DAT1 and Its Psychological Correlates in Children with Avoidant/Restrictive Food Intake Disorder: A Cross-Sectional Pilot Study
Abstract
1. Introduction
1.1. Dopamine Transporter Gene and Feeding Disorders
1.2. Bio-Psycho-Social Correlates of Avoidant/Restrictive Food Intake Disorder in Early Childhood
1.3. The Present Study
2. Materials and Methods
2.1. Study Design
2.2. Procedure
2.3. Participants
2.4. Measures
2.5. DNA Isolation and Genotyping
2.6. Analysis of DNA Methylation
2.7. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Association between Children’s Dopamine Trasnporter Genotype and Children’s Diagnoses
3.3. Children’s Dopamine Transporter Methylation, Emotional-Behavioral Dysregulation, and Maternal Psychopathological Risk in the Four Groups
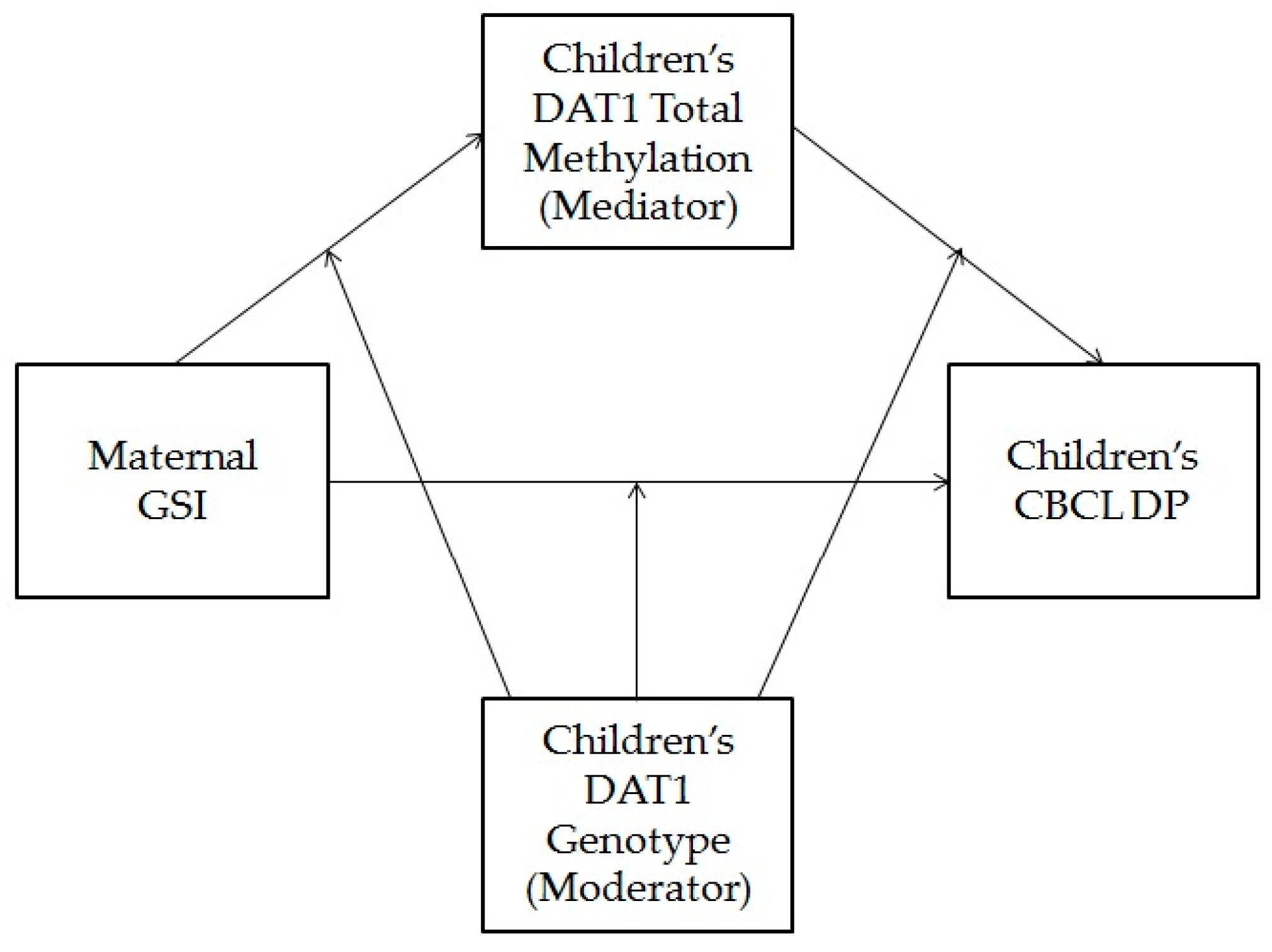
3.4. Children’s Dopamine Transporter Methylation as a Mediator of the Association between Maternal Psychopathological Risk and Children’s Dysregulation, Moderated by Children’s Genotype
4. Discussion
4.1. Impulsive/Irritable Subtype
4.2. Sensory Food Aversions Subtype
4.3. Post Traumatic Feeding Disorder Subtype
4.4. The Complex Interplay between the Variables
4.5. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bressan, R.; Crippa, J. The Role of Dopamine in Reward and Pleasure Behaviour—Review of Data from Preclinical Research. Acta Psychiatr. Scand. 2005, 111, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Erlanson-Albertsson, C. How Palatable Food Disrupts Appetite Regulation. Basic Clin. Pharmacol. Toxicol. 2005, 97, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Fowler, J.S. The Role of Dopamine in Motivation for Food in Humans: Implications for Obesity. Expert Opin. Ther Targets 2002, 6, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Cordeira, J.W.; Frank, L.; Sena-Esteves, M.; Pothos, E.N.; Rios, M. Brain-Derived Neurotrophic Factor Regulates Hedonic Feeding by Acting on the Mesolimbic Dopamine System. J. Neurosci. 2010, 30, 2533–2541. [Google Scholar] [CrossRef]
- Heinz, A.; Goldman, D.; Jones, D.W.; Palmour, R.; Hommer, D.; Gorey, J.G.; Lee, K.S.; Linnoila, M.; Weinberger, D.R. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000, 22, 133–139. [Google Scholar] [CrossRef]
- Jacobsen, L.K.; Staley, J.K.; Zoghbi, S.S.; Seibyl, J.P.; Kosten, T.R.; Innis, R.B.; Gelernter, J. Prediction of dopamine transporter binding availability by genotype: A preliminary report. Am. J. Psychiatry 2000, 157, 1700–1703. [Google Scholar] [CrossRef]
- Franke, B.; Vasquez, A.A.; Johansson, S.; Hoogman, M.; Romanos, J.; Boreatti-Hümmer, A.; Heine, M.; Jacob, C.P.; Lesch, K.-P.; Casas, M.; et al. Multicenter Analysis of the SLC6A3/DAT1 VNTR Haplotype in Persistent ADHD Suggests Differential Involvement of the Gene in Childhood and Persistent ADHD. Neuropsychopharmacology 2010, 35, 656–664. [Google Scholar] [CrossRef]
- Pinsonneault, J.K.; Han, D.D.; Burdick, K.E.; Kataki, M.; Bertolino, A.; Malhotra, A.K.; Gu, H.H.; Sadee, W. Dopamine Transporter Gene Variant Affecting Expression in Human Brain Is Associated with Bipolar Disorder. Neuropsychopharmacology 2011, 36, 1644–1655. [Google Scholar] [CrossRef]
- Blum, K.; Oscar-Berman, M.; Barh, D.; Giordano, J.; Genetics, G.M.D. Dopamine genetics and Function in Food and Substance Abuse. J. Genet. Syndr. Gene. Ther. 2013, 4, 121. [Google Scholar] [CrossRef]
- Bello, N.T.; Dopamine, H.A. Dopamine and Binge Eating Behaviors. Pharmacol. Biochem. Behav. 2010, 97, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Rask-Andersen, M.; Benedict, C.; Schiöth, H.B. A Debate on Current Eating Disorder Diagnoses in Light of Neurobiological Findings: Is It Time for a Spectrum Model? BMC Psychiatry 2012, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Mizushima, H.; Hirano, M.; Shioe, K.; Nakazawa, M.; Hiejima, Y.; Ono, Y.; Kanba, S. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J. Psychiatry Neurosci. 2004, 29, 134–137. [Google Scholar] [PubMed]
- Frieling, H.; Römer, K.D.; Scholz, S.; Mittelbach, F.; Wilhelm, J.; De Zwaan, M.; Jacoby, G.E.; Kornhuber, J.; Hillemacher, T.; Bleich, S. Epigenetic Dysregulation of Dopaminergic Genes in Eating Disorders. Int. J. Eat. Disord. 2010, 43, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Leventakou, V.; Micali, N.; Georgiou, V.; Sarri, K.; Koutra, K.; Koinaki, S.; Vassilaki, M.; Kogevinas, M.; Chatzi, L. Is There an Association between Eating Behaviour and Attention-Deficit/Hyperactivity Disorder Symptoms in Preschool Children? J. Child Psychol. Psychiatry 2016, 57, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yang, J.; Reynolds, G.P.; Chen, B.; Shao, J.; Liu, R.; Qian, Q.; Liu, H.; Yang, R.; Wen, J.; et al. DAT1 methylation is associated with methylphenidate response on oppositional and hyperactive-impulsive symptoms in children and adolescents with ADHD. World J. Biol. Psychiatry 2017, 18, 291–299. [Google Scholar] [CrossRef]
- Adriani, W.; Romano, E.; Pucci, M.; Pascale, E.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Curatolo, P.; Granstrem, O.; Maccarrone, M.; et al. Potential for diagnosis versus therapy monitoring of attention deficit hyperactivity disorder: A new epigenetic biomarker interacting with both genotype and auto-immunity. Eur. Child Adolesc. Psychiatry 2018, 27, 241–252. [Google Scholar] [CrossRef]
- Cimino, S.; Cerniglia, L.; Ballarotto, G.; Marzilli, E.; Pascale, E.; D’Addario, C.; Adriani, W.; Tambelli, R. DNA Methylation at the DAT Promoter and Risk for Psychopathology: Intergenerational Transmission between School-Age Youths and Their Parents in a Community Sample. Front. Psychiatry 2018, 8, 303. [Google Scholar] [CrossRef]
- Cimino, S.; Cerniglia, L.; Ballarotto, G.; Marzilli, E.; Pascale, E.; D’Addario, C.; Adriani, W.; Maremmani, A.; Tambelli, R. Children’s DAT1 polymorphism moderates the relationship between parents’ psychological profiles, children’s DAT methylation, and their emotional/behavioral functioning in a normative sample. Int. J. Environ. Res. Public Health 2019, 16, 2567. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Peter, C.J.; Fischer, L.K.; Kundakovic, M.; Garg, P.; Jakovcevski, M.; Dincer, A.; Amaral, A.C.; Ginns, E.I.; Galdzicka, M.; Bryce, C.P.; et al. DNA methylation signatures of early childhood malnutrition associated with impairments in attention and cognition. Biol. Psychiatry 2016, 80, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.T.; Cicchetti, D. Toward an integration of family systems and developmental psychopathology approaches. Dev. Psychopathol. 2004, 16, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Calkins, S.D.; Dollar, J.M. Emotion: Commentary. A biopsychosocial perspective on maternal psychopathology and the development of child emotion regulation. J. Pers. Disord. 2014, 28, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Golds, L.; de Kruiff, K.; MacBeth, A. Disentangling genes, attachment, and environment: A systematic review of the developmental psychopathology literature on gene-environment interactions and attachment. Dev. Psychopathol. 2020, 32, 357–381. [Google Scholar] [CrossRef]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5 (R)), 5th ed.; American Psychiatric Association Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Three, Z.T. Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood, 2nd ed.; ZERO TO THREE, National Center for Infants, Toddlers, & Families: Washington, DC, USA, 2005. [Google Scholar]
- Lucarelli, L.; Cimino, S.; D’Olimpio, F.; Ammaniti, M. Feeding Disorders of Early Childhood: An Empirical Study of Diagnostic Subtypes. Int. J. Eat. Disord. 2013, 46, 147–155. [Google Scholar] [CrossRef]
- Norris, M.L.; Spettigue, W.; Hammond, N.G.; Katzman, D.K.; Zucker, N.; Yelle, K.; Santos, A.; Gray, M.; Obeid, N. Building evidence for the use of descriptive subtypes In youth with avoidant restrictive food intake disorder. Int. J. Eat. Disord. 2018, 51, 170–173. [Google Scholar] [CrossRef]
- Chatoor, I.; Lucarelli, L. Feeding development and disorders. In Encyclopedia of Infant and Early Childhood Development, 2nd ed.; Benson, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 621–632. [Google Scholar]
- Cerniglia, L.; Marzilli, E.; Cimino, S. Emotional-Behavioral Functioning, Maternal Psychopathologic Risk and Quality of Mother-Child Feeding Interactions in Children with Avoidant/Restrictive Food Intake Disorder. Int. J. Environ. Res. Public Health 2020, 17, 3811. [Google Scholar] [CrossRef]
- Chatoor, I. Diagnosis and Treatment of Feeding Disorders in Infants, Toddlers, and Young Children; Zero to Three Press: Washington, DC, USA, 2009. [Google Scholar]
- American Psychiatric Association. Feeding and Eating Disorders: DSM-5 (R) Selections; American Psychiatric Association Publishing: Arlington, TX, USA, 2015. [Google Scholar]
- Dahl, M.; Sundelin, C. Feeding problems in an affluent society. Follow-up at four years of age in children with early refusal to eat. Acta. Paediatr. 1992, 81, 575–579. [Google Scholar] [CrossRef]
- Pennell, A.; Couturier, J.; Grant, C.; Johnson, N. Severe Avoidant/Restrictive Food Intake Disorder and Coexisting Stimulant Treated Attention Deficit Hyperactivity Disorder: Severe ARFID and Coexisting ADHD. Int. J. Eat. Disord. 2016, 49, 1036–1039. [Google Scholar] [CrossRef]
- Ammaniti, M.; Lucarelli, L.; Cimino, S.; D’Olimpio, F.; Chatoor, I. Feeding Disorders of Infancy: A Longitudinal Study to Middle Childhood. Int. J. Eat. Disord. 2012, 45, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lavender, J.M.; Wonderlich, S.A.; Engel, S.G.; Gordon, K.H.; Kaye, W.H.; Mitchell, J.M. Dimensions of Emotion Dysregulation in Anorexia Nervosa and Bulimia Nervosa: A Conceptual Review of the Empirical Literature. Clin. Psychol. Rev. 2015, 40, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Houser, M.E.; Voyer, A.P.; Grady, S.; Katzman, D.K. Children with avoidant/restrictive food intake disorder and anorexia nervosa in a tertiary care pediatric eating disorder program: A comparative study. Int. J. Eat. Disord. 2019, 52, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, L.; Sechi, C.; Cimino, S.; Chatoor, I. Avoidant/Restrictive Food Intake Disorder: A Longitudinal Study of Malnutrition and Psychopathological Risk Factors from 2 to 11 Years of Age. Front. Psychol. 2018, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Ammaniti, M.; Lucarelli, L.; Cimino, S.; D’Olimpio, F.; Chatoor, I. Maternal psychopathology and child risk factors in infantile anorexia. Int. J. Eat. Disord. 2010, 43, 233–240. [Google Scholar] [CrossRef]
- Champagne, F.A.; Curley, J.P. Epigenetic Mechanisms Mediating the Long-Term Effects of Maternal Care on Development. Neurosci. Biobehav. Rev. 2009, 33, 593–600. [Google Scholar] [CrossRef]
- Barker, E.D.; Walton, E.; Cecil, C.A.M. Annual Research Review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. J. Child Psychol. Psychiatry 2018, 59, 303–322. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Jovanovic, T.; Smith, A.K.; Binder, E.; Klengel, T.; Conneely, K.; Mercer, K.B.; Davis, J.S.; Kerley, K.; Winkler, J.; et al. Differential genetic and epigenetic regulation of catechol-O-methyltransferase is associated with impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef]
- Duman, E.A.; Canli, T. Influence of Life Stress, 5-HTTLPR Genotype, and SLC6A4 Methylation on Gene Expression and Stress Response in Healthy Caucasian Males. Biol. Mood Anxiety Disord. 2015, 5, 1. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Aseba Preschool Forms & Profiles: An Integrated System of Multi-Informant Assessment; Research Center for Children, Youth, & Families: Burlington, VT, USA, 2000. [Google Scholar]
- Frigerio, A.; Cattaneo, C.; Cataldo, M.; Schiatti, A.; Molteni, M.; Battaglia, M. Behavioral and emotional problems among Italian children and adolescents aged 4 to 18 years as reported by parents and teachers. Eur. J. Psychol. Assess. 2004, 20, 124–133. [Google Scholar] [CrossRef]
- Geeraerts, S.B.; Deutz, M.H.; Deković, M.; Bunte, T.; Schoemaker, K.; Espy, K.A.; Prinzie, P.; van Baar, A.; Matthys, W. The Child Behavior Checklist Dysregulation Profile in Preschool Children: A Broad Dysregulation Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Derogatis, L.R. SCL-90-R Symptom Checklist-90-R Administration, Scoring, and Procedures Manual; National Computer Systems: Minneapolis, MN, USA, 1994. [Google Scholar]
- Prunas, A.; Sarno, I.; Preti, E.; Madeddu, F.; Perugini, M. Psychometric Properties of the Italian Version of the SCL-90-R: A Study on a Large Community Sample. Eur. Psychiatry 2012, 27, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, L.; Cimino, S.; Bevilacqua, A.; Ballarotto, G.; Marzilli, E.; Adriani, W.; Tambelli, R. Patterns of DNA methylation at specific loci of the dopamine transporter 1 gene and psychopathological risk in trios of mothers, fathers and children. Eur. J. Dev. Psychol. 2020, 1–28. [Google Scholar] [CrossRef]
- Hayden, E.P.; Hanna, B.; Sheikh, H.I.; Laptook, R.S.; Kim, J.; Singh, S.M.; Klein, D.N. Child dopamine active transporter 1 genotype and parenting: Evidence for evocative gene-environment correlations. Dev. Psychopathol. 2013, 25, 163–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirata, T.; Uemura, T.; Shinohara, M.; Hirano, M. Association between Dopamine Transporter Gene (DAT1) Polymorphisms and Eating Disorders with Binge Eating Behavior. Open J. Psychiatr. 2017, 7, 329–343. [Google Scholar] [CrossRef][Green Version]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis, Second Edition: A Regression-Based Approach, 2nd ed.; Guilford Publications: New York, NY, USA, 2018. [Google Scholar]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and Its Comorbidity with Other Clinical Disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Maynard, L.; Jayne, M.; Fowler, J.S.; Zhu, W.; Logan, J.; Gatley, S.J.; Ding, Y.S.; Wong, C.; et al. Brain dopamine is associated with eating behaviors in humans. Int. J. Eat. Disord. 2003, 33, 136–142. [Google Scholar] [CrossRef]
- Tauscher, J.; Pirker, W.; Willeit, M.; de Zwaan, M.; Bailer, U.; Neumeister, A.; Asenbaum, S.; Lennkh, C.; Praschak-Rieder, N.; Brücke, T.; et al. [123I] beta-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol. Psychiatry 2001, 49, 326–332. [Google Scholar] [CrossRef]
- Peyre, H.; Speranza, M.; Cortese, S.; Wohl, M.; Purper-Ouakil, D. Do ADHD Children with and without Child Behavior Checklist-Dysregulation Profile Have Different Clinical Characteristics, Cognitive Features, and Treatment Outcomes? J. Atten. Disord. 2015, 19, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, D.; Yamashita, H.; Yamane, K.; Kanba, S.; Yoshida, K. Clinical Subtypes in Children with Attention-Deficit Hyperactivity Disorder According to Their Child Behavior Checklist Profile. Child Psychiatry Hum. Dev. 2020. [Google Scholar] [CrossRef]
- Faraone, S.V.; Bonvicini, C.; Scassellati, C. Biomarkers in the Diagnosis of ADHD—Promising Directions. Curr. Psychiatry Rep. 2014, 16, 11. [Google Scholar] [CrossRef]
- Lahey, B.B.; Rathouz, P.J.; Lee, S.S.; Chronis-Tuscano, A.; Pelham, W.E.; Waldman, I.D.; Cook, E.H. Interactions between early parenting and a polymorphism of the child’s dopamine transporter gene in predicting future child conduct disorder symptoms. J. Abnorm. Psychol. 2011, 120, 33–45. [Google Scholar] [CrossRef]
- Drury, S.S.; Theall, K.P.; Keats, B.J.B.; Scheeringa, M. The role of the dopamine transporter (DAT) in the development of PTSD in preschool children. J. Trauma Stress. 2009, 22, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Gervasini, G.; Gordillo, I.; García-Herráiz, A.; Flores, I.; Jiménez, M.; Monge, M.; Carrillo, J.A. Influence of dopamine polymorphisms on the risk for anorexia nervosa and associated psychopathological features. J. Clin. Psychopharmacol. 2013, 33, 551–555. [Google Scholar] [CrossRef]
- Anreiter, I.; Sokolowski, H.M.; Sokolowski, M.B. Gene–environment interplay and individual differences in behavior: Gene-environment interplay. Mind Brain Educ. 2018, 12, 200–211. [Google Scholar] [CrossRef]
- Belsky, J.; Pluess, M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol. Bull. 2009, 135, 885–908. [Google Scholar] [CrossRef]
- Hoexter, M.Q.; Fadel, G.; Felício, A.C.; Calzavara, M.B.; Batista, I.R.; Reis, M.A.; Shih, M.C.; Pitman, R.K.; Andreoli, S.B.; Mello, M.F.; et al. Higher striatal dopamine transporter density in PTSD: An in vivo SPECT study with [(99m)Tc]TRODAT-1. Psychopharmacology 2012, 224, 337–345. [Google Scholar] [CrossRef]
- Mongillo, E.A.; Briggs-Gowan, M.; Ford, J.D.; Carter, A.S. Impact of traumatic life events in a community sample of toddlers. J. Abnorm. Child Psychol. 2009, 37, 455–468. [Google Scholar] [CrossRef]
- Maguire, S.A.; Williams, B.; Naughton, A.M.; Cowley, L.E.; Tempest, V.; Mann, M.K.; Teague, M.; Kemp, A.M. A systematic review of the emotional, behavioural and cognitive features exhibited by school-aged children experiencing neglect or emotional abuse: Systematic review of school-aged neglect/emotional abuse. Child Care Health Dev. 2015, 41, 641–653. [Google Scholar] [CrossRef]
- Basten, M.M.; Althoff, R.R.; Tiemeier, H.; Jaddoe, V.W.; Hofman, A.; Hudziak, J.J.; Verhulst, F.C.; van der Ende, J. The dysregulation profile in young children: Empirically defined classes in the Generation R study. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 841–850.e2. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Cerniglia, L.; Porreca, A.; Ballarotto, G.; Marzilli, E.; Simonelli, A. Impact of parental binge eating disorder: Exploring children’s emotional/behavioral problems and the quality of parent-child feeding interactions. Infant Ment. Health J. 2018, 39, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, L.; Muratori, P.; Milone, A.; Paciello, M.; Ruglioni, L.; Cimino, S.; Levantini, V.; Tambelli, R. Paternal psychopathological risk and psychological functioning in children with eating disorders and Disruptive Behavior Disorder. Psychiatry Res. 2017, 254, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Parkes, A.; Sweeting, H. Direct, indirect, and buffering effects of support for mothers on children’s socioemotional adjustment. J. Fam. Psychol. 2018, 32, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Rollè, L.; Prino, L.E.; Sechi, C.; Vismara, L.; Neri, E.; Polizzi, C.; Trovato, A.; Volpi, B.; Molgora, S.; Fenaroli, V.; et al. Parenting stress, mental health, dyadic adjustment: A structural equation model. Front. Psychol. 2017, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Marzilli, E.; Tafà, M.; Cerniglia, L. Emotional-Behavioral Regulation, Temperament and Parent–Child Interactions Are Associated with Dopamine Transporter Allelic Polymorphism in Early Childhood: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 8564. [Google Scholar] [CrossRef]
- Paschall, K.W.; Mastergeorge, A.M. A review of 25 years of research in bidirectionality in parent–child relationships: An examination of methodological approaches. Int. J. Behav. Dev. 2016, 40, 442–451. [Google Scholar] [CrossRef]
- Lee, S.S.; Chronis-Tuscano, A.; Keenan, K.; Pelham, W.E.; Loney, J.; Van Hulle, C.A.; Cook, E.H.; Lahey, B.B. Association of maternal dopamine transporter genotype with negative parenting: Evidence for gene x environment interaction with child disruptive behavior. Mol. Psychiatry 2010, 15, 548–558. [Google Scholar] [CrossRef]
- Auerbach, J.G.; Zilberman-Hayun, Y.; Atzaba-Poria, N.; Berger, A. The contribution of maternal ADHD symptomatology, maternal DAT1, and home atmosphere to child ADHD symptomatology at 7 years of age. J. Abnorm. Child Psychol. 2017, 45, 415–427. [Google Scholar] [CrossRef]
- Cicchetti, D.; Blender, J.A. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Ann. N. Y. Acad. Sci. 2006, 1094, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.V.; Herzing, L.B.; Cook, E.H.; Gouze, K.R.; Hopkins, J.; Bryant, F.B. Gene × environment effects of serotonin transporter, dopamine receptor D4, and monoamine oxidase A genes with contextual and parenting risk factors on symptoms of oppositional defiant disorder, anxiety, and depression in a community sample of 4-year-old children. Dev. Psychopathol. 2013, 25, 555–575. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.A.; Groom, A.; Potter, C.; Coneyworth, L.J.; Ford, D.; Mathers, J.C.; Relton, C.L. Genetic and non-genetic influences during pregnancy on infant global and site specific DNA methylation: Role for folate gene variants and vitamin B12. PLoS ONE 2012, 7, e33290. [Google Scholar] [CrossRef] [PubMed]
- Maestro, S.; Cordella, M.R.; Intorcia, C.; Roversi, C.; Scardigli, S.; Silvestri, V.; Sara Calderoni, M.D. Parent-child interaction treatment for preschoolers with feeding disorders. Isr. J. Psychiatry Relat. Sci. 2016, 53, 63–72. [Google Scholar] [PubMed]
| Children’s Diagnoses | ||||||
|---|---|---|---|---|---|---|
| DAT1 Genotype | I/I | SFA | PTFD | NC | Total | |
| 9/x | N (%) | 19 (82.6) | 4 (17.4) | 21 (91.3) | 24 (96) | 68 |
| Exp. Val. | 16.6 | 16.6 | 16.6 | 18.1 | 68.0 | |
| St. R | 1.3 | −6.8 | 2.3 | 3.1 | ||
| 10/10 | N (%) | 4 (17.4) | 19 (82.6) | 2 (8.7) | 1 (4) | 26 |
| Exp. Val. | 6.4 | 6.4 | 6.4 | 6.9 | 26.0 | |
| St. R | −1.3 | 6.8 | −2.3 | −3.1 | ||
| Total | N | 23 | 23 | 23 | 25 | 94 |
| Children’s Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| I/I | SFA | PTFD | NC | |||||
| Variable | M | SD | M | SD | M | SD | M | SD |
| DAT1 Total methylation | 8.29 a | 1.07 | 6.01 b | 0.93 | 4.08 c | 1.18 | 6.08 b | 0.54 |
| CBCL DP | 33.69 a | 5.53 | 18.39 b | 5.67 | 35.43 a | 5.05 | 7.12 c | 2.83 |
| SCL-90/R GSI | 1.95 a | 0.22 | 0.60 b | 0.10 | 1.54 c | 0.17 | 0.18 d | 0.06 |
| Variable | B(SE) | LLCI | ULCI | |
| DAT methyl. | ||||
| GSI | 0.23 (0.11) * | 0.01 | 0.45 | |
| DAT1 genotype † | −0.14 (0.26) | −0.66 | 0.38 | |
| GSI x DAT1 genotype † | −0.31 (0.32) | −0.96 | 0.33 | |
| R2 = 0.04 | ||||
| F (3,90) = 1.51 | ||||
| CBCL DP | ||||
| GSI | 11.26 (0.73) ** | 9.80 | 12.71 | |
| DAT methylation | −1.06 (0.81) | −2.68 | 0.56 | |
| DAT1 genotype † | −3.39 (1.61) * | −6.60 | −0.19 | |
| GSI x DAT1 genotype † | 0.84 (2.35) | −3.83 | 5.52 | |
| DAT methyl x DAT1 genotype † | −6.48 (2.85) * | −12.15 | −0.80 | |
| R2 = 0.79 | ||||
| F (5,88) = 69.98 *** | ||||
| Conditional Direct Effect at Specific Levels of Moderator | ||||
| Predictor | Moderator | Direct Effect (SE) | LLCI | ULCI |
| GSI | 10/10 | 10.61 (2.25) ** | 6.12 | 15.10 |
| 9/9, 9/10 | 11.45 (0.66) ** | 10.13 | 12.77 | |
| R2 = 0.75F (3,90) = 93.75 ** | ||||
| Conditional Indirect Effect at Specific Levels of Moderator | ||||
| Mediator | Moderator | Indirect Effect (BootSE) | LLCI | ULCI |
| DAT methyl. | 10/10 | 1.85 (2.52) | −3.20 | 6.74 |
| 9/9, 9/10 | −0.41 (0.33) | −1.17 | 0.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimino, S.; Marzilli, E.; Babore, A.; Trumello, C.; Cerniglia, L. DAT1 and Its Psychological Correlates in Children with Avoidant/Restrictive Food Intake Disorder: A Cross-Sectional Pilot Study. Behav. Sci. 2021, 11, 9. https://doi.org/10.3390/bs11010009
Cimino S, Marzilli E, Babore A, Trumello C, Cerniglia L. DAT1 and Its Psychological Correlates in Children with Avoidant/Restrictive Food Intake Disorder: A Cross-Sectional Pilot Study. Behavioral Sciences. 2021; 11(1):9. https://doi.org/10.3390/bs11010009
Chicago/Turabian StyleCimino, Silvia, Eleonora Marzilli, Alessandra Babore, Carmen Trumello, and Luca Cerniglia. 2021. "DAT1 and Its Psychological Correlates in Children with Avoidant/Restrictive Food Intake Disorder: A Cross-Sectional Pilot Study" Behavioral Sciences 11, no. 1: 9. https://doi.org/10.3390/bs11010009
APA StyleCimino, S., Marzilli, E., Babore, A., Trumello, C., & Cerniglia, L. (2021). DAT1 and Its Psychological Correlates in Children with Avoidant/Restrictive Food Intake Disorder: A Cross-Sectional Pilot Study. Behavioral Sciences, 11(1), 9. https://doi.org/10.3390/bs11010009







