A Review on the Role of the Neuroscience of Flow States in the Modern World
Abstract
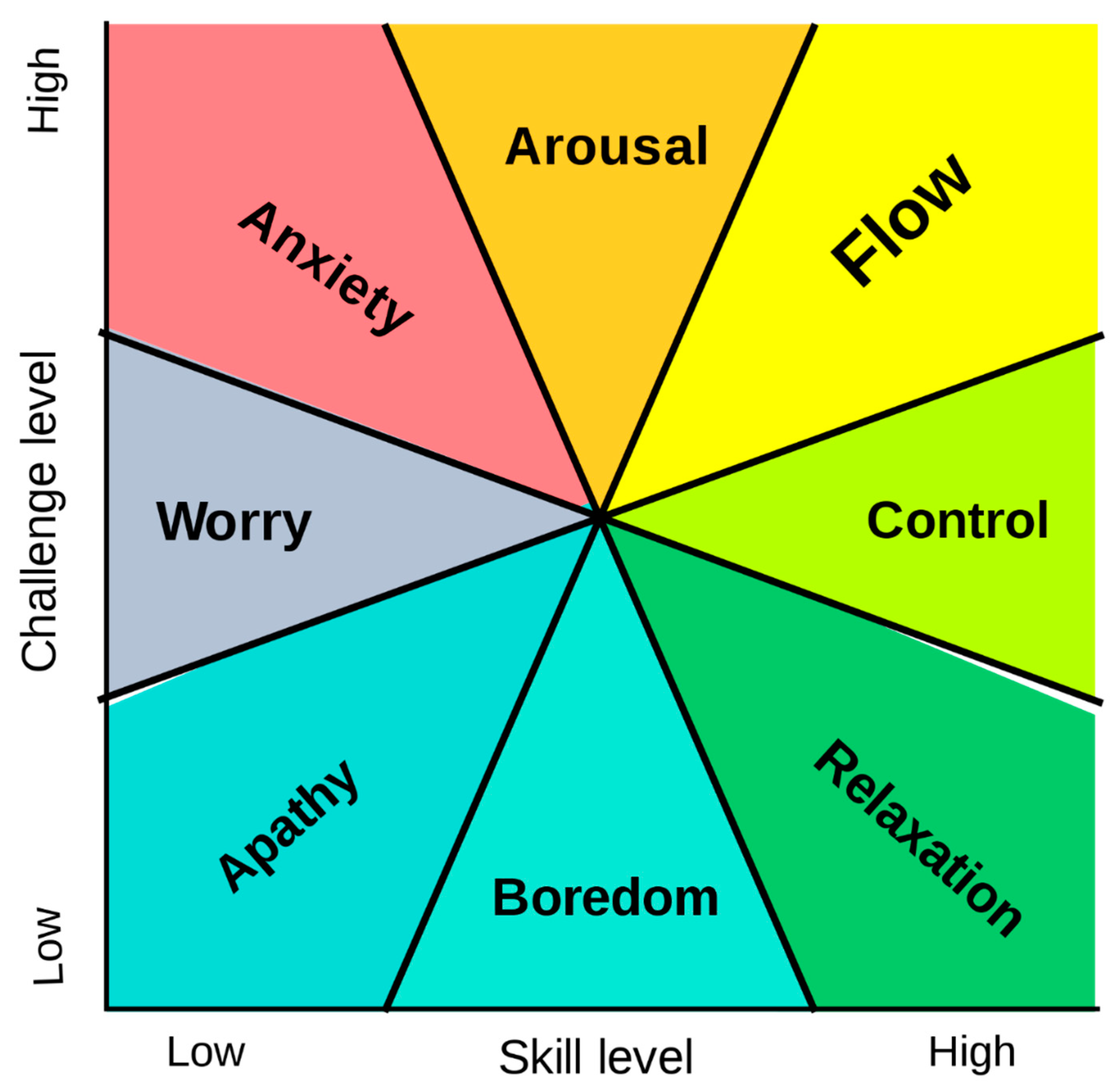
1. Introduction to Flow
1.1. Environmental Influences on Flow
1.2. Flow Measurement
1.3. Flow Neurocognitive Mechanisms
1.4. Neurocognitive Models of Flow
2. Exploration of Flow Functions
3. Flow Facilitation
4. Conclusions
Funding
Conflicts of Interest
References
- Rheinberg, F.; Vollmeyer, R.; Engeser, S. Die Erfassung des Flow-Erlebens (The Assessment of Flow); Stiensmeier-Pelster, J., Rheinberg, F., Eds.; Hogrefe: Göttingen, Germany, 2003. [Google Scholar]
- Nakamura, J.; Csikszentmihalyi, M. The concept of flow. In Handbook of Positive Psychology; Oxford University Press: New York, NY, USA, 2002; pp. 89–105. [Google Scholar]
- Csikszentmihalyi, I.; Csikszentmihalyi, M. Optimal Experience: Psychological Studies of Flow in Consciousness; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Cranston, S.; Keller, S. Increasing the meaning quotient of work. McKinsey Q. 2013, 1, 48–59. [Google Scholar]
- Maslow, A.H. Religions, Values, and Peak-Experiences; Ohio State University Press: Columbus, OH, USA, 1964. [Google Scholar]
- Csikszentmihalyi, M. Beyond Boredom and Anxiety: Experiencing Flow in Work and Play; Josey–Bass: San Francisco, CA, USA, 2000. [Google Scholar]
- Csikszentmihalyi, M. Finding Flow: The Psychology of Engagement with Everyday Life; Basic Books: New York, NY, USA, 1997. [Google Scholar]
- Csikszentmihalyi, M.; Abuhamdeh, S.; Nakamura, J. Flow. In Handbook of Competence and Motivation; Elliot, A.J., Dweck, C.S., Eds.; The Guilford Press: New York, NY, USA, 2005; pp. 598–608. [Google Scholar]
- Csikszentmihalyi, M. Flow: The Psychology of Optimal Experience; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Rheinberg, F. Intrinsic motivation and flow-experience. In Motivation and Action, 1st ed.; Heckhausen, H., Heckhausen, J., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 323–348. [Google Scholar]
- Jackson, S.A. Toward a Conceptual Understanding of the Flow Experience in Elite Athletes. Res. Q. Exerc. Sport 1996, 67, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.H.; Teichert, T. The Janus-Faced Role of Gambling Flow in Addiction Issues. Cyberpsychology Behav. Soc. Netw. 2017, 20, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Hedderson, C. Intrinsic Motivation and Flow in Skateboarding: An Ethnographic Study. J. Happiness Stud. 2009, 11, 277–292. [Google Scholar] [CrossRef]
- Rogatko, T.P. The Influence of Flow on Positive Affect in College Students. J. Happiness Stud. 2009, 10, 133–148. [Google Scholar] [CrossRef]
- Becker, K. Choosing and Using Digital Games in the Classroom; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Fong, C.J.; Zaleski, D.J.; Leach, J.K. The challenge—Skill balance and antecedents of flow: A meta-analytic investigation. J. Posit. Psychol. 2015, 10, 425–446. [Google Scholar] [CrossRef]
- Engeser, S.; Rheinberg, F. Flow, performance and moderators of challenge-skill balance. Motiv. Emot. 2008, 32, 158–172. [Google Scholar] [CrossRef]
- Atkinson, J.W. Motivational determinants of risk-taking behavior. Psychol. Rev. 1957, 64, 359. [Google Scholar] [CrossRef]
- Csikszentmihalyi, M.; LeFevre, J. Optimal experience in work and leisure. J. Personal. Soc. Psychol. 1989, 56, 815–822. [Google Scholar] [CrossRef]
- Viljoen, C. The Experience of Flow in Professional and Semi-Professional Orchestral Musicians. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Kabat-Zinn, J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life; Hachette Books: New York, NY, USA, 2009. [Google Scholar]
- De Charms, R.d. Personal Causation; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Deci, E.; Ryan, R.M. Intrinsic Motivation and Self-Determination in Human Behavior; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Canter, D.; Rivers, R.; Storrs, G. Characterizing user navigation through complex data structures. Behav. Inf. Technol. 1985, 4, 93–102. [Google Scholar] [CrossRef]
- Webster, J.; Martocchio, J.J. Microcomputer Playfulness: Development of a Measure with Workplace Implications. MIS Q. 1992, 16, 201–226. [Google Scholar] [CrossRef]
- Ghani, J.A.; Deshpande, S.P. Task Characteristics and the Experience of Optimal Flow in Human—Computer Interaction. J. Psychol. 1994, 128, 381–391. [Google Scholar] [CrossRef]
- Larson, R.; Csikszentmihalyi, M. The Experience Sampling Method. In Flow and the Foundations of Positive Psychology; Springer: Dordrecht, The Netherlands, 2014; pp. 21–34. [Google Scholar]
- Swann, C. Flow in sport. In Flow Experience; Springer: New York, NY, USA, 2016; pp. 51–64. [Google Scholar]
- Jackson, S.A.; Eklund, R.C. The Flow Scales Manual; Fitness Information Technology: Morgantown, WV, USA, 2004. [Google Scholar]
- Peifer, C.; Schulz, A.; Schachinger, H.; Baumann, N.; Antoni, C.H. The relation of flow-experience and physiological arousal under stress—Can u shape it? J. Exp. Soc. Psychol. 2014, 53, 62–69. [Google Scholar] [CrossRef]
- Brewer, B.W.; Van Raalte, J.L.; Linder, D.E.; Van Raalte, N.S. Peak Performance and the Perils of Retrospective Introspection. J. Sport Exerc. Psychol. 1991, 13, 227–238. [Google Scholar] [CrossRef]
- Dietrich, A. Neurocognitive mechanisms underlying the experience of flow. Conscious. Cogn. 2004, 13, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Sun, R. Duality of the Mind: A Bottom-Up Approach toward Cognition; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2001. [Google Scholar]
- Ashby, F.G.; Maddox, W.T. Stimulus categorization. In Measurement, Judgment and Decision Making; Elsevier: New York, NY, USA, 1998; pp. 251–301. [Google Scholar]
- Jiménez, L.; Vaquero, J.M.M.; Lupiañez, J. Qualitative differences between implicit and explicit sequence learning. J. Exp. Psychol. Learn. Mem. Cogn. 2006, 32, 475–490. [Google Scholar] [CrossRef]
- Berry, D.C.; Broadbent, D.E. Interactive tasks and the implicit-explicit distinction. Br. J. Psychol. 1988, 79, 251–272. [Google Scholar] [CrossRef]
- Köhler, W. The Mentality of Apes; Harcourt, Brace & Co.: New York, NY, USA, 1925. [Google Scholar]
- Crick, F.; Koch, C. Consciousness and neuroscience. Cereb. Cortex 1998, 8, 97–107. [Google Scholar] [CrossRef]
- Ashby, F.G.; Casale, M.B. The cognitive neuroscience of implicit category learning. Investig. Phenom. Conscious. Res. 2003, 48, 109–142. [Google Scholar]
- Dehaene, S.; Naccache, L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 2001, 79, 1–37. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Packard, M.G. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 2003, 41, 245–251. [Google Scholar] [CrossRef]
- Dietrich, A. Functional neuroanatomy of altered states of consciousness: The transient hypofrontality hypothesis. Conscious. Cogn. 2003, 12, 231–256. [Google Scholar] [CrossRef]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Mandon, S.; Freiwald, W.A.; Kreiter, A.K. Coherent oscillatory activity in monkey area v4 predicts successful allocation of attention. Cereb Cortex 2005, 15, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Executive frontal functions. Exp. Brain Res. 2000, 133, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef]
- Fuster, J.M. Temporal processing—Structure and function of the human prefrontal cortex. Ann. N. Y. Acad. Sci. 1995, 769, 173–181. [Google Scholar] [CrossRef]
- Sarter, M.; Givens, B.; Bruno, J.P. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res. Rev. 2001, 35, 146–160. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Lhermitte, F.; Pillon, B.; Serdaru, M. Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: A neuropsychological study of 75 patients. Ann. Neurol. 1986, 19, 326–334. [Google Scholar] [CrossRef]
- Crews, F.T.; Boettiger, C.A. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav. 2009, 93, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gaspelin, N.; Luck, S.J. The Role of Inhibition in Avoiding Distraction by Salient Stimuli. Trends Cogn. Sci. 2018, 22, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, M. Psychophysiological Patterns Related to Individual Zone Optimal Performance in Self-Paced Task: A Step forward with Map Model; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2014; Semantic Scholar; Corpus ID: 141471373. [Google Scholar]
- Katahira, K.; Yamazaki, Y.; Yamaoka, C.; Ozaki, H.; Nakagawa, S.; Nagata, N. EEG Correlates of the Flow State: A Combination of Increased Frontal Theta and Moderate Frontocentral Alpha Rhythm in the Mental Arithmetic Task. Front. Psychol. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Castellar, E.P.N.; Antons, J.; Marinazzo, D.; Van Looy, J. Mapping attention during gameplay: Assessment of behavioral and ERP markers in an auditory oddball task. Psychophysiology 2019, 56, e13347. [Google Scholar] [CrossRef]
- Fairclough, S.H. BCI and physiological computing: Similarities, differences and intuitive control. In Proceedings of the Workshop on BCI and Computer Games, Florence, Italy, 5–10 April 2008. [Google Scholar]
- Ulrich, M.; Keller, J.; Grön, G. Neural signatures of experimentally induced flow experiences identified in a typical fMRI block design with BOLD imaging. Soc. Cogn. Affect. Neurosci. 2016, 11, 496–507. [Google Scholar] [CrossRef]
- Ulrich, M.; Keller, J.; Hoenig, K.; Waller, C.; Grön, G. Neural correlates of experimentally induced flow experiences. NeuroImage 2014, 86, 194–202. [Google Scholar] [CrossRef]
- Hirao, K. Prefrontal hemodynamic responses and the degree of flow experience among occupational therapy students during their performance of a cognitive task. J. Educ. Eval. Health Prof. 2014, 11, 24. [Google Scholar] [CrossRef]
- Weber, R.; Tamborini, R.; Kantor, B.; Westcott-Baker, A. Theorizing Flow and Media Enjoyment as Cognitive Synchronization of Attentional and Reward Networks. Commun. Theory 2009, 19, 397–422. [Google Scholar] [CrossRef]
- Harmat, L.; De Manzano, O.; Theorell, T.; Högman, L.; Fischer, H.; Ullén, F. Physiological correlates of the flow experience during computer game playing. Int. J. Psychophysiol. 2015, 97, 1–7. [Google Scholar] [CrossRef]
- Posner, M.I.; Inhoff, A.W.; Friedrich, F.J.; Cohen, A. Isolating attentional systems: A cognitive-anatomical analysis. Psychobiology 1987, 15, 107–121. [Google Scholar]
- Huskey, R.; Craighead, B.; Miller, M.B.; Weber, R. Does intrinsic reward motivate cognitive control? A naturalistic-fMRI study based on the synchronization theory of flow. Cogn. Affect. Behav. Neurosci. 2018, 18, 902–924. [Google Scholar] [CrossRef]
- Huskey, R.; Wilcox, S.; Weber, R. Network Neuroscience Reveals Distinct Neuromarkers of Flow during Media Use. J. Commun. 2018, 68, 872–895. [Google Scholar] [CrossRef]
- Wolf, S.; Brölz, E.; Keune, P.M.; Wesa, B.; Hautzinger, M.; Birbaumer, N.; Strehl, U. Motor skill failure or flow-experience? Functional brain asymmetry and brain connectivity in elite and amateur table tennis players. Boil. Psychol. 2015, 105, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Klasen, M.; Weber, R.; Kircher, T.; Mathiak, K.A.; Mathiak, K. Neural contributions to flow experience during video game playing. Soc. Cogn. Affect. Neurosci. 2012, 7, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Baars, B.J. A Cognitive Theory of Consciousness; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Karmiloff-Smith, A. Beyond Modularity: A Developmental Perspective on Cognitive Science; MIT Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Reber, A.S. Implicit learning and tacit knowledge. J. Exp. Psychol. Gen. 1989, 118, 219. [Google Scholar] [CrossRef]
- Schacter, D.L.; Buckner, R.L. On the Relations among Priming, Conscious Recollection, and Intentional Retrieval: Evidence from Neuroimaging Research. Neurobiol. Learn. Mem. 1998, 70, 284–303. [Google Scholar] [CrossRef][Green Version]
- Spiering, B.J.; Ashby, F.G. Initial training with difficult items facilitates information integration, but not rule-based category learning. Psychol. Sci. 2008, 19, 1169–1177. [Google Scholar] [CrossRef]
- Ashby, F.G.; Gott, R.E. Decision rules in the perception and categorization of multidimensional stimuli. J. Exp. Psychol. Learn. Mem. Cogn. 1988, 14, 33–53. [Google Scholar] [CrossRef]
- Allen, S.W.; Brooks, L.R. Specializing the operation of an explicit rule. J. Exp. Psychol. Gen. 1991, 120, 3–19. [Google Scholar] [CrossRef]
- Regehr, G.; Brooks, L.R. Perceptual manifestations of an analytic structure: The priority of holistic individuation. J. Exp. Psychol. Gen. 1993, 122, 92–114. [Google Scholar] [CrossRef]
- Wilson, C.J. The contribution of cortical neurons to the firing pattern of striatal spiny neurons. In Models of Information Processing in the Basal Ganglia; Houk, J.C., Davis, J.L., Beiser, D.G., Eds.; Bradford: Cambridge, MA, USA, 1995; pp. 29–50. [Google Scholar]
- Shook, B.L.; Schlag-Rey, M.; Schlag, J. Primate supplementary eye field. II. Comparative aspects of connections with the thalamus, corpus striatum, and related forebrain nuclei. J. Comp. Neurol. 1991, 307, 562–583. [Google Scholar] [CrossRef] [PubMed]
- Ashby, F.G.; Ennis, J.M.; Spiering, B.J. A neurobiological theory of automaticity in perceptual categorization. Psychol. Rev. 2007, 114, 632–656. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, G.W.; Ingham, C.A.; Wickens, J.R. Dopamine and synaptic plasticity in the neostriatum. J. Anat. 2000, 196, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wickens, J. A Theory of the Striatum; Pergamon Press: New York, NY, USA, 1993. [Google Scholar]
- Malenka, R.C.; Nicoli, R.A. Long-term potentiation—A decade of progress? Science 1999, 285, 1870–1874. [Google Scholar] [CrossRef]
- Maddox, W.T.; Ashby, F.G.; Bohil, C.J. Delayed feedback effects on rule-based and information-integration category learning. J. Exp. Psychol. Learn. Mem. Cogn. 2003, 29, 650. [Google Scholar] [CrossRef]
- Halford, G.S.; Wilson, W.H.; Phillips, S. Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behav. Brain Sci. 1998, 21, 803–831. [Google Scholar] [CrossRef]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef]
- Jenkins, I.H.; Brooks, D.; Nixon, P.D.; Frackowiak, R.S.; Passingham, R.E. Motor sequence learning: A study with positron emission tomography. J. Neurosci. 1994, 14, 3775–3790. [Google Scholar] [CrossRef]
- Gazzaniga, S.M.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience; W.W. Norton: New York, NY, USA, 1998. [Google Scholar]
- Mishkin, M.; Malamut, B.; Bachevalier, J. Memory and habit: Two neural systems. In Neurobiology of Learning and Memory; Lynch, G., McGaugh, J.J., Weinberger, N.M., Eds.; Guilford Press: New York, NY, USA, 1984; pp. 66–77. [Google Scholar]
- Ashby, F.G.; Turner, B.O.; Horvitz, J.C. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn. Sci. 2010, 14, 208–215. [Google Scholar] [CrossRef]
- Shiffrin, R.M.; Schneider, W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol. Rev. 1977, 84, 127–190. [Google Scholar] [CrossRef]
- Happe, F. Automaticity of Action, Psychology of. In International Encyclopedia of the Social & Behavioral Sciences; Smelser, N.J., Baltes, P.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 11. [Google Scholar]
- Attri, R.K. The Models of Skill Acquisition and Expertise Development: A Quick Reference of Summaries; Speed to Profiency Research, S2Pro: Singapore, 2019. [Google Scholar]
- De Manzano, O.; Cervenka, S.; Jucaite, A.; Hellenäs, O.; Farde, L.; Ullén, F. Individual differences in the proneness to have flow experiences are linked to dopamine D2-receptor availability in the dorsal striatum. NeuroImage 2013, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gyurkovics, M.; Kotyuk, E.; Katonai, E.R.; Horvath, E.Z.; Vereczkei, A.; Szekely, A. Individual differences in flow proneness are linked to a dopamine D2 receptor gene variant. Conscious. Cogn. 2016, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Boot, W.R.; Basak, C.; Neider, M.B.; Prakash, R.S.; Voss, M.W.; Graybiel, A.M.; Simons, D.J.; Fabiani, M.; Gratton, G.; et al. Striatal Volume Predicts Level of Video Game Skill Acquisition. Cereb. Cortex 2010, 20, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, D.A. Perception and Communication; Pergamon: New York, NY, USA, 1958. [Google Scholar]
- Frackowiak, R.; Friston, K.; Frith, C.; Dolan, R.; Price, C.; Zeki, S. Brain systems mediating reward. Hum. Brain Funct. 2003, 445–470. [Google Scholar]
- Hatfield, B.D.; Landers, D.M.; Ray, W.J. Cognitive Processes during Self-Paced Motor Performance: An Electroencephalographic Profile of Skilled Marksmen. J. Sport Psychol. 1984, 6, 42–59. [Google Scholar] [CrossRef]
- Lawton, G.W.; Hung, T.M.; Saarela, P.; Hatfield, B.D. Electroencephalography and Mental States Associated with Elite Performance. J. Sport Exerc. Psychol. 1998, 20, 35–53. [Google Scholar] [CrossRef]
- Haufler, A.; Spalding, T.W.; Maria, D.S.; Hatfield, B.D. Neuro-cognitive activity during a self-paced visuospatial task: Comparative EEG profiles in marksmen and novice shooters. Boil. Psychol. 2000, 53, 131–160. [Google Scholar] [CrossRef]
- Gannon, T.; Landers, D.; Kubitz, K.; Salazar, W.; Petruizello, S. An Analysis of Temporal Electroemeeptialograpliic Patterning Prior to Initiation of the Arm Curl. J. Sport Exerc. Psychol. 1992, 14, 87–100. [Google Scholar] [CrossRef]
- Crews, D.J.; Landers, D.M. Electroencephalographic measures of attentional patterns prior to the golf putt. Med. Sci. Sports Exerc. 1993, 25, 116–126. [Google Scholar] [CrossRef]
- Landers, D.M.; Han, M.; Salazar, W.; Petruzzello, S.J. Effects of learning on electroencephalographic and electrocardiographic patterns in novice archers. Int. J. Sport Psychol. 1994, 25, 313–330. [Google Scholar]
- Wei, G.X.; Zhang, Y.; Jiang, T.; Luo, J. Increased Cortical Thickness in Sports Experts: A Comparison of Diving Players with the Controls. PLoS ONE 2011, 6, e17112. [Google Scholar] [CrossRef] [PubMed]
- Vernon, D.J. Can Neurofeedback Training Enhance Performance? An Evaluation of the Evidence with Implications for Future Research. Appl. Psychophysiol. Biofeedback 2005, 30, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Deeny, S.P.; Hillman, C.H.; Janelle, C.M.; Hatfield, B.D. Cortico-cortical Communication and Superior Performance in Skilled Marksmen: An EEG Coherence Analysis. J. Sport Exerc. Psychol. 2003, 25, 188–204. [Google Scholar] [CrossRef]
- Keller, J.; Bless, H.; Blomann, F.; Kleinböhl, D. Physiological aspects of flow experiences: Skills-demand-compatibility effects on heart rate variability and salivary cortisol. J. Exp. Soc. Psychol. 2011, 47, 849–852. [Google Scholar] [CrossRef]
- Earle, J.B.B.; Pikus, A.A. The effect of sex and task difficulty on EEG alpha activity in association with arithmetic. Biol. Psychol. 1982, 15, 1–14. [Google Scholar] [CrossRef]
- Rugg, M.; Dickens, A. Dissociation of alpha and theta activity as a function of verbal and visuospatial tasks. Electroencephalogr. Clin. Neurophysiol. 1982, 53, 201–207. [Google Scholar] [CrossRef]
- Shaw, J.C. Intention as a component of the alpha-rhythm response to mental activity. Int. J. Psychophysiol. 1996, 24, 7–23. [Google Scholar] [CrossRef]
- Klimesch, W.; Schimke, H.; Pfurtscheller, G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993, 5, 241–251. [Google Scholar] [CrossRef]
- Lindsay, P.; Maynard, I.; Thomas, O. Effects of Hypnosis on Flow States and Cycling Performance. Sport Psychol. 2005, 19, 164–177. [Google Scholar] [CrossRef]
- Baer, L. Effect of a Time-Slowing Suggestion on Performance Accuracy on a Perceptual Motor Task. Percept. Mot. Ski. 1980, 51, 167–176. [Google Scholar] [CrossRef]
- Liggett, D.R. Sport Hypnosis; Human Kinetics: Champaign, IL, USA, 2000. [Google Scholar]
- Crawford, H.J.; Gruzelier, J.H. A midstream view of the neuropsychophysiology of hypnosis: Recent research and future directions. In Contemporary Hypnosis Research; Fromm, E., Nash, M.R., Eds.; Guilford: London, UK, 1992; pp. 227–266. [Google Scholar]
- Gruzelier, J.; Warren, K. Neuropsychological evidence of reductions on left frontal tests with hypnosis. Psychol. Med. 1993, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tellegen, A.; Atkinson, G.; Auke, T.; Gilbert, A. Openness to absorbing and self-altering experiences (“absorption”), a trait related to hypnotic susceptibility. J. Abnorm. Psychol. 1974, 83, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Frischholz, E.; Braun, B.G.; Sachs, R.G.; Schwartz, D.R.; Lewis, J.; Shaeffer, D.; Pasquotto, J. Construct validity of the Dissociative experiences scale (DES): I. Relatsh. DES Self-Rep. Meas. DES Dissociation Prog. Dissociative Disord. 1991, 4, 185–188. [Google Scholar]
- Lutz, A.; Slagter, H.A.; Rawlings, N.B.; Francis, A.D.; Greischar, L.L.; Davidson, R.J. Mental training enhances attentional stability: Neural and behavioral evidence. J. Neurosci. 2009, 29, 13418–13427. [Google Scholar] [CrossRef]
- Jacobs, J.; Kahana, M.J.; Ekstrom, A.; Fried, I. Brain Oscillations Control Timing of Single-Neuron Activity in Humans. J. Neurosci. 2007, 27, 3839–3844. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Buhle, J. Typologies of attentional networks. Nat. Rev. Neurosci. 2006, 7, 367–379. [Google Scholar] [CrossRef]
- Newberg, A.B.; Iversen, J. The neural basis of the complex mental task of meditation: Neurotransmitter and neurochemical considerations. Med. Hypotheses 2003, 61, 282–291. [Google Scholar] [CrossRef]
- Miller, A.; Tomarken, A.J. Task-dependent changes in frontal brain asymmetry: Effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology 2001, 38, 500–511. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Sherwood, R.J.; Henriques, J.B.; Davidson, R.J. Frontal Brain Asymmetry and Reward Responsiveness: A Source-Localization Study. Psychol. Sci. 2005, 16, 805–813. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J. Frontal EEG asymmetry as a moderator and mediator of emotion. Boil. Psychol. 2004, 67, 7–50. [Google Scholar] [CrossRef]
- Davidson, R.J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Boil. Psychol. 2004, 67, 219–234. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.B.; Mattu, U.; Grafman, J.; Lomarev, M.; Sato, S.; Wassermann, E.M. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005, 64, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Vanderhasselt, M.A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ammann, C.; Spampinato, D.; Márquez-Ruiz, J. Modulating Motor Learning through Transcranial Direct-Current Stimulation: An Integrative View. Front. Psychol. 2016, 7, 1981. [Google Scholar] [CrossRef]
- Gibson, B.C.; Mullins, T.S.; Heinrich, M.D.; Witkiewitz, K.; Yu, A.B.; Hansberger, J.T.; Clark, V.P. Transcranial direct current stimulation facilitates category learning. Brain Stimul. 2020, 13, 393–400. [Google Scholar] [CrossRef]
- Shadmehr, R.; Wise, S.P. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Clark, V.P.; Coffman, B.A.; Mayer, A.R.; Weisend, M.P.; Lane, T.D.; Calhoun, V.D.; Raybourn, E.M.; Garcia, C.M.; Wassermann, E.M. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. NeuroImage 2012, 59, 117–128. [Google Scholar] [CrossRef]
- Mazzoni, P.; Krakauer, J.W. An Implicit Plan Overrides an Explicit Strategy during Visuomotor Adaptation. J. Neurosci. 2006, 26, 3642–3645. [Google Scholar] [CrossRef]
- Scheldrup, M.; Greenwood, P.M.; McKendrick, R.; Strohl, J.; Bikson, M.; Alam, M.; McKinley, R.A.; Parasuraman, R. Transcranial direct current stimulation facilitates cognitive multi-task performance differentially depending on anode location and subtask. Front. Hum. Neurosci. 2014, 8, 665. [Google Scholar] [CrossRef]
- Bullard, L.M.; Browning, E.S.; Clark, V.P.; Coffman, B.A.; Garcia, C.M.; Jung, R.E.; Weisend, M.P. Transcranial direct current stimulation’s effect on novice versus experienced learning. Exp. Brain Res. 2011, 213, 9–14. [Google Scholar] [CrossRef]
- Tseng, P.; Hsu, T.Y.; Chang, C.F.; Tzeng, O.J.; Hung, D.L.; Muggleton, N.G.; Juan, C.H. Unleashing potential: Transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J. Neurosci. 2012, 32, 10554–10561. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Klaus, M.; Nitsche, M.A.; Paulus, W.; Altenmüller, E. Ceiling effects prevent further improvement of transcranial stimulation in skilled musicians. J. Neurosci. 2014, 34, 13834–13839. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.S.; Erickson, B.; Kim, Y.E.; Mirman, D.; Hamilton, R.H.; Kounios, J. Anodal tDCS to right dorsolateral prefrontal cortex facilitates performance for novice jazz improvisers but hinders experts. Front. Hum. Neurosci. 2016, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Niemann, J.; Boland, M.; Kammer, T.; Niemann, F.; Grön, G. The neural correlates of flow experience explored with transcranial direct current stimulation. Exp. Brain Res. 2018, 236, 3223–3237. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.; Ciorciari, J. A Transcranial Stimulation Intervention to Support Flow State Induction. Front. Hum. Neurosci. 2019, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Yeung, A.Y.; Poolton, J.M.; Lee, T.M.; Leung, G.K.; Masters, R.S. Cathodal transcranial direct current stimulation over left dorsolateral prefrontal cortex area promotes implicit motor learning in a golf putting task. Brain Stimul. 2015, 8, 784–786. [Google Scholar] [CrossRef]
- Kasten, F.H.; Herrmann, C.S. Transcranial Alternating Current Stimulation (tACS) Enhances Mental Rotation Performance during and after Stimulation. Front. Hum. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef]
- Jausovec, N.; Jaušovec, K. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Boil. Psychol. 2014, 96, 42–47. [Google Scholar] [CrossRef]
|
|
|
|
|
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gold, J.; Ciorciari, J. A Review on the Role of the Neuroscience of Flow States in the Modern World. Behav. Sci. 2020, 10, 137. https://doi.org/10.3390/bs10090137
Gold J, Ciorciari J. A Review on the Role of the Neuroscience of Flow States in the Modern World. Behavioral Sciences. 2020; 10(9):137. https://doi.org/10.3390/bs10090137
Chicago/Turabian StyleGold, Joshua, and Joseph Ciorciari. 2020. "A Review on the Role of the Neuroscience of Flow States in the Modern World" Behavioral Sciences 10, no. 9: 137. https://doi.org/10.3390/bs10090137
APA StyleGold, J., & Ciorciari, J. (2020). A Review on the Role of the Neuroscience of Flow States in the Modern World. Behavioral Sciences, 10(9), 137. https://doi.org/10.3390/bs10090137






