Health. The New York Times, 2019. Available online: https://www.nytimes.com/2019/01/28/upshot/pharmacists-drugs-health-unsung-role.html (accessed on 21 September 2021).
- Shin, L.; Bowling, F.L.; Armstrong, D.G.; Boulton, A.J. Saving the Diabetic Foot During the COVID-19 Pandemic: A Tale of Two Cities. Diabetes Care 2020, 43, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Benkel, I.; Arnby, M.; Molander, U. Living with a chronic disease: A quantitative study of the views of patients with a chronic disease on the change in their life situation. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Gerland, H.-M.E.; Prell, T. Association Between the Health Locus of Control and Medication Adherence: An Observational, Cross-Sectional Study in Primary Care. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, C.I.; García-González, J.M.; Adams, D.P.; Fernandez-Lazaro, D.; Mielgo-Ayuso, J.; Caballero-Garcia, A.; Racionero, F.M.; Córdova, A.; Miron-Canelo, J.A. Adherence to treatment and related factors among patients with chronic conditions in primary care: A cross-sectional study. BMC Fam. Pract. 2019, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Scialli, A.R.; Saavedra, K.; Fugh-Berman, A. The Benefits and Risks of Adherence to Medical Therapy. J. Sci. Pract. Integr. 2021, 21386. [Google Scholar] [CrossRef]


| Drug | Mechanism of Action | Risk of Hypoglycemia | Weight | Secondary Effects |
|---|---|---|---|---|
| Metformin (*) | ↓ hepatic glucose synthesis | Not associated | ↓ | GI changes (diarrhea and vomiting) and vitamin B12 deficiency |
| α-Glucosidase inhibitors (**) | Prevent the breakdown of complex carbohydrates in the small intestine, delaying their absorption | Not associated | = | Diarrhea, flatulence or abdominal discomfort |
| Sodium-glucose cotransporter inhibitors (SGLT2) | ↑ elimination of glucose in the urine and block its renal absorption | Not associated | ↓ | ↑ risk of genitourinary infections, hypovolemia with hypotension, increased LDL cholesterol and may even lead to a transient increase in creatinine |
| Glucagon-like peptide−1 agonists (GLP−1 agonists) | ↑ insulin secretion, by decreasing glucagon secretion, delaying gastric emptying, also promoting the feeling of satiety | Low | ↓ | Nausea, diarrhea, vomiting, and headache |
| Dipeptidyl peptidase 4 inhibitors (iDPP4) | Inhibit the degradation of incretins which promote↑ secretion of insulin and the ↓ of glucagon secretion | Not associated | = | Well tolerated |
| Thiazolidinediones | ↑ peripheral insulin sensitivity in liver, fat and skeletal muscle cells | Not associated | ↑ but ↓ visceral obesity | ↑ risk of fluid retention (edema), congestive heart failure and an increased risk of bone fractures |
| Sulfonylureas and Glinides (***) | Secretagogues (↑ insulin secretion) | Increased risk | ↑ | Well tolerated |
| Insulins (****) | Activates insulin receptors | High | ↑ | Possibility of local allergic reactions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.; Roque, F.; Morgado, S.; Dinis, C.; Herdeiro, M.T.; Morgado, M. Behavioral Sciences in the Optimization of Pharmacological and Non-Pharmacological Therapy for Type 2 Diabetes. Behav. Sci. 2021, 11, 153. https://doi.org/10.3390/bs11110153
Lopes A, Roque F, Morgado S, Dinis C, Herdeiro MT, Morgado M. Behavioral Sciences in the Optimization of Pharmacological and Non-Pharmacological Therapy for Type 2 Diabetes. Behavioral Sciences. 2021; 11(11):153. https://doi.org/10.3390/bs11110153
Chicago/Turabian StyleLopes, António, Fátima Roque, Sandra Morgado, Cristina Dinis, Maria Teresa Herdeiro, and Manuel Morgado. 2021. "Behavioral Sciences in the Optimization of Pharmacological and Non-Pharmacological Therapy for Type 2 Diabetes" Behavioral Sciences 11, no. 11: 153. https://doi.org/10.3390/bs11110153
APA StyleLopes, A., Roque, F., Morgado, S., Dinis, C., Herdeiro, M. T., & Morgado, M. (2021). Behavioral Sciences in the Optimization of Pharmacological and Non-Pharmacological Therapy for Type 2 Diabetes. Behavioral Sciences, 11(11), 153. https://doi.org/10.3390/bs11110153







