The Experience of Post-Stroke Pain and The Impact on Quality of Life: An Integrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Data Extraction
2.4. Critical Appraisal
2.5. Data Analysis and Qualitative Synthesis
3. Results
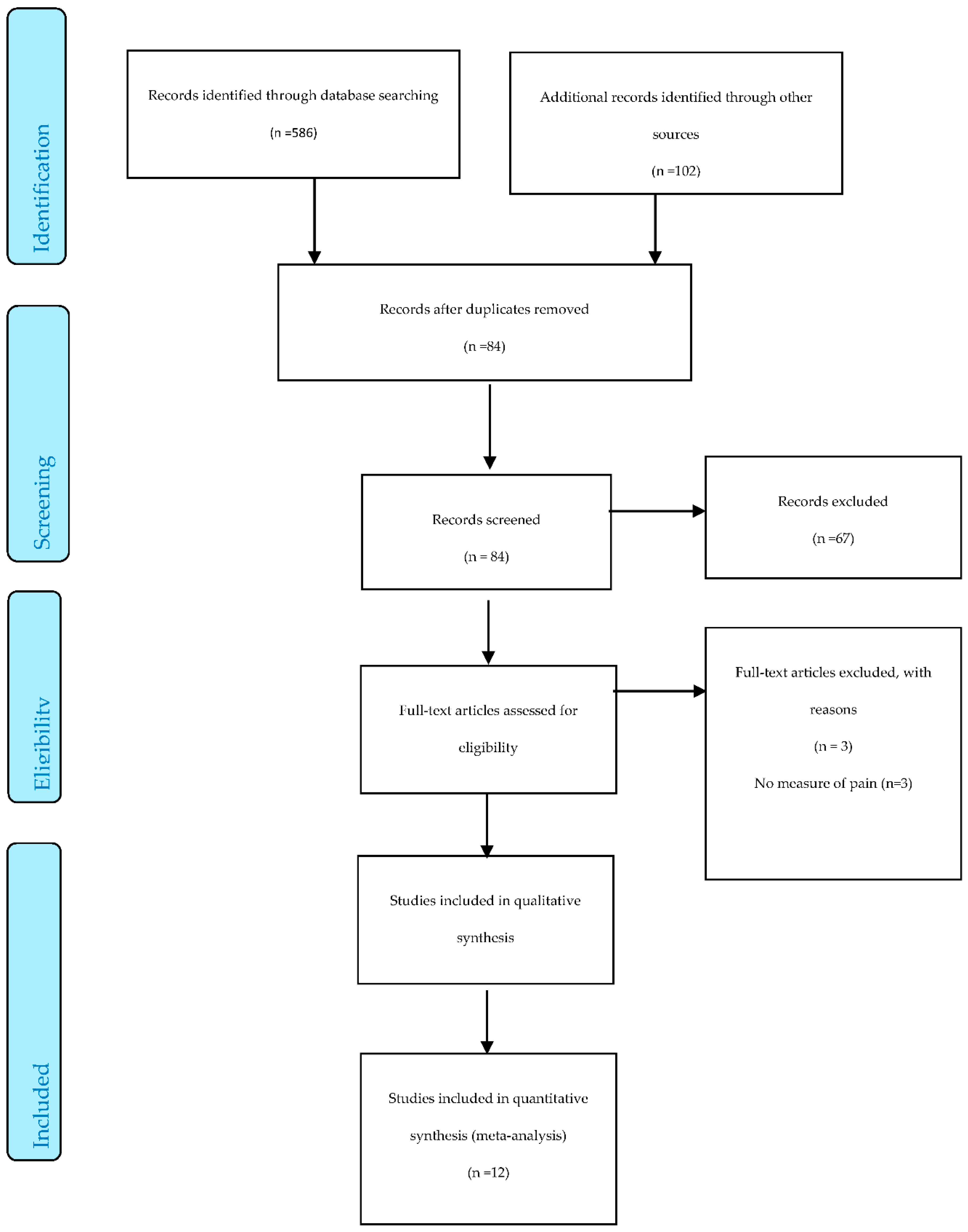
3.1. Search
3.2. Demographics
3.3. Critical Appraisal
3.3.1. Between Study Quantitative Appraisal
3.3.2. Within Study Quantitative Appraisal
3.3.3. Between Study Qualitative Appraisal
3.3.4. Within Study Qualitative Appraisal
3.4. Synthesis
3.4.1. Depression and Pain
Scales Used to Assess Depression
Depression and Pain; Quantitative Evidence
Depression and Pain, Qualitative Evidence
Evidence Summary
3.4.2. The effect of Depression on QOL
Scales Used to Assess QOL
Depression and QOL; Quantitative Evidence
Depression and QOL, Qualitative Evidence
Evidence Summary
3.4.3. Anxiety and Pain
Scales used to assess anxiety
Anxiety and Pain, Quantitative Evidence
Anxiety and Pain, Qualitative Evidence
Evidence Summary
3.4.4. The Effect of Anxiety on QOL
3.4.5. Fatigue
Scales Used to Assess Fatigue
Fatigue and Pain, Quantitative Evidence
Fatigue and Pain, Qualitative Evidence
Evidence Summary
3.4.6. The Effect of Fatigue on QOL
Scales Used to Assess QOL
Fatigue and QOL; Quantitative Evidence
Fatigue and QOL, Qualitative Evidence
Evidence Summary
3.4.7. Cognitive Function and Pain
Scales Used to Assess Cognitive Function
Cognitive Function and Pain, Quantitative Evidence
Cognitive Function and Pain; Qualitative Evidence
Evidence Summary
3.4.8. The Effect of Reduced Cognitive Function on QOL
Scales Used to Assess QOL
Cognitive Function and QOL, Quantitative Evidence
Cognitive Function and QOL, Qualitative Evidence
Evidence Summary
3.4.9. Physical Function and Pain
Scales Used to Assess Physical Function
Physical Function and Pain; Quantitative Evidence
Physical Function and Pain, Qualitative Evidence
Evidence Summary
3.4.10. The Effect of Reduced Physical Function on QOL
Scales Used to Assess QOL
Physical Function and QOL, Quantitative Evidence
Physical Function and QOL, Qualitative Evidence
Evidence Summary
3.4.11. The Effect of Pain on QOL
Scales Used to Assess QOL
Pain and QOL, Quantitative Evidence
Pain and QOL, Qualitative Evidence
Evidence Summary
Interactions and Associations
4. Discussion
4.1. Emotional Distress
4.1.1. Depression
4.1.2. Anxiety
4.2. Other Factors
4.2.1. Fatigue
4.2.2. Cognitive Function
4.2.3. Physical Function
4.3. Impact on QOL
4.3.1. Depression
4.3.2. Fatigue
4.3.3. Cognitive Function
4.3.4. Physical Function
4.3.5. Pain
4.4. Implications
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klit, H.; Finnerup, N.; Overvad, K.; Andersen, G.; Jensen, T. Pain Following Stroke: A Population-Based Follow-Up Study. PLoS ONE 2011, 6, e27607. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Diener, H.-C.; Sacco, R.L.; Panju, A.A.; Vinisko, R.; Yusuf, S. Chronic pain syndromes after ischemic stroke. Stroke 2013, 44, 1238–1243. [Google Scholar] [CrossRef]
- Westerlind, E.; Singh, R.; Persson, H.C.; Sunnerhagen, K.S. Experienced pain after stroke: A cross-sectional 5-year follow-up study. BMC Neurol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Harrison, R.A.; Field, T.S. Post Stroke Pain: Identification, Assessment, and Therapy. Cereb. Dis. 2015, 39, 190–201. [Google Scholar] [CrossRef]
- Widar, M.; Ahlstrom, G. Disability after a stroke and the influence of long-term pain on everyday life. Scand. J. Caring Sci. 2002, 16, 302–310. [Google Scholar] [CrossRef]
- Delpont, B.; Blanc, C.; Osseby, G.V.; Hervieu-Begue, M.; Giroud, M.; Bejot, Y. Pain after stroke. A review. Revue Neurol. 2017, 174, 671–674. [Google Scholar] [CrossRef]
- Treister, A.K.; Hatch, M.N.; Cramer, S.C.; Chang, E.Y. Demystifying Poststroke Pain: From Etiology to Treatment. PM R. 2016, 9, 63–75. [Google Scholar] [CrossRef]
- Jensen, M.P.; Chodroff, M.J.; Dworkin, R.H. The impact of neuropathic pain on health-related quality of life. Review and implications. Neurology 2007, 68, 1178–1182. [Google Scholar] [CrossRef]
- Golomb, B.A.; Vickrey, B.G.; Hays, R.D. A review of health-related quality-of-life measures in stroke. Pharmacoeconomics 2001, 19, 155–185. [Google Scholar] [CrossRef]
- Tang, S.-C.; Lee, L.J.-H.; Jeng, J.-S.; Hsieh, S.-T.; Chaiang, M.-C.; Yeh, S.-J.; Hsueh, H.-W.; Chao, C.-C. Pathophysiolgy of central post stroke pain. Motor cortex disinhibition and its clinical and sensory correlates. Stroke 2019, 50, 2851–2857. [Google Scholar] [CrossRef]
- Dysvik, E.; Lindstrom, T.; Eikeland, O.; Natvig, G. Health-related quality of life and pain beliefs among people suffering from chronic pain. Pain Manag. Nurs. 2004, 52, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, M.; Goh, H.-T. Post-stroke fatigue: A review on prevalence, correlates, measurement and management. Top. Stroke Rehabil. 2015, 22, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 1317, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Halaicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Neuropsychological Changes in Complex Regional Pain Syndrome CRPS. Behav. Neurol. 2019, 456, 1–30. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Teltzlaff, J.; Altman, D.G. PRIMSA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER Tool for Qualitative Evidence Synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef]
- Hénon, G. Pain after stroke: A neglected issue. J. Neurol. Neurosurg. Psychiatry 2006, 77, 569. [Google Scholar] [CrossRef]
- WHO. WHOQOL: Measuring Quality of Life. 2020. Available online: https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/index4.html (accessed on 24 March 2020).
- McGowan, J.; Sampson, M.; Salzwedel, D.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Soundy, A.; Roskell, C.; Elder, T.; Collett, J.; Dawes, H. The Psychological Processes of Adaptation and Hope in Patients with Multiple Sclerosis: A Thematic Synthesis. Open J. Rehabil. 2016, 4, 22–47. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated Criteria for Reporting Qualitative Research COREQ: A 32-Item Checklist for Interviews and Focus Groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Emberson, J.R. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Choi-Kwon, S.; Choi, J.; Kwon, S.; Kang, D.; Kim, J. Factors that Affect the Quality of Life at 3 Years Post-Stroke. J. Clin. Neurol. 2006, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Galligan, N.; Hevey, D.; Coen, R.; Harbison, J. Clarifying the associations between anxiety, depression and fatigue following stroke. J. Health Psychol. 2016, 2112, 2863–2871. [Google Scholar] [CrossRef]
- Jonsson, A.; Lindgren, I.; Hallstrom, B.; Norrving, B.; Lindgren, A. Prevalence and intensity of pain after stroke: A population based study focusing on patients’ perspectives. J. Neurol. Neurosurg. Psychiatry 2006, 77, 590–595. [Google Scholar] [CrossRef]
- Kong, K.H.; Woon, V.C.; Yang, S.Y. Prevalence of Chronic Pain and Its Impact on Health-Related Quality of Life in Stroke Survivors. Arch. Phys. Med. Rehabil. 2004, 85, 35–40. [Google Scholar] [CrossRef]
- Lindgren, I.; Gard, G.; Brogårdh, C. Shoulder pain after stroke—Experiences, consequences in daily life and effects of interventions: A qualitative study. Disabil. Rehabil. 2018, 40, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Naess, H.; Lunde, L.; Brogger, J. The Triad of Pain, Fatigue and Depression in Ischemic Stroke Patients: The Bergen Stroke Study. Cereb. Dis. 2012, 33, 461–465. [Google Scholar] [CrossRef]
- Naess, H.; Lunde, L.; Brogger, J. The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: The Bergen Stroke Study. Vasc. Health Risk Manag. 2012, 8, 407–413. [Google Scholar] [CrossRef]
- Şahin-Onat, S.; Ünsal-Delialioğlu, S.; Kulaklı, F.; Özel, S. The effects of central post-stroke pain on quality of life and depression in patients with stroke. J. Phys. Sci. 2016, 28, 96–101. [Google Scholar] [CrossRef]
- Tang, W.K.; Lau, C.G.; Mok, V.; Ungvari, G.S.; Wong, K.S. The impact of pain on health-related quality of life 3 months after stroke. Top. Stroke Rehabil. 2015, 22, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Widar, M.; Samuelsson, L.; Karlsson-Tivenius, S.; Ahlstrom, G. Long-term pain conditions after stroke. J. Rehabil. Med. 2002, 34, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Widar, M.; Ahlstrom, G.; Ek, A. Health-related quality of life in persons with long-term pain after a stroke. J. Clin. Nurs. 2004, 13, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Widar, M.; Ek, A.; Ahlstrom, G. Coping with Long-Term Pain after a Stroke. J. Pain Symptom Manag. 2004, 27, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; Sutton, A.; Shaw, R. Appraising qualitative research for inclusion in systematic reviews: A quantitative and qualitative comparison of three methods. J. Health Serv. Res. Policy 2007, 121, 42–47. [Google Scholar] [CrossRef]
- Button, K.; Kounali, D.; Thomas, L.; Wiles, N.; Peters, T.; Welton, N.; Ades, A.; Lewis, G. Minimal clinically important difference on the Beck Depression Inventory-II according to the patient’s perspective. Psychol. Med. 2015, 45, 3269–3279. [Google Scholar] [CrossRef]
- Sawilowsky, S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 579–599. [Google Scholar] [CrossRef]
- Hsieh, Y.-W.; Wang, C.-H.; Wu, S.-C.; Chen, P.-C.; Sheu, C.-F.; Hsieh, C.-L. Establishing the Minimal Clinically Important Difference of the Barthel Index in Stroke Patients. Neurorehabil. Neural Repair 2007, 213, 233–238. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 1998, 133, 581–624. [Google Scholar] [CrossRef]
- Sieber. The elderly patient-04Who is that? Internist Berl. 2007, 4811, 1192–1194. [Google Scholar]
- Van Almenkerk, S.; Depla, M.F.I.A.; Smalbrugge, M.; Eefsting, J.; Hertogh, C.M.P.M. Pain among institutionalised stroke patients its relationship to emotional distress and social engagement. Int. J. Geriatr. Psychiatry 2015, 30, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A. Anxiety: A short history, Baltimore: John Hopkins University Press. Sociol. Health Illn. 2013, 37, 164–165. [Google Scholar]
- Markham, R.; Darke, S. The effects of anxiety on verbal and spatial task performance. Aust. J. Psychol. 1991, 43, 107–111. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network SIGN. Management of Patients with Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning: A National Clinical Guideline. Available online: https://www.sign.ac.uk/assets/sign118.pdf (accessed on 3 June 2020).
- Hinkle, J.; Becker, K.; Kim, J.; Choi-Kwon, S.; Saban, K.; McNair, N.; Mead, G. Poststroke Fatigue: Emerging Evidence and Approaches to Management: A Scientific Statement for Healthcare Professionals from the American Heart Association. Stroke 2017, 48, e159–e170. [Google Scholar] [CrossRef]
- Miller, K.; Combs, S.; Van Puymbroeck, M.; Altonburger, P.; Kean, J.; Dierks, T.; Schmid, A. Fatigue and Pain: Relationships with Physical Performance and Patient Beliefs after Stroke. Top. Stroke Rehabil. 2015, 20, 347–355. [Google Scholar] [CrossRef]
- Su, Y.S.; Veeravagu, A.; Grant, G. Chapter 8 Neuroplasticity after traumatic brain injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; Taylor and Francis: London, UK, 2018. [Google Scholar]
- Stroemal-Scheder, C.; Kundermann, B.; Lautenbacher, S. The effects of recovery sleep on pain perception: A systematic review. Neurosci. Behav. Rev. 2020, 113, 408–425. [Google Scholar] [CrossRef]
- Eccleson, C.; Crombez, G. Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychol. Bull. 1999, 125, 356–366. [Google Scholar] [CrossRef]
- Hart, R.P.; Martelli, M.F.; Zasler, N.D. Chronic pain and neuropsychological functioning. Neuropsychol. Rev. 2000, 10, 131–149. [Google Scholar] [CrossRef]
- Deary, V.; Chalder, T.; Sharpe, M. The cognitive behavioural model of medically unexplained symptoms: A theoretical and empirical review. Clin. Psychol. Rev. 2007, 27, 781–797. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- McCory, P. Headaches and exercise. Sports Med. 2012, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.; Vestergaard, K.; Ingemann-Nielsen, M.; Lauritzen, L. Risk factors for post-stroke depression. Acta Psychiatr. Scand. 1995, 92, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Barge-Schaapveld, D.; Nicolson, N.; Berkhof, J.; WdeVries, M. Quality of life in depression: Daily life determinants and variability. Psychiatry Res. 1999, 88, 173–189. [Google Scholar] [CrossRef]
- Brown, C.; Hasson, H.; Thyselius, V.; Almborg, A.H. Post-stroke depression and functional independence: A conundrum. Acta Neurolgica Scand. 2012, 126, 45–51. [Google Scholar] [CrossRef]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, P.; Hung, Y.; Iannetti, G.; Zhang, Z. A novel approach to predict subjective pain perception from single-trial laser-evoked potentials. Neuro Image 2013, 81, 283–293. [Google Scholar] [CrossRef]
| Factor | Quantitative Evidence | Qualitative Evidence | Summary |
|---|---|---|---|
| Depression and PSP |
|
|
|
| Depression and QOL |
|
| |
| Anxiety and PSP |
|
|
|
| Fatigue and PSP |
|
|
|
| Fatigue and QOL |
|
| |
| Cognitive function and PSP |
|
|
|
| Cognitive function and QOL |
|
|
|
| Physical function and PSP |
|
|
|
| Physical function and QOL |
|
|
|
| Interactions and associations |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payton, H.; Soundy, A. The Experience of Post-Stroke Pain and The Impact on Quality of Life: An Integrative Review. Behav. Sci. 2020, 10, 128. https://doi.org/10.3390/bs10080128
Payton H, Soundy A. The Experience of Post-Stroke Pain and The Impact on Quality of Life: An Integrative Review. Behavioral Sciences. 2020; 10(8):128. https://doi.org/10.3390/bs10080128
Chicago/Turabian StylePayton, Hannah, and Andrew Soundy. 2020. "The Experience of Post-Stroke Pain and The Impact on Quality of Life: An Integrative Review" Behavioral Sciences 10, no. 8: 128. https://doi.org/10.3390/bs10080128
APA StylePayton, H., & Soundy, A. (2020). The Experience of Post-Stroke Pain and The Impact on Quality of Life: An Integrative Review. Behavioral Sciences, 10(8), 128. https://doi.org/10.3390/bs10080128






